Abstract
A tetrameric N-acetyl galactosaminyl (GalNAc) peptidomimetic was constructed by N-acetylation of repeating proline-based hydroxamic acid units, followed by a convergent ‘click chemistry’ coupling. This novel glycopeptidomimetic was determined to effectively antagonize the interaction between a transmembrane hepatic lectin and GalNAc on the cellular level.
Similar content being viewed by others
Introduction
Transmembrane glycoprotein receptors (TGRs) are calcium (II)-dependent sugar-binding proteins that can recognize and/or endocytose glycoproteins (proteins decorated with a sugar chain) from circulation. These functional receptors including hepatic lectins, selectins, etc., collectively termed the C-type lectins, are critically involved in immunological processes related to inflammation, viral invasion and cancer immunity1,2.
For instance, the galactose/N-acetyl-galactosamine (GalNAc)-specific asialoglycoprotein receptor (ASGP-R), which is the first TGR identified on the hepatocyte membrane3, is responsible for endocytosis of asialoglycoproteins (a circulating desialylated glycoprotein)4,5, clearance of IgA6 and trapping of activated lymphocytes7. However, ASGP-R has also been located as an entry site for hepatotropic viruses8 and is overexpressed during some hepatic diseases such as liver inflammation9.
As a consequence, molecular tools that can efficiently antagonize the TGRs under various physiological and pathological circumstances may help improve the understanding of the functions of these receptors and facilitate receptor-targeted drug development and delivery10,11.
Given the oligomeric structure of the majority of TGRs that cluster at the cell membrane, a diverse range of multivalent glycoconjugates have been designed and constructed to achieve high binding avidities with these lectins12,13. Of the various molecular backbones used, peptides are the most prevailing due to their modular synthetic pathway and, most importantly, high biocompatibility. Nevertheless, such backbones connected by repeating natural amide bonds are prone to be cleaved in the serum and have, generally, poor water solubility limiting their medicinal and pharmacological values.
Hydroxamic acids are a new class of structural analogs of the amino acids, where a carbon atom of the backbone of the latter is replaced by an oxygen atom14. Interestingly, peptides that consist of the hydroxamic acids have been reported to adopt more rigid and better pre-organized conformations, leading to improved stability against enzymatic cleavage and enhanced binding affinity with proteins15. Yang et al.16,17 also determined that hydroxamic acid-based peptidomimetics can function as anion receptors and channels.
Enlightened by these elegant studies, the incorporation of the hydroxamic acid functionality to sugars was exploited, leading to the formation of a series of sugar hydroxamic acid building blocks18,19,20,21,22,23,24,25. Here, we wish to report the construction of the first functional hydroxamic acid-based oligomeric glycopeptidomimetic that is able to antagonize sugar-TGR interactions on the cellular level.
Results
To begin with, hydroxyl l-proline 126 (Fig. 1) was used as a starting material as it features three easily modifiable handles (a carboxylic group, a secondary amino group and a hydroxyl group). Mitsunobu reaction27 of 1 with N-hydroxyphthalimide gave hydroxamic acid compound 2 in 90% yield. Removal of the Boc group of 2 with HCl, followed directly by addition of chloroacetyl chloride, led to 3 with a chlorine precursor. Treatment of 3 with sodium azide gave the azido hydroxamic acid 4 in a three-step yield of 62%. Then, orthogonal deprotections of the tert-butyl and phthalimide groups in the presence of TFA/DCM (1:10, V/V, with trace amount of concentrated aqueous HCl) and hydrazine hydrate gave the hydroxamic intermediate 5 and carboxylic acid intermediate 6, respectively.
As shown in Fig. 2, N-acetylation of 5 with 6 in the presence of DCC (dicyclohexylcarbodiimide) and HOBt (hydroxybenzotriazole) gave the azido dipeptidomimetic 7 in 62% yield. Orthogonal deprotection of 7 under the basic and acidic conditions above-employed led to dimeric intermediates 8 (hydroxamic free) and 9 (carboxylic free), respectively. Subsequently, N-acetylation of 8 with 9 produced the tetrameric peptidomimetic 10 in 50% yield.
Next, a previously prepared 1-α-O-propynyl N-acetylgalactosamine (A)28 was used to couple with the azido peptides via the Cu(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition (CuAAC) reaction (Fig. 3)29,30. Cycloaddition of A with the mono-azide 4 in the presence of [Cu(CH3CN)4]PF6 as catalyst gave the corresponding triazolyl compound 13 in 80% yield. Likewise, CuAAC of the dimer 7 and tetramer 10 with A produced the corresponding triazolyl dimeric (14) and tetrameric (15) glycopeptidomimetics in 62% and 61% yield, respectively.
As mentioned, the ASGP-R is a transmembrane C-type lectin expressed on hepatocytes, which can recognize GalNAc- or galactose-terminated glycoproteins, thereby leading to endocytosis of the proteins. As a consequence, molecular tools that can efficiently antagonize the ASGP-R-GalNAc/Gal interactions may not only help unravel the biological function of the receptor but also facilitate liver-specific drug delivery10,11.
Against this background, we interrogated the ability of our newly synthesized glycopeptidomimetics to block the ASGP-R-GalNAc interactions on the cellular level. A GalNAc-tailed rhodamine dye (B, Fig. 4b)28 that has proven to be capable of fluorescently imaging the ASGP-R expressed on a human hepatoma cell line (Hep-G2)31,32 was used as a reagent to stain the receptor. We envisioned that once the interaction was antagonized by the oligomers, the staining would be blocked resulting in suppression of the fluorescence of B on cells. By using unmodified GalNAc as a positive control, both the GalNAc peptido-dimer 14 and -tetramer 15 were incubated with the cells before staining with B.
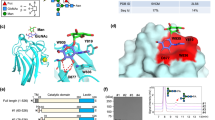
(a) Fluorescence imaging of the asialoglycoprotein receptors (ASGP-Rs) expressed on Hep-G2 cells by 10 μM of B (Cell nuclei were stained by Hoechst in blue); (b) Structure of GalNAc-rhodamine B; Fluorescence imaging of the ASGP-Rs expressed on Hep-G2 cells pre-incubated with 500 μM of (c) GalNAc, (e) dimer 14, (g) tetramer 15 and 100 μM of (d) GalNAc, (f) dimer 14, (h) tetramer 15 by 10 μM of B; Cell viability of (i) Hep-G2 and (j) 3T3-L1 cells in the presence of increasing glycosyl peptidomimetics 13, 14 and 15 determined by an MTS cytotoxicity assay.
As shown in Fig. 4a, the cells were effectively stained by the GalNAc dye (B) by taking advantage of the GalNAc-ASGP-R interactions in the absence of an antagonist. Interestingly, whereas the presence of increasing free GalNAc hardly suppressed the fluorescence of B (Fig. 4c and Fig. 4d), both the synthesized dimer 14 (Fig. 4e and Fig. 4f) and tetramer 15 (Fig. 4g and Fig. 4h) obviously weakened the staining in a concentration-dependent manner.
In addition, the tetramer exerted better suppressive effect than the dimer, meaning that increasing the valency of the oligomer could probably reinforce the ligand-receptor binding. The ability of the synthetic oligomers to suppress the cell staining was much stronger than the unmodified GalNAc. This suggests that the ‘click’ grafting of GalNAc onto the hydroxamic acid-based peptide backbone could enhance the binding affinity (avidity) of the oligomers with the hepatic lectin expressed on the hepatoma cells.
The cytotoxicity of the hydroxamic acid-based glycopeptidomimetics towards Hep-G2 as well as another normal cell line (3T3-L1, mouse embryonic fibroblast) was tested by an MTS assay. Results showed that the ‘clicked’ peptidomimetics 13, 14 and 15 are not toxic to neither cell lines examined and the cell viability was independent of compound concentrations (Fig. 4i for Hep-G2 and Fig. 4j for 3T3-L1). All the above-data positively imply that the tetrameric glycopeptidomimetic constructed here could be employed to antagonize ASGP-R-GalNAc interactions on the cellular level.
A GlcNAc (N-acetylglucosamine)-clicked dimeric peptidomimetic (16) was further synthesized (supporting information) as a negative control as the GlcNAc precursor of which might not have the ability to antagonize the interactions between ASGP-R and GalNAc28. As shown in Fig. S1 (supporting information), 16 did not show any antagonist effect against staining of Hep-G2 by B at the identical concentrations used for the counterpart dimer 14. To preliminarily interrogate the potential serum stability of the neo-glycopeptidomimetics, the tetramer 15 was incubated in a Tris-HCl buffer with trypsin (an enzyme abundantly existed in the serum to cleave peptides) for 1 h and 6 h. By HPLC tracking, we observed that the tetramer was not cleaved by the enzyme even by a co-incubation for 6 h (Fig. S2, supporting information).
Discussion
A number of multivalent glycopolymers and glycoconjugates have been synthesized to target the oligomeric ASGP-R of hepatocyte or hepatoma, achieving efficient target-specific chemo-therapy33,34. This study describes the construction of novel hydroxamic acid-based glycopeptidomimetics providing a new class of multivalent glycoconjugates to effectively target the ASGP-R. This class of neo-glycopeptides showed promising properties in terms of low toxicity and stability against trypsin cleavage. Notably, a tetramer could antagonize clearly the GalNAc-ASGP-R interactions on the cellular level. We believe that this unique class of neo-glycopeptidomimetics could serve as new molecular tools for study of the universal intercellular sugar-C-type lectin interactions and as a precursor for fabricating liver-specific drug delivering systems.
Methods
General
All purchased chemicals and reagents are of analytical grade. Solvents were purified by standard procedures. Reactions were monitored by TLC (thin-layer chromatography) using E-Merck aluminum precoated plates of Silica Gel. 1H and 13C NMR spectra were recorded on a Bruker AM-400 spectrometer using tetramethylsilane (TMS) as the internal standard (chemical shifts in parts per million). Optical rotations were measured using a Perkin-Elmer 241 polarimeter at room temperature and a 10 cm length cell of a 1 mL volume. High resolution mass spectra were recorded on a Waters LCT Premier XE spectrometer using standard conditions (ESI, 70 eV). Analytical HPLC was measured using Agilent 1100 Series equipment.
Cell culture and transfection
Hep-G2 cells were maintained in Dulbecco's Modified Eagle's Medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% Fetal bovine serum (Gibco, Gland Island, NY, USA) and passaged every 3–4 days based on 90% confluency. 3T3-L1 cells were maintained in Dulbecco's Modified Eagle's Medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% Fetal calf serum (Gibco, Gland Island, NY, USA).
Cellular fluorescence imaging
Hep-G2 cells were cultured in DMEM supplemented with 10% FBS. Cells (15 × 104) were seeded on a black 96-well microplate with optically clear bottom (Greiner bio-one, Germany) overnight. The cells were incubated with GalNAc or the glycopeptidomimetics (100 μM or 500 μM) for 30 min, followed by incubation with B (10 μM) and the nuclear staining reagent Hoechst (5 μg/ml) at 37°C in a humidified atmosphere of 5% CO2 in air for 5 min. Then, cells were gently washed with PBS three times and fixed using 4% para-formaldehyde. The fluorescence images were recorded using an Operetta high content imaging system (Perkinelmer, US).
MTS cytotoxicity assay
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) was purchased from Promega (Madison, WI, USA). For the MTS assay, cells (5,000 per well) were seeded into 96-well plates. After treatment with compounds of different concentrations for 72 h, cells were added with MTS at a final concentration of 0.5 mg/mL, followed by incubation for another 2 h. The optical density (OD) of each well was determined at 490 nm (background subtraction at 690 nm) by a SpectraMax 340 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The growth inhibitory ratio was calculated as follows: Growth inhibitory ratio = (Acontrol − Asample)/Acontrol (where A is the OD value per well).
References
Weis, W. I., Taylor, M. E. & Drickamer, K. The C-type lectin superfamily in the immune system. Immunol. Rev. 163, 19–34 (1998).
Marth, J. D. & Grewal, J. D. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 (2008).
Drickamer, K. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv. Enzymol. Relat. Areas Mol. Biol. 41, 99–128 (1974).
Spiess, M. The asialoglycoprotein receptor: a model for endocytic transport receptors. Biochemistry 29, 10009–10018 (1990).
Stockert, R. J. The asialoglycoprotein receptor: relationships between structure, function and expression. Physiol. Rev. 75, 591–609 (1995).
Rifai, A., Fadden, K., Morrison, S. L. & Chintalacharuvu, K. R. The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig)a1 and Iga2 isotypes. J. Exp. Med. 191, 2171–2182 (2000).
Guy, C. S., Rankin, S. L. & Michalak, T. I. Hepatocyte cytotoxicity is facilitated by asialoglycoprotein receptor. Hepatology 54, 1043–1050 (2011).
Dotzauer, A. et al. Hepatitis A virus-specific immunoglobulin a mediates infection of hepatocytes with hepatitis A virus via the asialoglycoprotein receptor. J. Virol. 74, 10950–10957 (2000).
Burgess, J. B., Baenziger, J. U. & Brown, W. R. Abnormal surface distribution of the human asialoglycoprotein receptor in cirrhosis. Hepatology 15, 702–706 (1992).
Rigopoulou, E. I. et al. Asialoglycoprotein receptor (ASGPR) as target autoantigen in liver autoimmunity: lost and found. Autoimmun. Rev. 12, 260–269 (2012).
Jain, K., Kesharwani, P., Gupta, U. & Jain, N. K. A review of glycosylated carriers for drug delivery. Biomaterials 33, 4166–4186 (2012).
Kiessling, L. L. & Grim, J. C. Glycopolymer probes of signal transduction. Chem. Soc. Rev. 42, 4476–4491 (2013).
Wittmann, V. & Pieters, R. J. Bridging lectin binding sites by multivalent carbohydrates. Chem. Soc. Rev. 42, 4492–4503 (2013).
Yang, D. et al. An unusual turn structure in peptides containing γ-aminoxyacids. J. Am. Chem. Soc. 118, 9794–9795 (1996).
Li, X., Wu, Y.-D. & Yang, D. α-Aminoxy acids: new possibilities from foldamers to anion receptors and channels. Acc. Chem. Res. 41, 1428–1438 (2008).
Yang, D., Li, X., Sha, Y. & Wu, Y-D. A cyclic hexapeptide comprising alternating α-aminoxy and α-amino acids is a selective chloride ion receptor. Chem. Eur. J. 11, 3005–3009 (2005).
Li, X., Shen, B., Yao, X.-Q. & Yang, D. A small synthetic molecule forms chloride channels to mediate chloride transport across cell membranes. J. Am. Chem. Soc. 129, 7264–7265 (2007).
Sharma, G. V. M., Manohar, V., Dutta, S. K., Subash, V. & Kunwar, A. C. Design of a “new motif” with β-amino acids and α-aminoxy acids: synthesis of hybrid peptides with 12/10-helix. J. Org. Chem. 73, 3689–3698 (2008).
Chandrasekhar, S. et al. β-Sugar aminoxy peptides as rigid secondary structural scaffolds. J. Org. Chem. 73, 9443–9446 (2008).
Andreini, M., Taillefumier, C., Chrétien, F., Thery, V. & Chapleur, Y. Synthesis and solution conformation of homo-β-peptides consisting of N-mannofuranosyl-3-ulosonic acids. J. Org. Chem. 74, 7651–7659 (2009).
Sharma, G. V. M. et al. Self-assembling cyclic tetrapeptide from alternating C-linked carbo-β-amino acid [(S)-β-Caa] and α-aminoxy acid [(R)-Ama]: a selective chloride ion receptor. J. Org. Chem. 75, 1087–1094 (2010).
Gong, Y., Sun, H. & Xie, J. Synthesis of oligosaccharide mimetics with glycoaminoxy acids. Eur. J. Org. Chem. 2009, 6027–6033 (2009).
Gong, Y., Peyrat, S., Sun, H. & Xie, J. Synthesis of nucleoside aminoxy acids. Tetrahedron 67, 7114–7120 (2011).
Song, Z., He, X.-P., Chen, G.-R. & Xie, J. 6-O-amino-2-O-carboxymethyl glycopyranoside as novel glycoaminoxy acid building block for the construction of oligosaccharide mimetics. Synthesis 17, 2761–2766 (2011).
Peyrat, S. & Xie, J. Synthesis of thymidine dimers from 5′-O-aminothymidine. Synthesis 44, 1718–1724 (2012).
Makoto, T., Han, G. & Hruby, V. J. Synthesis of 4-cis-phenyl-L-proline via hydrogenolysis. J. Org. Chem. 66, 3593–3596 (2001).
Mitsunobu, O. The use of diethyl azodicarboxylate and triphenylphosphine in synthesis and transformation of natural products. Synthesis 13, 1–28 (1981).
Zhang, H.-L. et al. Fluorogenic probing of specific recognitions between sugar ligands and glycoprotein receptors on cancer cells by an economic graphene nanocomposite. Adv. Mater. 25, 4097–4101 (2013).
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 41, 2596–2599 (2002).
Tornøe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
Li, Z. et al. Capturing intercellular sugar-mediated ligand-receptor recognitions via a simple yet highly biospecific interfacial system. Sci. Rep. 3, 2293 (2013).
Shi, D.-T. et al. Substitution pattern reverses the fluorescence response of coumarin glycoligands upon coordination with silver (I). Sci. Rep. 4, 4252 (2014).
Lepenies, B., Lee, J. & Sonkaria, S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv. Drug Delivery Rev. 65, 1271–1281 (2013).
Ma, W. et al. Target-specific imaging of transmembrane receptors using quinonyl glycosides functionalized quantum dots. Anal. Chem. 86, 5502–5507 (2014).
Acknowledgements
We thank the 973 project (2013CB733700), the National Science Fund for Distinguished Young Scholars (81125023), the National Natural Science Foundation of China (21176076, 21202045 and 81173033) and the Key Project of Shanghai Science and Technology Commission (13NM1400900).
Author information
Authors and Affiliations
Contributions
G.-R.C., J.X., J.L. and X.-P.H. discussed and conceived the idea. H.-L.Z. synthesized the compounds; Y.Z. performed the biological tests; X.-P.H. and Y.Z. wrote the paper. H.T. G.-R.C. and J.L. supervised the research. All authors commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supporting Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Zhang, HL., Zang, Y., Xie, J. et al. A ‘Clicked’ Tetrameric Hydroxamic Acid Glycopeptidomimetic Antagonizes Sugar-Lectin Interactions On The Cellular Level. Sci Rep 4, 5513 (2014). https://doi.org/10.1038/srep05513
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05513
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.