Abstract
The receptor-tyrosine-kinase-like orphan receptor 1 (ROR1) is a transmembrane protein belongs to receptor tyrosine kinase (RTK) family. This study aimed to examine the expression of ROR1 in human ovarian cancer and investigate the relationship between its expression and the prognosis of ovarian cancer patients. In this present study, one-step quantitative reverse transcription-polymerase chain reaction (15 ovarian cancer samples of high FIGO stage, 15 ovarian cancer samples of low FIGO stage and nine normal ovary tissue samples) and immunohistochemistry by tissue microarrays (100 ovarian cancer samples and 50 normal ovary samples) were performed to characterize expression of the ROR1 gene in ovarian cancer. Kaplan-Meier survival and Cox regression analyses were executed to evaluate the prognosis of ovarian cancer. The results of qPCR and IHC analysis showed that the expression of ROR1 in ovarian cancer was significantly higher than that in normal ovary tissues (all p < 0.05). Survival analysis showed that ROR1 protein expression was one of the independent prognostic factors for disease-free survival and overall survival (both p < 0.05). The data suggest that ROR1 expression is correlated with malignant attributes of ovarian cancer and it may serve as a novel prognostic marker in ovarian cancer.
Similar content being viewed by others
Introduction
Ovarian cancer is the most lethal gynecologic malignancy among women worldwide and its incidence has been increasing persistently in Asian countries, including China1,2. Approximately over 200,000 new cases of ovarian cancer occurred worldwide in 20113. Ovarian cancer generally originates from the malignant transformation of the ovarian surface epithelium, which is a single continuous layer of epithelial cells surrounding the ovary. The majority of ovarian cancer patients are diagnosed at advanced stages because of asymptomatic characteristic and lack of susceptible detection at early stage4. Currently, surgery is still necessary for appropriate staging of ovarian cancer and for improving chemotherapy results and survival rate. Chemotherapy is an important strategy in the treatment of ovarian cancer. Platinum-taxane combination has been used as the reference standard for the first-line chemotherapy of postsurgical ovarian cancer5. Although the standard platinum-taxane regimen shows effectiveness with a response rate of 80% in advanced ovarian cancer patients, most of these patients relapse because of drug resistance6,7. Therefore, the identification of novel and specific biomarkers that have clinicopathologic and prognostic significance in ovarian cancer is remarkably important.
The receptor-tyrosine-kinase-like orphan receptor 1 (ROR1) is a transmembrane protein that belongs to the receptor tyrosine kinase (RTK) family. ROR1 consists of an extracellular frizzled-like, cysteine-rich domain, an extracellular, membrane proximal kringle domain and an intracellular tyrosine-kinase-like domain8,9. ROR1 protein is evolutionarily conserved among various species and it is primarily expressed during embryogenesis. ROR1-deficient mice do not display any morphological abnormalities of the skeleton or heart or face, but they die within 24 h after birth probably because of respiratory failure10. Although the exact biological function of ROR1 is not fully understood, an increasing number of studies indicated that ROR1 is highly associated with human cancers11,12,13,14,15 and ROR1 may serve as a potential target for cancer therapy16,17,18. Moreover, there is emerging data suggests that the Wnt/β-catenin pathway plays an important role in carcinogenesis of all ovarian cancer subtypes19,20. Wnt5a, a substantial ligand of ROR1, also participates in the ROR1-dependent signaling pathway in enhancing cancer cell growth12,13. Hence, we assume that there may be intriguing relationship between ROR1 expression and certain clinicopathological significance of ovarian cancer. The potential of ROR1 as a candidate for molecular-targeted therapy of ovarian cancer requires further investigation.
In this present research, we detected the expression of ROR1 mRNA in fresh ovarian cancer tissue via one-step quantitative reverse transcription-polymerase chain reaction (qPCR). Subsequently, we examined the expression of ROR1 protein in ovarian cancer with tissue microarray (TMA) by immunohistochemistry (IHC) analysis. Finally, we evaluated the correlation of ROR1 expression with the clinicopathologic features and survival of ovarian cancer.
Results
Clinical features of 100 ovarian cancer patients
The main clinicopathologic characteristics of ovarian cancer patients are shown in Table 1. The age of the 100 patients with ovarian carcinoma ranged from 21 years to 82 years (mean age, 50.8 years). The tumor diameter of 64 patients was >5 cm, whereas that of the remaining 36 patients was <5 cm. In terms of the distribution of FIGO stage, 56 patients were at stages I and II, while 44 patients were at stages III and IV. Regarding the histologic tumor grade, 25 patients were at grade 1, 50 were at grade 2 and 25 were at grade 3. The distribution of histological type was as follows: 76 patients had serous-papillary type, 6 had clear cell type, 10 had mucinous type and 8 had endometrioid type. The serum CA-125 level of 52 patients was >35 U/ml, whereas that of the other 48 patients was <35 U/ml. A total of 63 patients presented positive ascites syndrome while the remaining 37 showed negative ascites. There were 35 patients encountered positive lymph node metastasis and the other 65 showed negative lymph node metastasis. 43 patients experienced cancer relapse while the other 57 did not. All 100 patients with ovarian cancer shared an overall survival rate of 39% and a disease-free survival rate of 28%.
Analysis of ROR1 mRNA expression in ovarian cancer by qPCR
Three reference genes, peptidylprolyl isomerase A (PPIA), β-actin (ACTB) and TATA-Box binding protein (TBP), were used as internal control in qPCR test. Total RNA was extracted from the 30 ovarian cancer tissues (15 high FIGO stage and 15 low FIGO stage) and subjected to one-step qPCR to evaluate the expression of ROR1 mRNA. We also detected samples from nine normal ovary tissues for comparing the expression of the mRNA with that of non-cancerous tissue. When normalized to PPIA, the expression of ROR1 mRNA (means ± SEM) in ovarian cancer of high stage, low stage and corresponding normal ovary were 4.02 ± 0.785, 3.20 ± 0.515 and 1.14 ± 0.292 respectively. The expression of ROR1 mRNA in high (t = 2.745, p = 0.002) and low (t = 2.910, p = 0.022) stage ovarian cancer samples was significantly higher than in non-cancerous tissues. Similarly, when normalized to ACTB and TBP, the ROR1 expression in ovarian cancer samples (both high and low stage) was statistically higher than that of in normal ovary samples (Figure 1).
One-step quantitative real-time polymerase chain reaction (qPCR) was employed to evaluate ROR1 mRNA expression levels in ovarian cancer compared with normal ovary tissues.
The ROR1 mRNA levels in both high and low FIGO stage ovarian cancer samples were significantly higher than that in normal ovary tissue, when normalized to PPIA, ACTB and TBP mRNA levels (*p < 0.05).
Detection of ROR1 protein expression in ovarian cancer by IHC
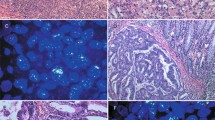
IHC was employed to investigate the expression of ROR1 in ovarian cancer. High ROR1 expression was detected in 55 of 100 (55%) ovarian cancer tissues, whereas only 6 cases of 50 normal ovary tissues (12%) exhibited ROR1 expression. A significant difference in ROR1 expression was found between ovarian cancer tissues and normal ovary tissues (p < 0.001). Positive staining was mainly localized in the cytoplasm and partly localized in the nucleus of cancer cells. The observed typical IHC staining shapes for ROR1 expression in ovarian cancer are shown in Figure 2.
Representative pattern of ROR1 protein expression in ovarian cancer and normal ovary tissue with tissue microarray (TMA) sections.
(A1), (A2) and (A3) High immunohistochemical (IHC) staining of ROR1 in serous-papillary type of ovarian cancer sample. (B1), (B2) and (B3) High IHC staining of ROR1 in clear cell type of ovarian cancer tissue sample. (C1), (C2) and (C3) High IHC staining of ROR1 in mucinous type of ovarian cancer tissue sample. (D1), (D2) and (D3) High IHC staining of ROR1 in endometrioid type of ovarian cancer tissue sample. (E1), (E2) and (E3) Low IHC staining of ROR1 in normal ovary tissue sample. Original magnification ×40 in (A1), (B1), (C1), (D1) and (E1); ×200 in (A2), (B2), (C2), (D2) and (E2); ×400 in (A3), (B3), (C3), (D3) and (E3).
Relationship between ROR1 expression and clinicopathological parameters
The relationship between ROR1 protein expression and the clinicopathological parameters of 100 ovarian cancer patients was demonstrated in Table 1. High ROR1 expression was associated with FIGO stage (p = 0.019), tumor grade (p = 0.027) and positive lymph node metastasis (p = 0.015). In contrast, no significant correlation was discovered between ROR1 expression and other clinical parameters, such as age, tumor diameter, histological type, serum CA-125 level, ascites condition and relapse status.
Survival analysis
According to univariate analysis, the disease-free survival of the ovarian cancer patients was correlated with ROR1 expression (p = 0.005), FIGO stage (p = 0.001) and lymph node metastasis (p = 0.001), whereas the overall survival was correlated with ROR1 expression (p = 0.001), FIGO stage (p = 0.001), tumor grade (p = 0.009) and lymph node metastasis (p = 0.001) in overall survival (Tables 2 and 3). Multivariate analysis using Cox regression model indicated that ROR1 protein expression and FIGO stage may serve as independent prognostic factors for disease-free survival (both p < 0.05) and overall survival (both p < 0.05) (Tables 2 and 3). Kaplan-Meier survival curves also demonstrated that ovarian cancer patients with high ROR1 expression and advanced FIGO stage presented a significantly unfavorable disease-free survival time and overall survival time (Figure 3).
Survival analysis of ovarian cancer patients by Kaplan-Meier method.
(A) Disease-free survival rate in patients with high ROR1 expression (red line) was significantly lower than that in patients with low and no ROR1 expression (black line). (B) Disease-free survival rate in patients with grade 2 and 3 of FIGO stage (red line) was significantly lower than that in patients with grade 1 of FIGO (black line). (C) Overall survival rate in patients with high ROR1 expression (red line) was significantly lower than that in patients with low and no ROR1 expression (black line). (D) Overall survival rate in patients with grade 2 and 3 of FIGO stage (red line) was significantly lower than that in patients with grade 1 of FIGO (black line).
Discussion
Despite the substantial advances in surgery and chemotherapy for ovarian cancer over the last few decades21,22,23,24, therapeutic failure and disease progression are still quite frequent25. Searching for novel and validated biomarkers correlated with the clinical and prognostic characteristics of ovarian cancer is important for diagnosis and treatment. A few studies have suggested several molecular biomarkers of ovarian cancer26,27. In our previous research, we have identified several oncogenic biomarkers, constructed related human monoclonal antibody-based drugs and tested their anti-tumor effectiveness28,29,30,31. Similarly, we have been searching a novel and valuable marker which associates with ovarian cancer and could be used as a potential candidate for targeted therapy with monoclonal antibodies.
RTKs have significant functions in cell differentiation, proliferation, angiogenesis and migration32,33. In this regard, RTK-like ROR1 is an evolutionarily conserved, type-I membrane protein that is primarily expressed during embryogenesis and it is significant for the morphogenesis of several organs34. Although the exact function of ROR1 in malignancies is not well understood, an increasing number of studies have reported that ROR1 expression is highly correlated with the development, progression and metastasis of various types of human cancers. At the same time, the possible mechanism of ROR1 characteristics is being explored continuously. ROR1 reportedly interacts with casein kinase 1 epsilon to activate phosphoinositide 3-kinase-mediated AKT phosphorylation and cAMP-response-element-binding protein, which are associated with enhanced tumor-cell growth. Also, Wnt5a, a ligand of ROR1, also participates in the ROR1-dependent signaling pathway in enhancing cancer cell growth12,13. Constitutive silencing of ROR1 tumor cells impairs significant proliferation in vitro and induces remarkable inhibition of tumorigenesis in vivo; and the critical function for ROR1 in malignant phenotypes is sustained by the Met oncogene15,35. Treatment with specific antibody for ROR1 in tumor cells induces the downmodulation of vimentin and inhibits cancer migration and invasion in cells as well as tumor metastasis in mice. ROR1 may regulate epithelial-mesenchymal transition and metastasis; antibodies that target ROR1 can inhibit cancer progression and metastasis14. Nevertheless, the potential relationship between ROR1 expression and ovarian cancer remains obscure. The present study is the first to investigate the association between ROR1 and ovarian cancer.
The qPCR result with three reference genes all confirmed that the ROR1 mRNA expressions in ovarian cancer tissues were higher than those in normal ovary tissues. The data is consistent with the previous reports, which showed that the mRNA of ROR1 was highly upregulated in cancer cell lines11,13,15. For further analysis, we prepared TMA with ovarian cancer specimens. IHC analysis revealed higher ROR1 protein expression in ovarian cancer tissues than that in normal ovary tissues. This result is similar to the studies of various malignancies that indicated high expression of ROR1 in cancer tissues11,12,13. Besides, high ROR1 expression in ovarian cancer was correlated with certain clinical pathologic parameters, including FIGO stage, tumor grade and positive lymph node metastasis. Kaplan-Meier analysis demonstrated that the life span of patients with positive ROR1 expression was shorter than that of patients with negative expression. Univariate analysis showed that ROR1 expression, FIGO stage and lymph node metastasis were correlated with life span (both in disease-free survival and overall survival) of ovarian cancer patients. Multivariate analysis further confirmed showed that high ROR1 expression and advanced FIGO stage independently predicted unfavorable disease-free survival and overall survival of ovarian cancer patients. Further studies that enroll a larger number of clinical samples of ovarian cancer tissues are necessary to confirm our findings concerning the effectiveness of ROR1 expression on the prognosis of human cancers. In addition, a fully human anti-ROR1 antibody is undergoing construction and evaluation by our group.
In conclusion, to the best of our knowledge, this is the first study to report that high ROR1 expression is correlated with aggressive malignant phenotype of ovarian cancer. Our data indicate that ROR1 may serve as a novel prognostic marker for ovarian cancer and targeting ROR1 may provide a promising strategy for targeted therapy in ovarian cancer treatment.
Methods
Patient specimens
A total of 100 paraffin-embedded tissue samples of ovarian cancer were collected from the archives of the Department of Pathology, Nanjing Maternal and Children Care Hospital Affiliated to Nanjing Medical University, between July 2003 and July 2010. 50 normal ovary samples from hysterectomy specimens resected for non-ovarian disease in our hospital were collected for control in IHC analysis. The stage of tumors was evaluated according to the International Federation of Gynecology and Obstetrics (FIGO) system. Tumors were graded according to the Silverberg grading system. All the cases were reevaluated for grade and histological type by two independent pathologists (LX and FSL). When a conclusion differed, the final decision was made by consensus. The original clinical data were obtained from hospital medical records, including patient age, tumor size, FIGO stage, tumor grade, histological type, serum CA-125 level, ascites, lymph node metastasis, relapse status, disease-free survival and overall survival. Besides, another 15 ovarian cancer tissue samples of high FIGO stage (FIGO III–IV), 15 ovarian cancer tissue samples of low FIGO stage (FIGO I–II) and nine normal ovary tissue samples were collected for qPCR analysis. None of the patients had received radiotherapy, chemotherapy or immunotherapy before surgery. Written informed consent was obtained from the patients for publication of this study and any accompanying images. Study protocol was approved by the Ethics Committee of Nanjing Maternal and Children Care Hospital Affiliated to Nanjing Medical University and all experiments were performed in accordance with approved guidelines of Nanjing Medical University.
One-step qPCR test
Total RNA from ovarian cancer tissues and normal ovary tissues were extracted using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's guidelines. One-step qPCR analysis was performed with a SensiMixTM One-Step Kit (Quantace, London, UK) using a Real Time PCR system (Bio-Rad IQ5, Hercules, CA, USA) according to the standard protocol. Total RNA extraction, quality control and one-step qPCR were performed as previously described36. The primers for ROR1 which designed by Primer Express Software were as follows: forward primer 5′- TAA TCG GAG AGC AAC TTCA -3′ and reverse primer 5′- TGT AGT AAT CAG CGG AGT AA -3′. Three reference genes, peptidylprolyl isomerase A (PPIA), β-actin (ACTB) and TATA-Box binding protein (TBP), were employed to standardize the analysis of the target gene in qPCR test. The primers of three reference genes were as follows: PPIA forward primer GTG GTG TTT GGC AAA GTG AA, reverse primer TCG AGT TGT CCA CAG TCA GC; ACTB, forward primer GCC AAC ACA GTG CTG TCT GG, reverse primer GCT CAG GAG GAG CAA TGA TCT TG; TBP forward primer TGC ACA GGA GCC AAG AGT GAA, reverse primer CAC ATC ACA GCT CCC CAC CA37. Amplification conditions consisted of 30 minutes at 42°C for reverse transcription and 2 minutes at 94°C for Taq activation, followed by 35 cycles of 95°C for 20 seconds, 56°C for 20 seconds and elongation at 72°C for 30 seconds.
TMA construction and IHC analysis
100 ovarian cancer and 50 normal ovary tissues were prepared and utilized in this present study. TMA was produced by Alenabio Biotech (Xi'an, China). Core tissue biopsies (2 mm in diameter) were taken from individual paraffin-embedded sections and arranged in the new recipient paraffin blocks. Tissue microarray was cut into 4-μm sections and placed on super frost charged glass microscope slides.
IHC analysis was performed as described previously38. Paraffin tissue sections (4 μm) were deparaffinized in 100% xylene and re-hydrated in graded ethanol solutions. TMA sections were incubated with a primary polyclonal rabbit anti-ROR1 antibody (1:200, Abcam, England) in TBS containing 1% bovine serum albumin for 1 hour. After washing, sections were then washed with TBS and incubated with horseradish peroxidase-conjugated anti-rabbit antibody (Dako Cytomation, Carpinteria, CA). Negative controls were included by replacement of the primary antibody with phosphate-buffered saline (PBS). ROR1 immunostaining was scored by blinded observers according to intensity and percentage of ROR1-positive cells. Staining intensity was scored as follows: 0 (negative), 1 (weakly positive), 2 (moderately positive) and 3 (strongly positive). The percentage of ROR1-positive cells was also scored according to 4 categories, where 1 was given for 0–10%, 2 for 11–50%, 3 for 51–80% and 4 for 81–100%. The product of the intensity and percentage scores was used as the final ROR1 staining score.
The cutoff point for the ROR1 expression score that was statistically significant in terms of survival was set using the X-tile software program (The Rimm Lab at Yale University; http://www.tissuearray.org/rimmlab) as described previously38. The degree of ROR1 staining was quantified using a two-level grading system and staining scores were defined as follows: 0–1, low expression and 2–9, high expression.
Statistical analysis
Statistical analyses were performed by employing STATA Version 12.0 (Stata Corporation, College Station, TX) and SPSS 18.0 statistic software (SPSS Inc, Chicago, IL). The ROR1 mRNA expression in fresh ovarian cancer tissues compared with normal ovary tissues was analyzed with the Wilcoxon signed rank nonparametric test. The influence of ROR1 expression on clinicopathological features of ovarian cancer was analyzed by chi-square test. Univariate and multivariate analyses were performed using Cox's proportional hazards regression models to determine factors that were independently associated with disease-free survival and overall survival. The Kaplan-Meier method was used to assess the associations between ROR1 expression and patients' outcomes. For all tests, p < 0.05 was considered as statistical significance.
References
Jemal, A. et al. Global cancer statistics. CA. Cancer. J. Clin. 61, 69–90 (2011).
Chen, Y. L. et al. Interferon-gamma in ascites could be a predictive biomarker of outcome in ovarian carcinoma. Gynecol. Oncol. 131, 63–68 (2013).
White, K. L. et al. Ovarian cancer risk associated with inherited inflammation-related variants. Cancer. Res. 72, 1064–1069 (2012).
Koyanagi, T. et al. In vivo delivery of siRNA targeting vasohibin-2 decreases tumor angiogenesis and suppresses tumor growth in ovarian cancer. Cancer. Sci. 104, 1705–1710 (2013).
Tsofack, S. P. et al. Low expression of the X-linked ribosomal protein S4 in human serous epithelial ovarian cancer is associated with a poor prognosis. BMC. Cancer. 13, 303 (2013).
Marsh, S. Pharmacogenomics of taxane/platinum therapy in ovarian cancer. Int. J. Gynecol. Cancer. 19, S30–S34 (2009).
Kartalou, M. & Essigmann, J. M. Mechanisms of resistance to cisplatin. Mutat. Res. 478, 23–43 (2001).
Green, J. L., Kuntz, S. G. & Sternberg, P. W. Ror receptor tyrosine kinases: orphans no more. Trends. Cell. Biol. 18, 536–544 (2008).
Katoh, M. & Katoh, M. Identification and characterization of rat Ror1 and Ror2 genes in silico. Int. J. Mol. Med. 15, 533–538 (2005).
Broome, H. E., Rassenti, L. Z., Wang, H. Y., Meyer, L. M. & Kipps, T. J. ROR1 is expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia. Leuk. Res. 35, 1390–1394 (2011).
Daneshmanesh, A. H. et al. Orphan receptor tyrosine kinases ROR1 and ROR2 in hematological malignancies. Leuk. Lymphoma. 54, 843–850 (2013).
Zhang, S. et al. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS. One. 7, e31127 (2012).
Zhang, S. et al. The onco-embryonic antigen ROR1 is expressed by a variety of human cancers. Am. J. Pathol. 181, 1903–1910 (2012).
Cui, B. et al. Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer. Res. 73, 3649–3660 (2012).
Gentile, A., Lazzari, L., Benvenuti, S., Trusolino, L. & Comoglio, P. M. Ror1 is a pseudokinase that is crucial for Met-driven tumorigenesis. Cancer. Res. 71, 3132–3141 (2011).
Rebagay, G., Yan, S., Liu, C. & Cheung, N. K. ROR1 and ROR2 in Human Malignancies: Potentials for Targeted Therapy. Front. Oncol. 2, 34 (2012).
Daneshmanesh, A. H. et al. Ror1, a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. Int. J. Cancer. 123, 1190–1195 (2008).
Hojjat-Farsangi, M. et al. Inhibition of the receptor tyrosine kinase ROR1 by anti-ROR1 monoclonal antibodies and siRNA induced apoptosis of melanoma cells. PLoS. One. 8, e61167 (2013).
Boyer, A., Goff, A. K. & Boerboom, D. WNT signaling in ovarian follicle biology and tumorigenesis. Trends. Endocrinol. Metab. 21, 25–32 (2010).
Arend, R. C., Londoño-Joshi, A. I., Straughn, J. M., Jr & Buchsbaum, D. J. The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol. Oncol. 131, 772–779 (2013).
Petrillo, M. et al. Timing and pattern of recurrence in ovarian cancer patients with high tumor dissemination treated with primary debulking surgery versus neoadjuvant chemotherapy. Ann. Surg. Oncol. 20, 3955–3960 (2013).
Feigenberg, T. et al. Is routine appendectomy at the time of primary surgery for mucinous ovarian neoplasms beneficial? Int. J. Gynecol. Cancer. 23, 1205–1209 (2013).
Canevari, S., Raspagliesi, F. & Lorusso, D. Bevacizumab treatment and quality of life in advanced ovarian cancer. Future. Oncol. 9, 951–954 (2013).
Song, G., Gao, H. & Yuan, Z. Effect of leuprolide acetate on ovarian function after cyclophosphamide-doxorubicin-based chemotherapy in premenopausal patients with breast cancer: results from a phase II randomized trial. Med. Oncol. 30, 667 (2013).
Zhao, S. H. et al. Basigin-2 is the predominant basigin isoform that promotes tumor cell migration and invasion and correlates with poor prognosis in epithelial ovarian cancer. J. Transl. Med. 11, 92 (2013).
Köbel, M. et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS. Med. 5, e232 (2008).
Nolen, B. M. & Lokshin, A. E. Biomarker testing for ovarian cancer: clinical utility of multiplex assays. Mol. Diagn. Ther. 17, 139–146 (2013).
Lin, H. et al. A novel human Fab antibody for Trop2 inhibits breast cancer growth in vitro and in vivo. Int. J. Cancer. 134, 1239–1249 (2014).
Lin, H. et al. Selection and characterization of human anti-MAGE-A1 scFv and immunotoxin. Anticancer. Agents. Med. Chem. 13, 1259–1266 (2013).
Chen, R. et al. A human Fab-based immunoconjugate specific for the LMP1 extracellular domain inhibits nasopharyngeal carcinoma growth in vitro and in vivo. Mol. Cancer. Ther. 11, 594–603 (2012).
Zhang, D. et al. Generation and characterization of a novel recombinant antibody against LMP1-TES1 of Epstein-Barr virus isolated by phage display. Viruses. 5, 1131–1142 (2013).
Yamaguchi, T. et al. NKX2-1/TITF1/TTF-1-induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer. Cell. 21, 348–361 (2012).
Klemm, F. et al. Beta-catenin-independent WNT signaling in basal-like breast cancer and brain metastasis. Carcinogenesis. 32, 434–442 (2011).
Hojjat-Farsangi, M. et al. Inhibition of the receptor tyrosine kinase ROR1 by anti-ROR1 monoclonal antibodies and siRNA induced apoptosis of melanoma cells. PLoS. One. 8, e61167 (2013).
Choudhury, A. et al. Silencing of ROR1 and FMOD with siRNA results in apoptosis of CLL cells. Br. J. Haematol. 151, 327–335 (2010).
Chen, R. et al. Increased expression of Trop2 correlates with poor survival in extranodal NK/T cell lymphoma, nasal type. Virchows. Arch. 463, 713–719 (2013).
Cai, J. et al. The Use of Laser Microdissection in the Identification of Suitable Reference Genes for Normalization of Quantitative Real-Time PCR in Human FFPE Epithelial Ovarian Tissue Samples. PLoS. One. 9, e95974 (2014).
Huang, J. et al. VCAM1 expression correlated with tumorigenesis and poor prognosis in high grade serous ovarian cancer. Am. J. Transl. Res. 5, 336–346 (2013).
Acknowledgements
This work is supported by the grants from Medical Science and Technique Development Fund, Nanjing, Jiangsu (No.ZKX09015), the Key Project of Nanjing Medical University (No.08NMUZ030) and the National Natural Science Foundation for Youth of China (No.81301951).
Author information
Authors and Affiliations
Contributions
Y.M. and J.Z. designed the study; H.L.Z. and J.R.Q. collected the tissue samples and carried out the qPCR analysis; F.S.L., N.X., X.J.T. and L.X. performed the IHC analysis; H.L.Z., J.R.Q., C.P.Y., D.Z.Y., L.J.G., Y.P.S. and D.W.Z. collected clinical data and participated in the evaluation of the IHC; Y.M. drafted the manuscript; Y.M., F.S.L. and J.Z. supervised the study. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Zhang, H., Qiu, J., Ye, C. et al. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci Rep 4, 5811 (2014). https://doi.org/10.1038/srep05811
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05811
This article is cited by
-
ROR1-STAT3 signaling contributes to ovarian cancer intra-tumor heterogeneity
Cell Death Discovery (2023)
-
Multiomics characterization implicates PTK7 in ovarian cancer EMT and cell plasticity and offers strategies for therapeutic intervention
Cell Death & Disease (2022)
-
Functional and clinical significance of ROR1 in lung adenocarcinoma
BMC Cancer (2020)
-
Glucocorticoids induce differentiation and chemoresistance in ovarian cancer by promoting ROR1-mediated stemness
Cell Death & Disease (2020)
-
Caveola-forming proteins and prostate cancer
Cancer and Metastasis Reviews (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.