Abstract
In the attempt to find valid alternatives to classic antibiotics and in view of current limitations in the efficacy of antimicrobial-coated or loaded biomaterials, this work is focused on the development of a new glass-ceramic with antibacterial performance together with safe biocompatibility. This bactericidal glass-ceramic composed of combeite and nepheline crystals in a residual glassy matrix has been obtained using an antimicrobial soda-lime glass as a precursor. Its inhibitory effects on bacterial growth and biofilm formation were proved against five biofilm-producing reference strains. The biocompatibility tests by using mesenchymal stem cells derived from human bone indicate an excellent biocompatibility.
Similar content being viewed by others
Introduction
Glass-ceramics are defined as polycrystalline materials, with residual amorphous phase, obtained from glass melting and controlled crystallization. They offer numerous advantages compared with parent glasses such as improved thermo-physical properties, higher strength and wear resistance. In general, glass-ceramics show favorable chemical, thermal, dielectric and biological properties superior to metals and various polymers1. They are ideal materials for the design and manufacture of bioceramics with superior mechanical properties. Most biomedical glass-ceramics are based on compositions similar to those of Hench's bioactive glasses (Bioglass®), having P2O5 as a common ingredient in all of them2. In the last few decades the important clinical applications of glass-ceramic are related to the repair and reconstruction of skeletal system (bone, joints and teeth) owing to their biocompatibility with living tissues. One of the main problems associated with the clinical use of these biomaterials in orthopedic surgery is contamination by infectious microorganisms. In fact, bone and joint have been listed among the most frequent sites of infections for which antibacterials are prescribed3.
Biomaterial-associated infections are a major problem in modern medicine. They have an enormous impact in terms of morbidity of the patients and costs to national health systems. Bacterial colonization and biofilm formation on the implanted device may lead to acute and chronic infection of the underlying bone and the adjacent soft tissues4. Bacteria start implant infection by adhering to biomaterials surfaces and/or interfaces producing biofilms, which represent a major reason for bacterial persistence. Moreover, the formation of a biofilm increases the resistance of the bacteria to antibiotics. The variety of bacterial species implicated in the etiology of implant infections commonly enlists Gram-positive bacteria of the genera Staphylococcus as well as Gram-negative microorganisms of the genera Pseudomonas, but often includes also other less frequent opportunistic pathogens with varying degree of virulence5. The multifactorial nature of the variables influencing the development of biomaterial-associated infections makes the assessment of clinical efficacy of anti-infective biomaterials an extremely difficult task. Various strategies have been adopted to retard biofilm development on implant surfaces: (a) modification of the biomaterial surface to give anti-adhesive and bacteria repelling properties, (b) antibacterial coatings, (c) nanostructured materials, (d) molecules interfering with bacterial biofilm6,7.
Once a mature bacteria biofilm has been established, conventional medical therapies based on systemic antibiotics are not efficacious and implant removal often represents the only chance to eradicate the infection. Antibiotic-loaded biomaterials are currently part of standard medical procedures for both prophylaxis and local treatment of implant infection. Considering the large risk of antibiotic resistance associated with antibiotic loaded coatings, non-antibiotics agents in the coating became very attractive alternatives4.
Pointing out the need for further research in the strategies to treat infection, this work is focused on the development of an antibacterial glass-ceramic that could be used for medical applications. This glass-ceramic can be obtained from several soda-lime glasses belonging to the SiO2-Na2O-CaO-B2O3 system with increasing content of calcium oxide. These family glasses were probed to be efficient antimicrobials in a previous work8. There are many examples in the literature of glass/glass-ceramic with bactericidal properties, but at least to the author's knowledge, there is no previous work in the literature about the efficacy to inhibit biofilm formation by a glass-ceramic which is not loaded with antibiotics or metallic nanoparticles (silver, copper).
Bactericidal activity is rarely highly specific and uniquely oriented towards prokaryotic cells. Often it is associated to a certain degree of cytotoxicity. However, several research works have proposed biocompatible glasses and glass ceramic with antibacterial properties9,10. It is critical to find a correct balance between bactericidal effects and biocompatibility properties. On this regard, a strong requirement is to ensure a proper stability with the high level of biological safety. Therefore, both the bactericidal activity and cytotoxicity of this glass-ceramic were evaluated in the present study.
Results
Characterization of the glass ceramic
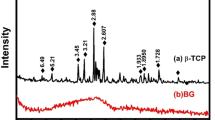
Thermal properties of the precursor glasses were investigated by differential thermal analysis (DTA-Tg) (Fig. 1.A). No exothermic peaks are observed in the case of the inert glass (G1). On the contrary, thermograms of the antimicrobial glasses exhibits two well defined crystallization exotherms at around 645 and 700°C, suggesting that at least two crystalline phases are being formed when these glasses are reheated. The XRD analyses allow us to identify the formed crystalline phases (Fig. 1.B). Before sintering, antimicrobial glasses are completely amorphous, present the characteristic broad peak at about 2θ = 25°, but the sintered materials exhibited a diffraction pattern characteristic of a glass-ceramic structure. The XRD study conducted on both antimicrobial glasses after heat treatment at 750°C showed that devitrification of these glasses lead to a glass-ceramic material composed of combeite (Na4Ca4(Si6O18), PDF 75-1687) and nepheline (Na6.65Al6.24Si9.76O32, PDF 83-2372) dispersed in a glassy matrix. Analysis of the relative intensities of the peaks concluded that the same glass-ceramic can be obtained from both antimicrobial glasses. Therefore, the rest of the work was carried out using only the glass-ceramic obtained from the antimicrobial glass G3. In the case of the inert glass after thermal treatment, peaks of quartz (PDF 001-0649) are clearly visible.
SEM image (Fig. 2) illustrates the presence of dense arrangement of crystals and it also reveals the presence of hexagonal shaped and rod likes crystals. Pores are also perceptible. The specific surface areas (SBET) of the glassy pellets were calculated from the N2 adsorption isotherms using the multipoint Brunauer-Emmett-Teller (BET) technique. Both glassy pellets present similar SBET: 0.33 m2/g the glass-ceramic and 0.31 m2/g the inert control glass. Higher average surface roughness values (Ra) was found for the glass-ceramic discs (0.97 ± 0.09) than for the inert glass discs (0.04 ± 0.03).
TEM/EDS provide more detailed microstructural characterization of the glass-ceramic. In TEM image (Figure 3) both crystalline phases can be clearly identified in close agreement with the XRD results (Fig. 1B). Nepheline crystals (Fig. 3, n°2) show larger sizes than combeite crystals ((Fig. 3, n°1). The EDS analysis of crystal 1 showed that the Ca/Si ratio reached values corresponding to combeite. Likewise, Al/Si ratio in crystal 2 is consistent with nepheline. The CaO content in the residual glassy phase (Fig. 3, n°3) is similar to that of the parent glass.
Bactericidal property of the glass ceramic
Bacterial growth
Total colony forming units of all studied strains from inert glass powder (control) achieved 108–109 CFU/mL in all study time period (0–72 h). Colony forming units from glass-ceramic cultures were significantly lower (p < 0.0001) than those from inert soda-lime glass cultures for all strains and at all study time period, except for S. aureus at 24 h (p = 0.02), S. aureus at 48 h (p = 0.004), E. coli at 24 h (p = 0.004). Figure 4 shows the logarithm reduction (CFU/mL) observed in the glass-ceramic cultures respect to inert glass cultures. Glass-ceramic powder significantly reduced the number of bacterial cells of all ATCC strains with respect to control glass powder by 99.99% at 48 and 72 h except for S. aureus where reductions of 96.6% and 99.7% were observed, respectively. S. epidermidis, M. lutea and E. coli were the most affected strains achieving a 5 log10 reduction at 72 h.
In order to evaluate the role of each crystalline phase in the bactericidal capability, antibacterial tests were also carried out with the phases fully crystallized. As it is clearly seen (Figure 5) no bactericidal activity against E. coli was obtained for nepheline, whereas in the case of combeite a quite similar behavior to the glass-ceramic was observed.
Biofilm formation
Total colony forming units of biofilms formed in the inert glass discs (control) achieved 106–108 CFU/mL for all strains and at all study time period (0–72 h). Colony forming units from control discs were significantly higher (p < 0.0001) than those from glass-ceramic discs for all ATCC strains. Figure 6 shows the logarithm reduction (CFU/mL) observed in the glass-ceramic discs respect to inert glass discs. Glass-ceramics significantly reduced the adherence of all ATCC strains with respect to inert glass by 99.99% at 24 h, except for S. epidermidis where a reduction of 99.87% was observed. It is noteworthy the case of M. lutea strain, antibiofilm activity increased over time achieving a 5–6 log10 reduction at 48 and 72 h.
Biocompatibility of antibacterial glass ceramic
Cells performed generally better when cultured on the antibacterial glass-ceramic substrates than on the control surfaces. The values obtained for the MTT assay (Figure 7) marked an optimal and gradually increasing proliferation values for the glass-ceramic (p-value < 0.05, t-student test, 95% confidence level). Such results are in harmony with the apoptotic rates (Figure 8). A nominal apoptotic rate is always found in cell cultures even in cell friendly surfaces from culture flasks, however, soda-lime glass used as control is showing a greater apoptotic rate than expected when compared to a more biocompatible surface like the glass-ceramic.
Cells need to attach in order to proceed with the colonization of any biomaterial surface, while attached, DNA concentration could be extracted and calculated in order to gain an idea of which surface is presenting a higher cell concentration. The glass-ceramic shows again (Figure 9) a good performance when compared to the inert glass (p-value < 0.05, t-student test, 95% confidence level), supporting the previous observation for apoptosis and proliferation. When cells are stained for a live/dead assay (Figure 10), glass-ceramic substrate shows a clear viability of hBMSCs.
Discussion
In the present work, a new glass-ceramic composed of combeite and nepheline in a residual soda-lime glassy matrix was fabricated by sintering at 750°C – 1 h of an antimicrobial glassy frit. Powders (<30 μm) were conformed to discs by conventional axial pressing.
The obtained antibacterial results (Fig. 4–6) clearly show that this glass-ceramic free of P2O5 not only is effective to diminish the growth of bacteria but also to inhibit adhesion and biofilm formation. It is well known that surface roughness promote a bacterial adhesion process11. In this particular case, the bactericidal properties are not affected although the glass ceramic is rougher than the inert glass used as control. The pH of the medium after the biocide test was measured. There was a slight increase of the pH of the medium but it was not significantly higher than the pH of the fermentation broth at the end of the growth. By way of example, the pH of the medium after the bactericidal test against E. coli is: 8.71 for the culture, 8.87 for the inert glass and 8.97 for the glass-ceramic. Therefore, it was not possible to conclude from these experiments that the antibacterial activity was due to a high pH per se. The antimicrobial activity of the parent glass arises from the capability to release calcium ions at the glass-particle interface, which leads to membrane depolarization and the subsequent death of the cell, as well reported in previous work8. It is well known that cellular calcium ions overload, or perturbation of intracellular Ca2+ compartmentalization, may cause cytotoxicity and result in either apoptotic, necrotic or autophagic cell death12,13,14. From those results we can infer that something similar happens with the glass-ceramic. Then role of calcium must be pointed out when the biocide activity is evaluated with the fully crystallized phases. Only the phase contains calcium (i.e., combeite [Na2Ca2Si3O9]) showed antibacterial capability. Calcium is contained in both combeite crystals and the residual glassy phase of the glass-ceramic (Fig. 3). Therefore, both phases are somehow involved in its antibacterial capability. Combeite is a complex structure constituted by piling of 6-fold silica tetrahedral rings. Sodium atoms are placed in the centre of these rings. Alternated calcium and sodium atoms are located in the open space between the rings columns of sodium15. Then, these calcium ions, as a consequence of its light bonding, can be easily exchanged by H+ (or H3O+) ions from the solution in a similar way than in the original glass structure. In this sense, we considered the operating mechanism must be very similar to that of the parent glass.
A similar situation has been previously observed by the authors16 in the case of a bioactive glass-ceramic made from sintered 45S5 Bioglass®, containing Na2Ca2Si3O9 as crystalline phase. Despite the amount of research and literature on antimicrobials, there is no previous work, at least to the author's knowledge, about the efficacy to inhibit biofilm formation by a glass-ceramic which is not loaded with antibiotics or metallic nanoparticles (e.g. silver, copper).
An ideal material for the treatment of infection should not only posses a bactericidal property, preventing microbial adhesion and colonization, but it must also have excellent biocompatibility. The cytotoxicity of the glass-ceramic was assessed by the MTT assay using the hBMSCS as a cell model. As shown in Fig. 7, the number of viable cells on the antibacterial glass-ceramic is noticeably higher than that on the inert glass after culturing the hBMSCs for 1, 2 and 5 days. In addition, the enhanced cell adhesion and proliferation on the bactericidal glass-ceramic (Fig. 8–9) indicates no obvious adverse effects on cell viability and proliferation. The results obtained in the present investigation open the possibility to fabricate a multifunctional biomaterial, which at the same time inhibits bacterial colonization (i.e. biofilm formation) as well as effective integrating with host tissues. This new glass-ceramic could found important medical applications such as coating (prosthesis, dental devices), scaffolds, etc.
On the other hand, this glass-ceramic acts as a delivery system providing sustained release of the ions which may affect and potentiate a more appropriate and rapid tissue response. The crystalline phase combeite (Na2Ca2Si3O9) has been formed and identified in other studies on sintered bioactive glasses17,18,19. It is known that this phase is bioactive and biodegradable in amorphous calcium phosphate upon immersion in simulated body fluid20,21. Certain compositions of the glass-ceramics have shown bioactivity, generally defined as the ability to elicit favorable cellular response. Phosphate content is considered important for the bioactivity but it was proved that P2O5 free CaO·SiO2 also showed bioactivity22. Base on this, it would be expected that this new antibacterial free of P2O5 glass-ceramic is also bioactive; however, even if the literature reports bioactivity properties also for P2O5-free glass compositions, in vitro bioactivity test will be/have to be performed for this new antimicrobial free of P2O5 glass-ceramic in order to verify its ability to create a chemical bond with tissue.
The results obtained in this investigation show the feasibility of the production by a sinter-crystallisation process of an antibacterial glass-ceramic composed of combeite and nepheline crystals in a residual glass matrix. This glass-ceramic is significantly able to inhibit bacterial growth, minimize bacterial adhesion and prevent biofilm formation. In addition, the in vitro biocompatibility assays with human stem cells demonstrate that this glass-ceramic has an excellent biocompatibility. This antibacterial glass-ceramic could find application in the production of anti-infective biomaterials. Final form of this glass-ceramic could be as powders, granules, dense pieces, coatings and porous scaffolds, thus providing a wide spectrum of alternatives for each specific clinical use.
Methods
Preparation of the antibacterial glass-ceramic
In previous studies8,23 the antimicrobial capability of several soda-lime glasses belonging to the SiO2-Na2O-CaO-B2O3 system, with increasing content of calcium oxide, were probed against different microorganisms. Two of those glasses with a significant biocide activity against bacteria and yeast were selected as precursors of the antibacterial glass-ceramic. Other one, with null effect against microorganism was selected as control in the bactericidal and biocompatibility tests.
Two antimicrobial soda-lime glasses (labeled as G2 and G3) were prepared by melting appropriate mixtures of reagent grade SiO2 (Cuarzos Industriales S.A., Santiago de Compostela), α-Al2O3 (Taimei Chemical Co. Ltd., Japan), H3BO3, Na2CO3 and CaCO3 (Sigma Aldrich). Their chemical compositions are shown in table 1. The starting materials were weighed, mixed and melted in a Pt crucible for 1 h at 850°C to favor decarbonation of samples and subsequently for 1 h at 1400°C. The melts were then quenched in water and grounded by ball milling to fine particulates and sieved to obtain particle size < 30 μm. The study of the thermal behavior of these glasses were performed with a thermogravimetric-differential thermal analyzer (TA Instruments, Q600). The heating rate was 10°C min−1 up to a maximum temperature of 1100°C in air. Based on the DTA data (Figure 1), the schedule of thermal treatment was designed to convert the glasses into glass-ceramics. Fractions of 1 g of powder were compacted at 250 MPa by uniaxial pressing to obtain discs (10 mm in diameter and 2 mm in height). Afterwards, the discs were sintered in air by heating at a rate of 10°C min−1 to 750°C and holding for 1 h.
Inert soda-lime glass (labeled as G1) with the chemical composition showed in table 1 was prepared in analogous procedure as the antimicrobial glasses. Fractions of 1 g of powder were compacted at 250 MPa by uniaxial pressing to obtain discs (10 mm in diameter and 2 mm in height). Afterwards, discs (10 × 2 mm) were sintered in air by heating at a range of 10°C min−1 to 700°C and holding for 1 h.
Fully crystallize phases were synthesized in order to evaluate the role of the crystalline phases of the glass-ceramic in the antibacterial capability. Combeite was synthesized by solid state reaction at 980°C from appropriate mixtures or reagent grade SiO2 (Cuarzos Industriales S.A., Santiago de Compostela), Na2CO3 and CaCO3 (Sigma Aldrich). Nepheline was also synthesized according to the Na2O-Al2O3-SiO2 system, by solid state reaction at 900°C starting from a proper proportions of SiO2 (Cuarzos Industriales S.A., Santiago de Compostela), Na2CO3 and Al2O3 (Sigma Aldrich).
Characterization of the antibacterial glass ceramic
The XRD analyses of glass-ceramic discs were carried out in a Bruker D8 diffractometer using CuKα radiation working at 40 kV and 30 mA in a step-scanning mode from 4° to 70° with a step width of 0.05° and a step time of 0.5 seconds. The morphology of the obtained glass-ceramic was studied by scanning electron microscopy (SEM) (Hitachi S-4300) and also characterized using transmission electron microscopy (TEM) (JEOL FXII) with an accelerating voltage of 200 kV. In order to estimate the surface morphology, the surface of the discs were measured with a surface profilometer (Talysurf CLI 500, Taylor Hobson, Leicester, UK) that maps the surface by putting a stylus in mechanical contact with the sample. The stylus arm has 90° conisphere diamond styli with 2 μm nominal radius tip. The data sampling interval in X and Y was 0.5 μm and 2.5 μm respectively. The resolution (Z) was 32 nm. The profilometer was used to determine the three-dimensional topographic map and to calculate the roughness factor or specific surface area (the ratio of the surface to the projected area). Three samples of each glass were scanned to evaluate the average surface roughness (Ra) of the surfaces at five different locations. Textural characterization was carried out by measuring the N2 adsorption isotherms at −196°C in an automatic apparatus (TriStar II 3020, Micromeritics Instrument Corporation). The Barret-Emmett-Teller (BET) method was utilized to calculate the specific surface areas (SBET).
Evaluation of the bactericidal effect
Bacterial strains
Five biofilm-producing reference strains: Staphylococcus aureus ATCC 29213, Staphylococcus epidermidis ATCC 35984, Pseudomonas aeruginosa ATCC 23389, Escherichia coli ATCC 25922 and Micrococcus lutea ATCC 9341, were used in both antibacterial assays (biocide and biofilm formation assays). They are the most frequent pathogens implicated in the etiology of biomaterials-associated infections24.
Biocide assays
A single colony from each microorganism was inoculated in Luria Bertani (LB) broth (Difco; BD Diagnostics, Sparks, MD, USA) and incubated overnight at 37°C. Each culture (10 mL) was diluted into fresh media (1 mL) and cultured at 37°C for 6 h; finally, fresh media (1 mL), with inert soda-lime glass (control) or glass-ceramic powders (75 μL of 200 mg/mL aqueous suspension), were inoculated with the above cultures (10 μL). Samples were taken at 0, 24, 48 and 72 hours to determine the viable bacteria and expressed as colony forming units (CFU/mL). All experiments were performed in triplicate. The limit of detection was 5 × 101 CFU/mL. Antibacterial effectiveness is expressed as the logarithm reduction in viable counts of the test bacteria (CFU/mL). It was calculated by subtracting the log10 colony counts in the glass ceramic cultures from those in the inert glass cultures (control). The percentage was calculated as %IR = 100 − (100 × In)/It, where %IR is percentage of inoculum reduction, In is the bacterial count (CFU/mL) in the glass-ceramic cultures and It is the inoculum (CFU/mL) in the control cultures.
Similarly, the antibacterial capability against E. coli of the fully crystallized phases (combeite and nepheline) was evaluated.
Biofilm formation assays
Colonies of ATCC strains from an overnight culture on Columbia sheep blood agar (Difco, BD Diagnostic Systems, Sparks, MD, USA) were allowed to grow in LB medium at 37°C to a density of 0.5–1 × 108 CFU/mL as measured by a UV-Visible spectrophotometer (GBC, Model Cintra 101, Australia) and diluted 1/10 in fresh LB medium. Glass-ceramic and inert soda-lime glass discs were placed in 24-well culture plates and 300 μL of the inoculum suspension were inoculated for two hours (adhesion phase). After the adhesion period, the medium was discarded (non-adherent cells were removed) and 300 μL of fresh LB medium were added in each well. The discs were incubated at 37°C for 72 h in a wet chamber. Samples were taken at 0, 24, 48 and 72 hours to determine the viable bacteria.
Viable counts were used to determine the quantity of viable adherent bacteria. After biofilm formation, discs were carefully washed three times with sterile saline solution to remove unattached cells and inserted in new well containing 1 mL of sterile saline solution. The discs were sonicated for 10 minutes in ultrasonic bath (FB15050; Thermo-Fischer Scientific) to disperse bacterial cells. Samples of 200 μL from sonicated biofilms were serially diluted in 0.9% saline solution and plated onto Columbia sheep blood agar to determine the total number of viable cells and expressed as CFU/mL. All experiments were performed in triplicate. The limit of detection was 2.5 × 102 CFU/mL. Preliminary experiments indicated that sonication was not associated with a loss of viability of the cells. Antibacterial effectiveness is expressed as the logarithm reduction in viable counts of the test bacteria (CFU/mL). It was calculated by subtracting the log10 colony counts in the glass ceramic discs from those in the inert glass discs. The percentage was calculated as %IR = 100 − (100 × In)/It, where %IR is percentage of inoculum reduction, In is the bacterial count (CFU/mL) in the glass-ceramic discs and It is the inoculum (CFU/mL) in the control discs.
Statistical analysis of the tests carried out with prokaryotic cells
All statistical analyses were performed by the unpaired Student's t test. A P-value of <0.05 was considered statistically significant.
Proliferation of hBMSCs
Cell culture
For investigating the cellular responses to the developed glass-ceramic, mesenchymal stem cells derived from human bone marrow were used (hBMSCs). hBMSCs were obtained by the Department of Orthopaedic Surgery (University of Santiago de Compostela Clinical Hospital) during routinary hip prosthesis surgeries by ethically approved protocols. Bone marrow was stored in heparin coated tubes and taken to the cell culture laboratory for proceeding with mesenchymal stem cell isolation. A Ficoll protocol as described by Yeo et al.25 was used. Briefly, a bone marrow as slowly poured on top of a Ficoll volume (1:1) in a 15 mL Falcon tube. Rapidly after this step a centrifugation takes place (2000 rpm, 30 min). Cells are pipetted onto fresh medium (D-MEM, 20%FCS, Gentamycin) and cultured for 2 weeks until a 90% confluence is reached. The total cell density used was 25000 cells/cm2. Human bone marrow cells response was evaluated by determining cell adhesion and cell proliferation.
Cell proliferation by MTT assays
The survival and proliferation of hBMSCs on glass-ceramic discs was examined with a MTT assay after 1, 2 and 5 days of culture. The MTT assays were carried out as per manufacturer's protocol (Sigma-Aldrich). Briefly, hBMSCs were seeded on the sample discs (d = 10 mm) at a density of 25 × 103 cells per well in a 24-well plate. At each time point, the medium were removed, MTT solution was added and cells were incubated overnight. After removal of the MTT solution, the purple formazan crystals were dissolved in 100 μL of dimethyl sulphoxide (DMSO) by shaking the plate for 15 min and 50 μL solution of each well was added into a new 24-well plate. The OD value was quantified by measuring the absorption at 570 nm using an automated plate reader (PerkinElmer).
Cellular adhesion
Viable cells should be adhered to the surface of the assayed biomaterials. An attachment assay gave us an insight of how many cells were attached on the material surface. Briefly, cells were incubated for 24 hours to allow the attachment to happen, after this first incubation, the culture media was removed to wash out any floating cell. Samples were gently washed with phosphate buffered saline (PBS), which helped to remove the non attached cells, right after this step, a 3.5% paraformaldehyde solution was added and incubated for 1 h. Finally, cells were stained with 0.1% toluidine blue for 3 h. The dye was washed out with destilled water and the cells lysed with 500 mL of 0.1% sodium dodecyl sulfate (SDS). The resulting aliquot was assed with an optical density at 595 nm.
Plates were incubated at 37°C in 5% CO2 for the indicated time period. Wells were flicked dry and were washed three times with PBS and adherent cells were fixed in 100 μL of 10% formalin for 15 minutes at RT. Plates were stained overnight with 100 μL of toluidine blue in 10% formalin. Plates were washed three times with copious amounts of distilled water before lysis in 2% SDS for 10 minutes. Optical density (OD) was read at 600 nm with a microtiter plate reader.
Apoptosis: caspase activity assay
Caspase-3 activity was measured in supernatants by a Quantikine immunoassay (ELISA) active caspase-3 kit (R&D Systems, Minneapolis, MN). Briefly, cytoplasmic protein extract was incubated with biotin-ZVKD-fluoromethylketone inhibitor, which covalently binds and makes detectable the active caspase-3. A monoclonal antibody specific for active caspase-3 was precoated onto a microplate and active protease concentrations were assessed in cell lysates, according to the manufacturer's instructions.
Confocal microscopy. live/dead staining
Calcein-AM and propidium iodide were used to stain the hBMSCs cultured on the glass ceramic pellets. After 48 h of incubation the samples were gently washed with D-MEM. The viable and non viable cells were visualized by using a confocal laser scanning microscope (CLSM-SP2, Leica), the viable cells appear fluorescent green, whereas nonviable cells appear fluorescent red.
Statistical analysis of the tests carried out with eukaryotic cells
Mintab 16 (Minitab INC) was used for the statistical data analysis. T-student tests were carried out with an α = 0.05.
References
Höland, W. & Beall, G. H. Glass-ceramic technology: Second Edition (John Wiley and Sons, New Jersey, 2012).
de Aza, P. N., de Aza, A. H., Pena, P. & de Aza, S. Bioactive glasses and glass-ceramics. B. Soc. Esp. Ceram. V 46, 45–55 (2007).
Campoccia, D., Montanaro, L., Speziale, P. & Arciola, C. R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 31, 6363–6377 (2010).
Goodman, S. B., Yao, Z., Keeney, M. & Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 34, 3174–3183 (2013).
Arciola, C. R., An, Y. H., Campoccia, D., Donati, M. E. & Montanaro, L. Etiology of implant orthopedic infections: a survey on 1017 clinical isolates. Int. J. Artif. Organs. 28, 1091–1100 (2005).
Campoccia, D., Montanaro, L. & Arciola, C. R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 34, 8533–8554 (2013).
Simchi, A., Tamjid, E., Pishbin, F. & Boccaccini, A. R. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomed. Nanotechnol. 7, 22–39 (2011).
Moya, J. S., Esteban, L., Pecharromán, C., Mello-Castanho, S. R. H., da Silva, A. C. & Malpartida, F. Glass powders with a high content of calcium oxide: A step towards a “green” universal biocide. Adv. Eng. Mater. 13, B256–B260 (2011).
Zhang, D. et al. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. Part A 93, 475–483 (2010).
Gomes, C. H. et al. Assesment of antimicrobial effect of biosilicate® against anaerobic, microaerophilic and facultative anaerobic microorganisms. J. Mater. Sci: Mater. Med. 22, 1439–1446 (2011).
Teughels, W., Van Assche, N., Sliepen, I. & Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implan. Res. 17, 68–81 (2006).
Rizzuto, R., Stefani, D., Raffaello, A. & Mammucari, C. Mitochondria as sensors and regulators of calcium signaling. Nat. Rev. Mol. Cell Bio. 13, 566–578 (2012).
Zhivotovsky, B. & Orrenius, S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium 50, 211–221 (2011).
Krebs J., & Michalak M. (Eds). Calcium: A matter of life or death. Elsevier 2007.
Ohsato, H., Takeuchi, Y. & Maki, I. Structure of Na4Ca4(Si6O18). Acta Crystallogr. Sect. C: Cryst. Struct. Commun. C42, 934–937 (1986).
Cabal, B. et al. The development of bioactive glass-ceramic substrates with biocide activity. Adv. Eng. Mater. 13, B462–B466 (2011).
Rahaman, M. N. et al. Bioactive glass in tissue engineering. Acta Biomater. 7, 2355–2373 (2011).
Salman, S. M., Salama, S. N. & Abo-Mosallam, H. A. The role of strontium and potassium on crystallization and bioactivity of Na2O-CaO-P2O5-SiO2 glasses. Ceram. Int. 38, 55–63 (2012).
Belluci, D., Cannillo, V. & Sola, A. An overview of the effects of thermal processing on bioactive glasses. Sci. Sinter. 42, 307–320 (2010).
Chen, Q. Z., Thompson, I. D. & Boccaccini, A. R. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 27, 2414–2425 (2006).
Du, R. & Chang, J. Preparation and characterization of bioactive sol-gel-derived Na2Ca2Si3O9. J. Mater. Sci. Mater. M. 15, 1285–1289 (2004).
Ebisawa, Y. & Kokubo, T. Bioactivity of CaO SiO2-based glasses: in vitro evaluation. J. Mater. Sci. Mater. M. 1, 239–244 (1990).
Moya, J. S. et al. Mechanism of calcium lixiviation in soda-lime glasses with a strong biocide activity. Mater. Lett. 70, 113–115 (2012).
Moriarty, F. Z. & Sebastian, A. J. Biomaterials associated infection. [Busscher H. J. (ed.)] (Springer, New York, 2013).
Yeo, C. et al. Ficoll-Paque versus Lymphoprep: A comparative study of two density gradient media for therapeutic bone marrow mononuclear cell preparations. Regen. Med. 4, 689–696 (2009).
Acknowledgements
This work was supported by the Spanish Ministry of Economy and Competitiveness (MINECO) under the project MAT2012-38645 and by CSIC project ref. n° 201360E012. B. Cabal acknowledges financial support from JAE-Doc program (CSIC, cofounded by FSE).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: B.C., L.A., D.S., F.G., R.T. and J.S.M. Performed the experiments: B.C., F.C., R.C. and L.E.-T. Analyzed the data: B.C., L.A., R.C., D.S., R.T. and J.S.M. Wrote the paper: B.C., L.A., R.C., D.S. and J.S.M. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Cabal, B., Alou, L., Cafini, F. et al. A New Biocompatible and Antibacterial Phosphate Free Glass-Ceramic for Medical Applications. Sci Rep 4, 5440 (2014). https://doi.org/10.1038/srep05440
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05440
This article is cited by
-
Carbon-fiber reinforced PEEK instrumentation for spondylodiscitis: a single center experience on safety and efficacy
Scientific Reports (2021)
-
Sol–gel based synthesis and biological properties of zinc integrated nano bioglass ceramics for bone tissue regeneration
Journal of Materials Science: Materials in Medicine (2021)
-
Novel antimicrobial phosphate-free glass–ceramic scaffolds for bone tissue regeneration
Scientific Reports (2020)
-
Real-time sensors for live monitoring of disease and drug analysis in microfluidic model of proximal tubule
Microfluidics and Nanofluidics (2020)
-
Histological response of soda-lime glass-ceramic bactericidal rods implanted in the jaws of beagle dogs
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.