Abstract
Solidagocanadensis is an aggressive invader in China. Solidago invasion success is partially attributed to allelopathic compounds release and more benefits from AM fungi, which potentially makes the properties of Solidago litter different from co-occurring natives. These properties may comprehensively affect litter decomposition of co-occurring natives. We conducted a field experiment to examine litter mixing effects in a Phragmites australis dominated community invaded by Solidago in southeast China. Solidago had more rapid mass and N loss rate than Phragmites when they decomposed separately. Litter mixing decreased N loss rate in Phragmites litter and increased that of Solidago. Large decreases in Phragmites mass loss and smaller increases in Solidago mass loss caused negative non-additive effect. Solidago litter extracts reduced soil C decomposition and N processes, suggested an inhibitory effect of Solidago secondary compounds. These results are consistent with the idea that nutrient transfer and secondary compounds both affected litter mixtures decomposition.
Similar content being viewed by others
Introduction
Biological invasions alter ecosystem services across the globe. Invasive plants are frequently fast-growing, use high levels of soil nutrients1 and produce higher quality litter (e.g. higher N content, lower C:N ratio)2,3,4. Higher quality litter following invasions can be important in altering ecosystem C and N cycling through litter decomposition2,5. Litter from invasive plants with higher N concentration and lower C:N ratio usually decomposes faster and releases more N than native species, in line with a number of cases that an N-fixing species invades communities where N fixers are absent2,4,6,7. On the other hand, secondary metabolites released by decomposing litter might reduce nutrient releasing rate of co-occurring plant litter via litter mixing8,9. While many studies examining the impacts of invasive species focus on the decomposition of litter from a given species decomposing in isolation, native ecosystems are generally invaded over a prolonged time period and invasions are spatially patchy so different plant litters often co-occur and decompose in mixtures in natural conditions. Therefore, inferences from the studies of individual invasive species are limited given that there may be chemical and/or biological interactions among different co-occurring species2,10.
Solidagocanadensis L. (Canada goldenrod, hereafter “Solidago”) is an invasive perennial forb introduced from North America into China as an ornamental plant in 1913 and subsequently escaped to surrounding areas11 and is aggressively invading Phragmitesaustralis (Cav.) Trin. ex Steud. (common reed, hereafter “Phragmites”) stands in southeast China12. Phragmites is a perennial grass that maintains living rhizomes year round. Phragmites has thick aboveground stalks that create micro-environments in which little sunlight reaches the ground and few other native species can survive. Several explanations have been given for Solidago's successful invasion. Consistent with Enhanced Mutualism Hypothesis11,13, invasive Solidago plants benefited more from AM fungi than native plants, potentially leading to higher soil nutrient up-taking ability and hence higher litter quality13,14. Other studies showed that release of allelopathic compounds (e.g. phenolics, flavones, saponins) by invasive Solidago plants exhibited inhibitory effects on native plant neighbors and soil pathogens in the introduced range, providing the evidence in support of Novel Weapons Hypothesis15,16. Given that litter of invasive Solidago has higher nutrients concentration and allelopathic compounds, these chemical traits may have mixing effect on litter decomposition in the invasion stand of Phragmites communities by Solidago15,16,17,18.
Non-additive effects of litter mixing on litter mass loss and nutrient release relative to the litters of component species decomposed in isolation have been well documented10,17,18,19. Overall decomposition of litter mixtures may be accelerated or retarded through non-additive effects17,18,19,20. The first review on litter mixture decomposition studies found that positive non-additive effects as a result of synergistic interactions among component litter types are the most common result of litter mixture studies19. A key factor driving synergistic interactions could be transfer of nutrients among component litter types. Through nutrient transfers mediated by physical or biological means (e.g. leaching, fungi) in mixtures, nutrients released from rapidly decaying, higher quality litter can facilitate decay in adjacent, more recalcitrant litters, resulting in an overall positive non-additive mixing effect. Since Solidago litter generally decomposes in mixtures with Phragmites when Solidago invades Phragmites stands, synergistic interactions between higher Solidago quality litter and lower Phragmites ones could reduce the quality differences between the two species litter types in mixtures17,21,22. Thereby, we may expect that nutrient transfer from Solidago to Phragmites would cause synergistic decomposition effects.
While synergistic interactions among different species litters are relatively common in mixture, indeed, other studies have reported antagonistic non-additive effects, in which decay rates of mixed litters were slower than expected from the component litters of mixes decomposed separately8,9,10,23,24. One possible explanation for antagonistic effects is given by the evidence that secondary compounds produced by one component species have inhibitory effects on the decomposition of other component litters in mixtures8,9. Movement of secondary compounds (e.g. phenolic compounds, tannins) among litter types can be caused simply by leaching, incurring an antagonistic interactions among litters in mixtures17,19. Based on results of allelopathic chemicals release and its inhibition effect on soil organic C decomposition and N process reported in Solidago15,16,25, we may expect that allelopathic compounds released by Solidago would result in an antagonistic non-additive effect on litter mixture decomposition26. Therefore, it would be of interest to know the effects of mixing litter from Solidago with both higher litter quality and secondary compounds content with that from native species Phragmites with lower litter quality and secondary compounds content on litter mixture decomposition.
Non-additive effects may vary over the course of decomposition due to changes in litter physical and chemical properties18,19,27,28. The literature on mixing effects and their variation over time emphasizes the need for more experiments investigating the effects of mixing litter of native and invasive species on litter mass and N dynamics. As litter decomposition proceeds in mixture over time, changes in the relative amount of component litters would alter the way litter decays in the mixed pool, especially when the invasive plant Solidago is potentially allelopathic to the co-occurring Phragmites species15. For instance, secondary chemicals released by decomposing Mikania micrantha in mixtures with Pinus massoniana litter causes a shift from a positive effect to an inhibitory effect on decomposition of mixtures after three months28.
Here, we investigated litter mixing effects in a natural Phragmites dominated community invaded by Solidago in southeast China. We conducted a pair of field experiments to examine potential effects of Solidago invasion on soil C and N processes from litter mixing, nutrient transfer and secondary compounds. Specifically, we proposed that: (1) Solidago litter with lower C:N ratio would decompose faster than Phragmites litter (H1); (2) positive non-additive mixing effects on litter mixtures would initially be observed by possible transfer of N from Solidago litter to Phragmites litter (H2); (3) secondary chemicals released by decomposing Solidago litter would inhibit soil C and N process and would subsequently have an antagonistic non-additive mixing effect on Phragmites litter decomposition in mixtures as decomposition proceeds28,29 (H3).
Results
Litter C, N concentration and C:N ratio
Initial litter C and N concentration both differed between Phragmites and Solidago species. While Phragmites litter was higher in C (441.9 ± 5.0 g kg−1 in Solidago vs. 537.7 ± 9.7 g kg−1 in Phragmites, F1,4 = 76.68, P = 0.0009), initial N concentration of Solidago litter (7.9 ± 0.03 g kg−1) was higher than that of Phragmites litter (7.3 ± 0.1 g kg−1; F1,4 = 45.86, P = 0.0025). Solidago litter was characterized by a significantly lower initial C:N ratio (56.0 ± 0.5) relative to that of Phragmites (73.8 ± 0.6; F1,4 = 564.15, P < 0.0001). Compared with litters decaying alone, Solidago N concentration was significantly lower in mixtures at the second and third measurements (P = 0.0021; Fig. 1b) but Phragmites N concentration was significantly higher in mixtures than in isolation (P = 0.0004; Fig. 1c). Except for differences in initial N concentration, Solidago N concentration did not significantly differ with that of Phragmites in mixtures (P = 0.67; Fig. 1d). Whether decaying alone or in mixture, the C:N ratio of litter decreased over time for both species. Over 12 months, C:N ratios of Solidago and Phragmites litters in single species bags dropped down to 42.0 ± 1.3 and 64.5 ± 5.1, respectively. In mixtures, C:N ratio of Solidago and Phragmites litters reached similar values over 12 months (48.3 ± 4.7 vs. 40.9 ± 1.2).
Litter N concentration dynamics (mean ± se) of Phragmites and Solidago when each was decomposed alone or in mixtures.
Results of MANOVA are shown in Table 3 and P-values referring to results of follow-up contrasts are shown.
Litter mass loss
Solidago litter decomposed more rapidly than Phragmites litter when they decomposed in single species bags (P < 0.0001; Fig. 2a). The first order decay constant (k) of Solidago litter was twice that of Phragmites (Table 1). Litter mixing accelerated litter mass loss rate of Solidago (P = 0.0003; Fig. 2b) but decreased that of Phragmites (P = 0.0003; Fig. 2c). For mass loss, litter mixing increased kSolidago and decreased kPhragmites by 53% and 38%, respectively, when calculated by the single negative exponential model (Table 1). During the first 6 months, non-additive mixing effect on litter mass was, on average, slightly positive but not statistically significant (Table 2). Over 12 months, however, significant negative effects on litter mass loss were observed. Compared with predicted remaining mass, up to 15% more mass remained over 12 months of litter decomposition in mixtures.
Litter mass loss dynamics (mean ± se) of Phragmites and Solidago when each was decomposed alone or in mixtures.
Results of MANOVA are shown in Table 3 and P-values referring to results of follow-up contrasts are shown. Statistical significance at the level of α = 0.025.
Litter N release
When decomposed alone, Solidago litter had faster N release than Phragmites litter (P = 0.0023; Fig. 3a). K for N release of Solidago litter was approximately two times that of Phragmites litter (Table 1). While Phragmites litter N decreased 17% decomposing alone, it was increased by 17% in litter mixtures over 12 months (P = 0.0004; Fig. 1c). An initial non-significant positive effect became a slightly significant negative effect over 12 months (Table 2). Nitrogen release over 12 months was 7% lower in mixtures than predicted from decomposition in isolation.
Litter N release dynamics (mean ± se) of Phragmites and Solidago when each was decomposed alone or in mixtures.
Results of MANOVA were shown in Table 3 and P-values referring to results of follow-up contrasts were shown. Statistical significance at the level of α = 0.025.
Litter extract effects on soil C and N processes
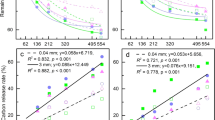
Solidago litter extracts had significant effects on soil organic carbon (SOC) concentration (F3,12 = 14.91, P = 0.0002), N mineralization (F3,8 = 41.51, P < 0.0001) and nitrification (F3,8 = 39.52, P < 0.0001) processes. After 6 months of extract treatments, SOC concentration was significantly greater for plots receiving the medium or high concentrations of extract treatments (Fig. 4a). Meanwhile, both nitrification and N mineralization rates with high level extracts were lower than those that received control, low or mid treatments (Fig. 4bc).
Discussion
In the present study, mixing of Solidago and Phragmites litter resulted in non-additive effects on decomposition compared to litter from each species decomposing in isolation. As predicted (H1), Solidago litter with higher initial N concentration and lower C:N ratio had faster mass loss and N release rate than that of native Phragmites when each was decomposed alone. Litter mixing decreased litter N concentration of Solidago but increased that of Phragmites, partially in support of the second hypothesis (H2), suggesting possible N transfer from Solidago litter to Phragmites litter, but further studies using isotope labeling method are necessary before solid conclusions could be drawn21. However, possible N transfer in mixtures was not associated with significant positive effect on litter mixture decomposition. In accordance with the third hypothesis (H3), instead, there was a negative non-additive mixing effect over 12 months of decay in mixtures, possibly due to release of inhibitory compounds by Solidago. For litter mass loss, there was a shift in mixing effect from a slightly but not significantly positive interaction to a significantly negative effect. A negative effect of secondary chemicals on C and N processes was supported by the litter extracts experiment, which is also consistent with the prediction of the third hypothesis (H3).
Our study demonstrated that litter mass loss and N release from Solidago were faster than that of Phragmites when each was decomposed separately (Table 1; Figs. 2a and 3a). Invasive species are frequently characterized by higher assimilation of soil nutrients relative to native species and consequently produce high quality litter30,31, leading to faster mass loss and N releasing rate. In this study, when each was decomposed alone, up to 64% and 71% of initial N was released by Phragmites and Solidago litter over 12 months decomposition, respectively (Fig. 3a), which is in keeping with some previous studies on nutrient release rates by invasive plants2,3,7. For example, a recent study on invasive shrub Lonicera maackii reported 96% of the initial N was released within the first year of decomposition3. Another study found that 50% of initial N was released from native plant litter during the period when the litter from the invasive shrub Berberis thunbergii lost 83% of initial N7. Increased N pool in standing biomass was reported in invasive Solidago gigantea relative to that of the adjacent natives14. Indeed, higher N concentration and lower C:N ratio of invasive species relative to native plant neighbors are usually associated with rapid growth of invasive plants. Thereby, decomposition of invasive plant litter may often be expected to proceed more rapidly than decomposition of native litter.
Mixing Solidago litter with that of Phragmites caused significant negative effects over 12 months of decomposition. Non-additive mixing effects were observed in most reviews of litter mixtures, with both positive and negative effects occurring17,19,28,32. Transfer of nutrients among litters in mixtures has been demonstrated leading to positive non-additive effects33, while the release of secondary compounds has been linked to negative effects26. For instance, during a succession in Alaskan taiga, secondary chemicals released by low quality balsam poplar litter were found to inhibit decomposition of alder litter and N mineralization in alder soils23. Since secondary compounds (e.g. polyacetylenes, diterpenoids, triterpenoid saponins and phenolics) have been reported in Solidago16,34, chemicals from the litter of invasive Solidago plants were also possibly released to the surrounding Phragmites litter when both were mixed together, retarding decomposition of Phragmites litter in this study. Unfortunately, chemical concentrations and transfer dynamics were not directly measured, which is a limitation of this study35,36,37.
Soil processes could be involved in inhibitory effects of Solidago secondary compounds from leaf litter on litter decomposition (e.g. enhancement of microbial substrate, reduced soil N mineralization)17,19,23. The results of litter extracts experiment showed that soil SOC decomposition and N mineralization were reduced by extract treatments, particularly in the high-level treatment (Fig. 4). In a previous study, soil SOC and ammonium contents in areas invaded by Solidago were 1.2 and 3.3 times those of the native uninvaded areas, respectively25. However, soil nitrate in the invaded area was only less than a half of that in the native areas25, indicating Solidago invasion might have inhibited nitrification process, which is consistent with our findings that both soil SOC decomposition and nitrification process were inhibited in the Solidago invaded area than in an area dominated only by Phragmites where Solidago was not present (Fig. 4). Such inhibitory effect on soil process of secondary compounds released from Solidago is consistent with the negative effects in litter mixture decomposition8,26.
The litter mixing effects varied over the time course of decay have been frequently observed in litter mixture studies18,19,27,28,38,39. As we predicted, litter mixing effects were shifted from a slight positive interaction within the earlier stages to a significant negative non-additive effect over 12 months in this study. The way in which this non-additive effect varied over the course of decomposition is possibly determined by a combined outcome of changes in concentrations of nutrient availability and carbohydrates during the different stages of decay. A shift from a positive effect to an inhibitory effect from mixing litters was also found after three months in the decomposition of litter of invasive Mikania micrantha mixing with that of native Pinus massoniana plants28. Despite of the limitation of this study that allelopathic chemical concentration and its transfer dynamics were not directly measured, our results suggest that simultaneously examining nutrient and secondary chemical dynamics of component litters in mixtures may give an insight into why the litter mixing effect was not accurately predicted by initial litter nutrient chemistry40,41, especially in invasion cases by potentially allelopathic species.
Solidago is a widespread, problematic invader in China, Australia and Europe42,43,44. Currently, Solidago is aggressively invading farmlands, roadsides and open areas in cities in southeast China12. To control invasive Solidago species or restore invaded ecosystems, it is necessary to understand the mechanisms of Solidago invasion and its impacts on ecosystem processes. Several hypotheses have been proposed and approved in invasive Solidago species in previous studies, such as Resource Availability Hypothesis, Enhanced Mutualism Hypothesis and Novel Weapons Hypothesis15,25,37,45, indicating that invasive Solidago has higher nutrients concentration and allelopathic compounds. The results of this study suggest that mixing effect of these properties by invasive Solidago on litter decomposition might have played an important role in its invasion success. Consistent with our hypothesis (H3), allelopathic compounds released by Solidago might have retarded litter decomposition of co-occurring natives and also restrained soil mineralization and nitrification processes at the high extract level, which may increase the soil C and N sink capacity retained in Solidago invaded ecosystems. On the other hand, faster nutrient cycling and more efficient use of nutrient through mutualistic soil-microbe interaction would give Solidago a competitive advantage over the co-occurring natives in the invasive range.
By examining litter mixing effects of phylogenetically paired native and invasive species, Hickman et al. posited the time course scheme of changes in nutrient cycling following invasion10. As proposed by Hickman et al.10, the invasive species impacts on nutrient cycling may be small during the earlier stage of an invasion, when the invasive plant is less abundant than native species. As invasion proceeds, increasingly abundant invasive plants will progressively remove native plants and thereby litter mixing effects of invasive plants with native plants would generate more profound impacts on nutrient cycling in the invaded area10. In this study, N mineralization and nitrification inhibited by Solidago litter extracts will effectively reduce N leaching loss in wetland ecosystem, which is supported by a field investigation, showing that Solidago invasion significantly increased soil organic carbon and ammonium contents and soil microbial parameters (biomass, activity and functional diversity) relative to the non-invaded area in the introduced Chinese range25. The slowing down SOC decomposition and N cycling might be advantageous for Solidago invasion by providing C source for soil microbial communities and N nutrients for plant growth. In particular, Solidago litter retarded decomposition of native Phragmites litter, also build up SOC and N pools available for the later recruitment of invasive Solidago. As Solidago aggressively displaces native Phragmites plants and ultimately forms monospecific stands, Solidago decomposing alone will increase decomposition rates. This study has implications for element cycling changes following native Phragmites communities invaded by Solidago species, particularly as the scale Solidago invasion increases with time10. Solidago invasion would result in a shift from native Phragmites dominated communities with slow litter decomposition rate to invasive Solidago communities with fast litter decomposition rate. As invasion proceeds, more C would be decomposed and input belowground, enhancing the SOC pools and C sequestration ability in the invaded areas. However, this implication could not be generalized before more species invading multi-site with multi-year time courses are considered in future studies.
Methods
Study site
Experiments were conducted in an invaded seasonal wetland ecosystem located at the Nanjing Agricultural University experimental station (6 m elevation, 32°01′N 118°37′E), Jiangsu province, China. Mean annual temperature is 25°C, with monthly means from −10°C (January) to 32.5°C (July). Annual precipitation is 980 mm. Soils have high clay content and are classified as Gleysols or hydromorphic soils. This location was flooded by the Yangtze River until the early 20th century. Some areas are dominated by native Phragmites australis, but Phragmites stands are currently threatened by Solidago invasions. Other plant species co-exist with Phragmites and Solidago in this location including Alternanthera philoxeroides, Eragrostis pilosa, Erigeron annuus, Conyza sumatrensis, Polygonum perfoliatum, Acalypha australis, Eleusine indica, Bidens pilosa, Digitaria sanguinalis, Abutilon theophrasti, Amaranthus mangostanu and Xanthium sibiricum. However, the most abundant species in this experimental station was Phragmites and it remains the most abundant species after Solidago invaded 5~ years ago. Currently, Phragmites coverage of this area is approximately 50% while Solidago takes around 30%.
Litterbag experiment
Litter decomposition rates of both focal species were examined via an in situ litterbag experiment19. In October 2009, we hand collected completely developed, mature leaf litter samples from different Solidago and Phragmites individuals located in the invaded area. Sun leaves and shade leaves were evenly collected by individuals, cut into a size of litter length at 3 ~ 6 cm and pooled together to produce a combined sample by species. Air dried litter samples (about 10 g) of a single species were placed in nylon litterbags (1 mm2 mesh, 10 × 15 cm). Another set of bags contained mixtures of Solidago and Phragmites litter (about 5 g of each species - thoroughly mixed). Litterbags were sealed and randomly deployed in March 2010 in a single block where both species co-occur. All litterbags were approximately 2 m from each other and at least 5 m from the edge of the plant standings. Litterbags were fixed to the soil surface by anchored flags after the original litter layer of the specific location was removed. Three replicates of each treatment were retrieved after decomposition of 3, 6 or 12 months and others were analyzed (0 months). When bags were retrieved, litter samples were processed by carefully removing dirt or other contaminants, separated by species, dried and weighed for mass and C and N measurement by an elemental analyzer. The morphological traits difference in Solidago and Phragmites leaf litter samples decomposed less than 12 months enabled us to identify component litter by species from litter mixtures. Leaf litter that decomposed 18 months was not used for analysis as litter fragments could not be readily identified. Litter samples at 0 months were directly dried for water, C and N estimation without deployment. Litter subsamples at each time points were oven dried at 60°C to constant weight and the litter data presented were converted to that oven dry weight (Supplementary Material).
Solidago litter extracts experiment
An additional experiment was performed to examine the effects of Solidago compounds on SOC and N processes. Within a patch dominated by Solidago at the center of invaded stand, twelve 0.5 × 0.5 m plots were established in March prior to the 2010 growing season and assigned to one of four concentration treatments with three replicates. The distance between plots was 2 m. Establishing plots within Solidago site dominated patch enabled us to exclude any possible effects of other plant litter extract on soil C and N process. When plots were established, Solidago litter, stems and roots were removed from plots and plots were isolated from the adjacent soils by PVC quadrat frame (0.5 × 0.5 m) buried flush with the soil surface. Solidago leaf litter collected during the 2009 growing season was air dried then soaked in distilled water (1 g per 10 ml) for 48 hours to extract chemical compounds46,47. The solution was filtered and diluted by distilled water to 3 concentrations (g ml−1): 0.002 (low), 0.050 (mid) and 0.100 (high). From April to October 2010, each plot received 800 ml of one solution biweekly (total = 9600 ml). Control plots received distilled water. Concentrations of the extract applied were decided according to an investigation of the mean annual litter input of Solidago in this area. Specifically, the low level extract treatment was related to one tenth of, with the mid and high level related to 2.5 and 5.5 times the mean annual litter input, respectively. After 6 months of treatment, top 20 cm soil samples were collected from each plot for SOC estimation by an elemental analyzer and N process study by lab incubation approach (Supplementary Material).
Data analysis
In the litter decomposition experiment, one-way ANOVAs were used to examine differences in initial litter C and N concentrations. For both litter types (i.e. alone or in mixture), we calculated litter decay constants (k, month−1) to examine litter mixing effects on litter decomposition rates. The k values were estimated using Maximum Likelihood from a negative exponential model using four time points over 12 months as follows48,49:

where Mt is litter mass (or Nt for N) remaining at time t (t = 3, 6, 12 months), M0 is initial litter biomass at time t = 0 (or N0 for N), k is a first-order litter decay constant (Table 1).
To examine effects of litter mixing on decomposition rates, we compared observed mass remaining in mixed-species bags to predicted mass remaining of litter mixtures based on the observed mass of single plant litter samples. We generated predicted values using a permutation approach with the set of all combinations of the two single-species results. The predicted litter mass remaining in mixed-species bags was calculated by an equation as follows50,51:

where R refers to the remaining litter mass of a species (S = Solidago, P = Phragmites) when they decomposed in isolation and M refers to the initial litter mass of a species in litter mixture.
Similar to other litter mixture analysis52,53, we used one-way ANOVAs for each time period to test for differences between predicted and observed percent mass remaining and N remaining of the litter in mixtures (Table 2). As pointed out by Gartner and Cardon19, nevertheless, an ANOVA of observed vs. predicted values could violate the assumption of homogeneity of variance in ANOVA. It should also be noted that design with blocking might help in accounting for a source of variation unrelated to our treatments. Repeated-measure ANOVAs (MANOVA) were used to test the dependence of N concentration, mass and N remaining on plant species and litter mixing treatments54, followed by a set of contrasts to examine differences as affected by species within litter type or by litter mixing within species (Table 3; Figs. 1–3). In the repeated-measure ANOVAs, the between subject effects refer to litters and the within subject effects refer to the three census times. Since litter mass and N remaining were analyzed twice, statistical significance was at the level of α = 0.05/2. Otherwise, α = 0.05 was used to test the statistical significance.
One-way ANOVA followed by a set of contrasts was used to examine extracts effect on SOC and N processes in the extract experiment (Fig. 4).
No transformation was conducted if data meet the assumption of ANOVA, otherwise data was square-root transformed as necessary in this study. Statistical analyses were conducted in SAS 9.0 and JMP 9.0 (SAS Inc., Cary, NC, USA).
References
Hobbie, S. E. Effects of plant-species on nutrient cycling. Trends Ecol Evol 7, 336–339 (1992).
Ashton, I. W., Hyatt, L. A., Howe, K. M., Gurevitch, J. & Lerdau, M. T. Invasive species accelerate decomposition and litter nitrogen loss in a mixed deciduous forest. Ecol Appl 15, 1263–1272 (2005).
Poulette, M. M. & Arthur, M. A. The impact of the invasive shrub Lonicera maackii on the decomposition dynamics of a native plant community. Ecol Appl 22, 412–424 (2012).
Allison, S. D. & Vitousek, P. M. Rapid nutrient cycling in leaf litter from invasive plants in Hawai'i. Oecologia 141, 612–619 (2004).
Liao, C. Z. et al. Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis. New Phytol 177, 706–714 (2008).
Ehrenfeld, J. G. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6, 503–523 (2003).
Ehrenfeld, J. G., Kourtev, P. & Huang, W. Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol Appl 11, 1287–1300 (2001).
McArthur, J. V., Aho, J. M., Rader, R. B. & Mills, G. L. Interspecific leaf interactions during decomposition in aquatic and floodplain ecosystems. J N Am Benthol Soc 13, 57–67 (1994).
Nilsson, M. C., Gallet, C. & Wallstedt, A. Temporal variability of phenolics and batatasin-III in Empetrum hermaphroditum leaves over an eight-year period: interpretations of ecological function. Oikos 81, 6–16 (1998).
Hickman, J. E., Ashton, I. W., Howe, K. M. & Lerdau, M. T. The native-invasive balance: implications for nutrient cycling in ecosystems. Oecologia 173, 319–328 (2013).
Jin, L., Gu, Y. J., Xiao, M., Chen, J. K. & Li, B. The history of Solidago canadensis invasion and the development of its mycorrhizal associations in newly-reclaimed land. Funct Plant Biol 31, 979–986 (2004).
Liu, J. et al. Invasive alien plants in China: role of clonality and geographical origin. Biol Invasions 8, 1461–1470 (2006).
Sun, Z. K. & He, W. M. Evidence for enhanced mutualism hypothesis: Solidago canadensis plants from regular soils perform better. PLoS ONE 5, e15418 (2010).
Vanderhoeven, S., Dassonville, N., Chapuis-Lardy, L., Hayez, M. & Meerts, P. Impact of the invasive alien plant Solidago gigantea on primary productivity, plant nutrient content and soil mineral nutrient concentrations. Plant Soil 286, 259–268 (2006).
Yuan, Y. G. et al. Enhanced allelopathy and competitive ability of invasive plant Solidago canadensis in its introduced range. J Plant Ecol 6, 253–263 (2013).
Butcko, V. M. & Jensen, R. J. Evidence of tissue-specific allelopathic activity in Euthamia graminifolia and Solidago canadensis (Asteraceae). Am Midl Nat 148, 253–262 (2002).
Hättenschwiler, S., Tiunov, A. V. & Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol S 36, 191–218 (2005).
Lecerf, A. et al. Incubation time, functional litter diversity and habitat characteristics predict litter-mixing effects on decomposition. Ecology 92, 160–169 (2011).
Gartner, T. B. & Cardon, Z. G. Decomposition dynamics in mixed-species leaf litter. Oikos 104, 230–246 (2004).
Berglund, S. L. & Ågren, G. I. When will litter mixtures decompose faster or slower than individual litters? A model for two litters. Oikos 121, 1112–1120 (2012).
Schimel, J. P. & Hättenschwiler, S. Nitrogen transfer between decomposing leaves of different N status. Soil Biol Biochem 39, 1428–1436 (2007).
Chapman, K., Whittaker, J. B. & Heal, O. W. Metabolic and faunal activity in litters of tree mixtures compared with pure stands. Agric Ecosyst Environ 24, 33–40 (1988).
Schimel, J. P., Cates, R. G. & Ruess, R. The role of balsam poplar secondary chemicals in controlling soil nutrient dynamics through succession in the Alaskan taiga. Biogeochemistry 42, 221–234 (1998).
Hättenschwiler, S. & Vitousek, P. M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15, 238–243 (2000).
Zhang, C. B., Wang, J., Qian, B. Y. & Li, W. H. Effects of the invader Solidago canadensis on soil properties. Appl Soil Ecol 43, 163–169 (2009).
Horner, J. D., Gosz, J. R. & Cates, R. G. The role of carbon-based plant secondary metabolites in decomposition in terrestrial ecosystems. Am Nat 132, 869–883 (1988).
Wardle, D. A., Nilsson, M. C., Zackrisson, O. & Gallet, C. Determinants of litter mixing effects in a Swedish boreal forest. Soil Biol Biochem 35, 827–835 (2003).
Chen, B. M., Peng, S. L., D'Antonio, C. M., Li, D. J. & Ren, W. T. Non-additive effects on decomposition from mixing litter of the invasive Mikania micrantha H.B.K. with native plants. PLoS ONE 8, e66289 (2013).
Kainulainen, P. & Holopainen, J. K. Concentrations of secondary compounds in Scots pine needles at different stages of decomposition. Soil Biol Biochem 34, 37–42 (2002).
Grotkopp, E., Rejmanek, M. & Rost, T. L. Toward a causal explanation of plant invasiveness: Seedling growth and life-history strategies of 29 pine (Pinus) species. Am Nat 159, 396–419 (2002).
Daehler, C. C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu Rev Ecol Evol S 34, 183–211 (2003).
Leishman, M. R., Haslehurst, T., Ares, A. & Baruch, Z. Leaf trait relationships of native and invasive plants: community- and global-scale comparisons. New Phytol 176, 635–643 (2007).
Bonanomi, G., Incerti, G., Antignani, V., Capodilupo, M. & Mazzoleni, S. Decomposition and nutrient dynamics in mixed litter of Mediterranean species. Plant Soil 331, 481–496 (2010).
De Marco, A., Meola, A., Maisto, G., Giordano, M. & De Santo, A. V. Non-additive effects of litter mixtures on decomposition of leaf litters in a Mediterranean maquis. Plant Soil 344, 305–317 (2011).
Berglund, S. L., Ågren, G. I. & Ekblad, A. Carbon and nitrogen transfer in leaf litter mixtures. Soil Biol Biochem 57, 341–348 (2013).
Zhang, S. S., Zhu, W. J., Wang, B., Tang, J. J. & Chen, X. Secondary metabolites from the invasive Solidago canadensis L. accumulation in soil and contribution to inhibition of soil pathogen Pythium ultimum. Appl Soil Ecol 48, 280–286 (2011).
Callaway, R. M. & Ridenour, W. M. Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2, 436–443 (2004).
Hansen, R. A. Red oak litter promotes a microarthropod functional group that accelerates its decomposition. Plant Soil 209, 37–45 (1999).
Hansen, R. A. & Coleman, D. C. Litter complexity and composition are determinants of the diversity and species composition of oribatid mites (Acari: Oribatida) in litterbags. Appl Soil Ecol 9, 17–23 (1998).
Hoorens, B., Stroetenga, M. & Aerts, R. Litter mixture interactions at the level of plant functional types are additive. Ecosystems 13, 90–98 (2010).
Anderson, J. M. & Hetherington, S. L. Temperature, nitrogen availability and mixture effects on the decomposition of heather [Calluna vulgaris (L.) Hull] and bracken [Pteridium aquilinum (L.) Kuhn] litters. Funct Ecol 13, 116–124 (1999).
Weber, E. Current and potential ranges of three exotic goldenrods (Solidago) in Europe. Conserv Biol 15, 122–128 (2001).
Weber, E. Invasive plant species of the world: a reference guide to environmental weeds. (CABI Pub, 2003).
Saltonstall, K. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Natl Acad Sci U S A 99, 2445–2449 (2002).
Abhilasha, D., Quintana, N., Vivanco, J. & Joshi, J. Do allelopathic compounds in invasive Solidago canadensis s.l. restrain the native European flora? J Ecol 96, 993–1001 (2008).
Chen, B. M., Peng, S. L. & Ni, G. Y. Effects of the invasive plant Mikania micrantha H.B.K. on soil nitrogen availability through allelopathy in South China. Biol Invasions 11, 1291–1299 (2009).
Lorenzo, P., Palomera-Perez, A., Reigosa, M. J. & Gonzalez, L. Allelopathic interference of invasive Acacia dealbata Link on the physiological parameters of native understory species. Plant Ecol 212, 403–412 (2011).
Olson, J. S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44, 322–331 (1963).
Wieder, R. K. & Lang, G. E. A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63, 1636–1642 (1982).
Briones, M. J. I. & Ineson, P. Decomposition of eucalyptus leaves in litter mixtures. Soil Biol Biochem 28, 1381–1388 (1996).
McTiernan, K. B., Ineson, P. & Coward, P. A. Respiration and nutrient release from tree leaf litter mixtures. Oikos 78, 527–538 (1997).
Blair, J. M., Parmelee, R. W. & Beare, M. H. Decay-rates, nitrogen fluxes and decomposer communities of single-species and mixed-species foliar litter. Ecology 71, 1976–1985 (1990).
Salamanca, E. F., Kaneko, N. & Katagiri, S. Effects of leaf litter mixtures on the decomposition of Quercus serrata and Pinus densiflora using field and laboratory microcosm methods. Ecol Eng 10, 53–73 (1998).
Gurevitch, J. & Chester, S. T. Analysis of repeated measures experiments. Ecology 67, 251–255 (1986).
Acknowledgements
This work was supported by the NSFC (41225003), the Fundamental Research Funds for the Central Universities (KYT201404, NAU), 111 project (B12009) by Ministry of Education and US-NSF (DEB0820560). Ling Zhang was supported by China Scholarship Council for his study in USA.
Author information
Authors and Affiliations
Contributions
L.Z., J.W.Z. and E.S. designed the research; L.Z. and Y.J.Z. performed the experiment; L.Z., J.W.Z. and E.S. analyzed data; L.Z., J.W.Z. and E.S. wrote the main manuscript text; L.Z., Y.J.Z., J.W.Z. and E.S. reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
R3-Supplementary materials
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Zhang, L., Zhang, Y., Zou, J. et al. Decomposition of Phragmites australis litter retarded by invasive Solidago canadensis in mixtures: an antagonistic non-additive effect. Sci Rep 4, 5488 (2014). https://doi.org/10.1038/srep05488
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05488
This article is cited by
-
Understory vegetation altered soil CO2 and N2O emissions and the correlation with plant and soil stoichiometry following N and P addition in Chinese fir plantations
Plant and Soil (2023)
-
The decomposition processes and return of carbon, nitrogen, and phosphorus of Phragmites australis litter with different detritus amount
Hydrobiologia (2023)
-
Soil Aggregate Size Distribution Alters CO2 but not N2O Emission Rates in Chinese Fir (Cunninghamia lanceolata) Plantations with N and P Additions
Journal of Soil Science and Plant Nutrition (2023)
-
Changing litter composition following the dual invasion of Amur honeysuckle and the emerald ash borer alters fungal driven decomposition in Midwestern forests
Biological Invasions (2023)
-
Soil sterilization and fertility impacts on urease and belowground mass specific phosphatase activity vary among Chinese tallow tree (Triadica sebifera) populations
Plant Ecology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.






