Abstract
Value-added intermediates produced by microorganisms during the catabolism of N-heterocycles are potential building blocks for agrochemical synthesis and pharmaceutical production. 6-Hydroxy-3-succinoyl-pyridine (HSP), an intermediate in nicotine degradation, is an important precursor for the synthesis of drugs and compounds with biological activities. In the present study, we show that an engineered biocatalyst, Pseudomonas putida P-HSP, efficiently produced HSP from the renewable raw material of tobacco-waste that contains a high concentration of nicotine. The genetically constructed strain P-HSP realized a high accumulation of HSP and HSP production was 3.7-fold higher than the non-engineered strain S16. Under optimal conditions, HSP was produced at high concentrations of 6.8 g l−1 and 16.3 g l−1 from tobacco-waste and nicotine, respectively. This work demonstrates a green strategy to block the catabolic pathway of N-heterocycles, which is a promising approach for the mutasynthesis of valuable compounds.
Similar content being viewed by others
Introduction
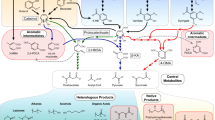
Microbial degradation of N-heterocycles, such as nicotine, may produce numerous intermediates and many of these intermediates are potential building blocks for agrochemical and pharmaceutical synthesis (Figure 1)1,2. Moreover, there is a rising interest in the microbial production of valuable chemicals from renewable raw materials. For example, tobacco production for cigarette and cigar manufacturing can generate approximately 75% residue (tobacco-waste), which is a renewable raw material that could potentially be used by bio-refineries to generate energy and other products3,4,5. Furthermore, tobacco-waste is considered to be an environmental pollutant because it contains a high concentration of nicotine, which is the main alkaloid in tobacco, constituting 0.6%–3% (w/w) of dry tobacco leaves3,6. Nicotine has not been seriously considered as a potential renewable resource except for its use as an insecticide. It may because nicotine is toxic and the selective functionalization at two N-heterocyclic rings makes nicotine difficult to control by chemical means7. Microbial degradation of nicotine produces several metabolic intermediates, most of which are pyridine derivatives.
Transformation of N-heterocycles and potential use in chemical synthesis.
(1) Arthrobacter nicotinovorans can transform nicotine to 6-hydroxynicotine (precursor of insecticides)16. (2–6) Pseudomonas putida S16 transforms nicotine to 3-succinoylpyridine, 6-hydroxy-3-succinoylpyridine and 2,5-dihydroxypyridine (precursor of 5-aminolevulinic acid) (red line)12. (7–11) Nicotinic acid can be transformed into 6-hydroxynicotinic acid (precursor of imidacloprid and nicotinoid insecticides) by Serratia marcescens IFO1264836, 2,5-dihydroxypyridine by Pseudomonas fluorescens TN537 and 2-hydroxynicotinic acid by Proteobacteria sp.38. (12, 13) 3-Cyanopyidine can be transformed into nicotinic acid and nicotinamide (a vitamin used as a food supplement) by Rhodococcus rhodochrous J139,40. (14, 15) Agrobacterium sp. DSM6336 has the capacity to transform 2-cyanopyrazine to 5-hydoxypyrazine-2-carboxylic acid41, which can be used for the synthesis of 5-chloropyrazine-2-carboxylic acid esters. (16–20) Microbial degradation of quinoline and isoquinoline produces several intermediates and these intermediates also have potential uses in pharmaceutical synthesis30. Solid-lined arrows: biological processes. Blue-dashed arrows: chemical processes.
Pyridine derivatives are extensively seen in natural products, pharmaceuticals and functional materials. However, pyridine derivatives are mainly produced by organic synthesis, which is often accompanied by the formation of by-products, resulting in high costs8. Biocatalysis is a useful supplementary technology for the chemical industry in the reactions that are not easily conducted in the organic chemistry methods1,7. For example, the reactions involved in the formation of 2-substituted pyridines and their derivatives from pyridine N-oxides are useful synthetic methods; however, these methods require strict conditions for high product yields9,10. In microorganisms, the pyridine 2-hydroxylation step is specifically catalyzed by a molybdenum-containing protein, such as nicotinic acid dehydrogenase (EC1.17.1.5) in nicotinic degradation pathway and nicotine hydroxylase (EC1.5.99.4) and Spm (Figure 2a) in nicotine degradation pathways11,12. This hydroxylation step can be introduced to replace the 2-substituted pyridine reaction in organic synthesis. Therefore, the bio-production of valuable intermediates from N-heterocycles has a promising potential for organic chemical synthesis.
Characteristics of P. putida P-HSP.
(a) Metabolic pathway of nicotine in P. putida P-HSP was locally blocked (a, top). Enzymatic steps 1–5 are catalyzed by NicA2, nicotine oxido-reductase; Pnao, pseudooxynicotine amine oxidase; Sapd, DSP dehydrogenase; Spm, SP monoxygenase and HspB, respectively. The HSP monooxygenase activities of strain P. putida S16 and P-HSP are shown in (a, bottom panels). The photographs (a, bottom panels) were taken in the lab by the first author Yu. (b) Construction and verification of engineered strain P-HSP.
6-Hydroxy-3-succinoyl-pyridine (HSP), a metabolic intermediate of nicotine, is a potential building block in the synthesis of drugs, insecticides and other compounds that possess biological activities, such as the analgesic molecule epibatidine (Figure 1)1. HSP, which has a hydroxyl group and a side-chain, is a promising precursor for the synthesis of 2,5-di-substituted and 2,3,5-tri-substituted pyridine derivatives13,14. These 2,5-/2,3,5-substituted pyridine units are also present in several currently marketed drugs, including pioglitazone (Actos, for diabetes), eszopiclone (Lunesta, for insomnia) and the recently approved crizotinib (Xalkori, for cancer)15. Additionally, HSP is involved in several nicotine degradation pathways and HSP production may facilitate investigation of these pathways16,17,18. No procedure for organic chemistry-mediated synthesis of HSP has been reported; however, biotransformation of HSP from nicotine is a practical commercial method1.
Compared with purified enzymes, whole-cell catalysts are readily available and relatively inexpensive and they are particularly useful for multi-enzymatic reactions19,20. Because most enzymes that catalyze the conversion of nicotine to HSP have not yet been purified and studied in vitro16,17, whole-cell catalysis is a better option for HSP production21,22. In previous studies, HSP concentration produced by whole cells of a wild strain of Pseudomonas putida S16 was rather low at a concentration of 1.45 g l−1, with a yield of 43.5%22. Although the growth and catalytic conditions could be optimized, it was very difficult to achieve high product yield and productivity. Therefore, microorganism engineering may be an attractive strategy for a high accumulation of HSP.
In this study, we constructed a whole-cell catalyst, P. putida P-HSP, by blocking the catabolic pathway of nicotine (Figure 2). The catalyst efficiently accumulated HSP and high yield and productivity were obtained in batch and fed-batch biotransformations using tobacco-waste and nicotine as substrates, under optimized production conditions.
Results
Construction of engineered P. putida P-HSP
HSP, converted from 3-succinoyl-pyridine, is transformed into 2,5-dihydroxy-pyridine (2,5-DHP) by the enzyme HspB in P. putida S1623. HspB is essential for the conversion of HSP in strain S16; therefore, it is estimated that the inactivation of hspB would cause HSP accumulation (Figure 2a). The hspB gene in strain S16 was disrupted by homologous recombination as described in the Methods section (Figure 2b) to produce P. putida P-HSP (P. putida S16 hspB::pK18mob). HspB activity was not detected in P. putida P-HSP (Figure 2a).
HSP transformation capacity was regained in an hspB-complemented strain (P. putidaS16 hspB::pK18mob (pME-hspB)) by transforming a plasmid containing hspB into strain P-HSP. Both engineered and unmodified strains were grown in LB media with 1 g l−1 nicotine for 24 hours. The culture color of wild strain S16 was not changed; however, the culture color of the hspB-complemented strain turned to saddle brown (the color of 2,5-DHP) (Figure 3a). The insertion of pK18mob in hspB blocks the transcription of the entire nic2 cluster17, which also blocks the expression of 2,5-DHP dioxygenase gene. Therefore, 2,5-DHP transformed from HSP in the hspB-complemented strain cannot be further converted because 2,5-DHP dioxygenase was not expressed. The resting cell experiment results (Figure 3b, c) confirm that the hspB-complemented strain regains the potential to convert HSP.
Degradation of nicotine and HSP by P. putida S16 and the hspB-complemented strain (P. putida S16 hspB::pK18mob (pME-hspB)).
(a) Both strains were grown in LB media with the addition of 1 g l−1 nicotine for 24 h. (b, c) Resting cells of P. putida S16 (black line) and hspB-complemented strain (red line) were used for nicotine and HSP degradation at 30°C, 3.4 g DCW l−1, in 0.05 mol l−1 sodium phosphate buffer (pH 7.0). Each value is the mean of three parallel replicates ± SD. Symbols: HSP,  ; nicotine,
; nicotine,  .
.
Reaction catalyzed by P. putida P-HSP
To determine the HSP production capacity of P. putida P-HSP, biotransformation was carried out using 3.4 g dry cell weight (DCW) l−1 whole cells of P. putida P-HSP as the biocatalyst, 3.0 g l−1 nicotine as the substrate and double-distilled H2O (ddH2O) (final pH 10.0) as the reaction buffer at 30°C with shaking at 120 rpm. After 5 h of reaction, the sample was centrifuged and the supernatant was analyzed by HPLC. A single peak with a retention time of 12.93 min was observed, which corresponds to the peak and spectrum of HSP (Figure 4a). The compound was analyzed by LC-MS ([M-H]−, m/z 194.0456) (Figure 4b) and NMR (1H NMR (DMSO, 400 MHz): δ 12.12, 8.25, 7.86, 6.38, 3.05, 2.5; 13C NMR (DMSO, 100 MHz): δ 194.57, 174.28, 162.93, 141.27, 138.67, 120.00, 116.61, 32.34, 28.25) (Figure 4c, d). The signals are consistent with the molecular weight and published NMR data of HSP. These results confirm that HSP was the only product generated from nicotine by whole cells of P. putida P-HSP.
Feasibility of HSP production and optimization of biotransformation conditions.
(a) HPLC analysis of the reaction sample at 0 min (black line) and 5 h (red line) for the catalysis of HSP from nicotine by whole cells of P. putida P-HSP. The inset shows the spectrum of the HPLC signal at 12.93 min. (b) LC-MS analysis of the sample after a 5-h reaction. (c, d)1H and 13C NMR profiles of the product. Effect of pH (e), temperature (f) and nicotine concentration (g) on HSP production. (h) Effect of biomass concentration on the specific biotranformation rate of HSP. Each value is the mean of three parallel replicates ± SD. t-test was used to calculate statistical significance. Symbols: ns, no significance (p > 0.05); **, p < = 0.01.
Transformation by whole cells of P. putida P-HSP
The reaction was carried out under various conditions. The reaction buffer (ddH2O) was adjusted with HCl to different pH values. The pH value (Figure 4e) and substrate concentration (Figure 4g) significantly affected HSP production. The optimal pH value was determined to be 9.0 and the optimal nicotine concentration was 6.0 g l−1. There was no significant change in HSP production at different reaction temperatures (Figure 4f), which is good for industrial production. From 1.7 g DCW l−1 to 3.4 g DCW l−1, a significant increase in HSP production was observed with an increase in the concentration of P. putida engineered cells. However, there was a minimal increase in HSP production with P. putida-engineered cell concentrations of over 3.4 g DCW l−1 (Figure 4h). Thus, the optimal concentration of P. putida engineered cells was determined to be 3.4 g DCW l−1. Taking into account the conversion rate and biomass, P. putida P-HSP was most productive in the late-exponential phase of cell growth.
HSP production by batch and fed-batch transformation
Large-scale biotransformation was performed at pH 9.0, 30°C, an initial nicotine concentration of 6.0 g l−1 and 3.4 g DCW l−1 of whole cells of P. putida P-HSP. Time courses of HSP production reactions in a cell-free system and with resting cells of wild strain S16, engineered strain of P-HSP and heat-killed strain of P-HSP were analyzed and are described in Figure 5. Figure 5a shows that HSP production with engineered strain (concentration, 6.40 ± 0.46 g l−1; yield, 99.4%) is much higher than that with unmodified strain (concentration, 1.73 ± 0.19 g l−1; yield, 38.9%) after 5 h biotransformation. It appears that the engineered strain is 3.7 times more efficient at HSP producing compared to the unmodified strain. Moreover, with the engineered strain, HSP concentration didn't decrease after it reached the maximum value; however, HSP concentration decreased in the reaction system of the unmodified strain from 7 h to 24 h. The results indicate that the engineered strain successfully blocked the pathway realizing the accumulation of HSP. Nicotine concentrations were basically unchanged in the control samples of the cell-free system and heat-killed resting cells of P. putida P-HSP.
Time course of batch biotransformation by P. putida S16 and P. putida P-HSP at pH 9.0, 30°C and 3.4 g DCW l−1.
(a) P. putida S16 (black line) and P. putida P-HSP (red line) were used for HSP production under the same conditions. Cell-free system (blue line) and heat-killed cells (pink line) were used as controls. (b) For batch biotransformation, the catalysts were used four times after collection by centrifugation. (c) Total HSP production by four reactions. Each value is the mean of three parallel replicates ± SD. Symbols: HSP,  ; nicotine,
; nicotine,  .
.
HSP rapidly accumulated when nicotine or crude tobacco-waste extract was used as a substrate under batch and fed-batch transformations. For batch transformation (Figure 5b, c), the same catalyst was used four times for 4.5 h, 4.5 h, 6.0 h and 20 h, respectively. HSP concentrations with the productive yield for the reactions were 6.81 ± 0.38 g l−1 (98%), 4.83 ± 0.070 g l−1 (100%), 2.98 ± 0.045 g l−1 (92%) and 1.46 ± 0.24 g l−1 (34%), respectively. Fed-batch biotransformation was conducted with intermittent substrate feeding to avoid substrate inhibition. The time course of nicotine feeding and HSP production are shown in Figure 6. The maximum HSP concentration obtained from nicotine was 16.3 ± 1.5 g l−1 at 23 h, with a productivity of 0.71 g l−1 h−1 and yield of 75% (Figure 6a). During the initial stage of biotransformation, stoichiometric formation of HSP from tobacco-waste and nicotine was observed. However, the yield decreased during the later stage of biotransformation, which suggests that the activity of resting cells and enzymes were affected by nicotine and high HSP concentration, respectively.
When crude tobacco-waste extract was used as the substrate, P-HSP cells produced 6.84 ± 0.36 g l−1 HSP at 27 h, with a productivity of 0.25 g l−1 h−1 and a yield of 36% (Figure 6b). The HSP concentration obtained using crude tobacco-waste extract was lower than that obtained using pure nicotine. This may be due to the fact that crude tobacco-waste extract has complex components, of which many are secondary metabolites of tobacco that could inhibit enzyme activity. However, preparation of crude tobacco-waste extract by steam distillation from tobacco-waste is a simple and green process that avoids the use of organic solvents for nicotine extraction.
Discussion
N-Heterocycles are common structural motifs in natural molecules and in man-made chemical active substances, some of which are environmental pollutants, such as nicotine from tobacco, pyrrole/pyridine/pyrrolidine/pyrazine derivatives that are used as drugs, herbicides, insecticides, paints and some carcinogenic azaarenes7,24,25,26. Microbial degradation is one of the best strategies to remove these pollutants when they have contaminated soil and water. Many microorganisms possessing different N-heterocycle degradation pathways have previously been isolated27,28. Meanwhile, microbial catabolism of N-heterocycles produces a lot of intermediates, which provide a huge pool of building blocks for the synthesis of agrochemicals and pharmaceuticals2,29,30. In some cases, the synthesis and modification of these intermediates are difficult via organic chemistry methods. Biocatalysis, born in the early 1900s and flourished with the concept of “green chemistry” in the 1990s, was introduced as a supplementary technology. Its highly chemo-, regio- and stereoselective bioconversions simplify certain manufacturing processes, making them economically attractive and environmentally acceptable1. Some biochemical pathways, which have the potential for biotransformation of N-heterocycles into value-added chemicals, are listed in Figure 128,31. Microbial catabolism has great potential for the production of N-heterocycle intermediates, which could be used as an important supplementary technology in the chemical industry.
Whole-cell catalysis is an important tool for biocatalysis, especially for multi-enzymatic reactions, although it is hindered by some shortcomings. Wang et al. developed a promising method for the production of HSP using whole cells of strain S1622. However, protein expression is sometimes inconsistent in the wild strains32; therefore, it is difficult to maintain the catalytic stability of native producers. Consequently, biotransformation conditions require extensive optimization to promote HSP accumulation and prevent its transformation to 2,5-DHP. However, it is notable that some HSP had been degraded before it reached the highest concentration when using wild strain S16 as the catalyst22. Metabolic engineering, which can enhance the accumulation of target compounds by blocking the catabolic pathways, is therefore a good strategy to overcome the shortcomings of whole-cell biocatalysis33. The results of these experiments demonstrated that engineered strain P-HSP realized a high accumulation of HSP. In fact, the metabolic engineering of strain S16 presents two advantages: (1) the engineered strain has high efficiency and yield and (2) the catalytic process is easy to control. Moreover, mutasynthesis was first used in the production of intermediates in nicotine degradation and it can be also used for the production of other intermediates, such as 3-succinoyl-pyridine and 2,5-DHP (Figure 1)12.
In summary, our results demonstrate the feasibility for efficient production of a value-added chemical, HSP, with high product yield and productivity from renewable waste by blocking the catabolic pathway in a metabolically-engineered microorganism. The green strategy introduced in this study may provide a technically and economically interesting route for value-added-chemical production from industrial waste or renewable sources.
Methods
Chemicals
l-(-)-Nicotine (99% purity) was purchased from Fluka Chemie GmbH (Buchs, Switzerland) and HSP was prepared by our lab as previously described22; both were used as standards. Crude tobacco-waste extract (~8.7% (w/v) nicotine) was obtained by steam distillation according to the method described by Nanohar and Sridharan5. Nicotine (~90%) was further purified by extraction with chloroform. All other reagents and solvents used in this study were of analytical grade and commercially available.
Strains and cultivation conditions
P. putida strain S16 from our own collection was cultivated as previously described34 and recently deposited at the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) (accession no. DSM 28022). The P. putida P-HSP strain was aerobically cultivated at 30°C in Luria-Bertani (LB) medium containing filtration-sterilized kanamycin (50 ng l−1) and nicotine (1 g l−1). Engineered strain P-HSP was deposited at the China Center for Type Culture Collection under accession no. CCTCC M 2014135.
Construction of engineered catalyst and complemented strain
A partial sequence of hspB (fragment PH) was amplified by PCR with the primers PH-F (5′-ccgGAATTCggggacaaatgtggtggtg-3′) and PH-R (5′-cgcGGATCCcaagaactacccgaacaga-3′) (Figure 2b). Fragment PH and plasmid pK18mob were digested with the restriction enzymes EcoRI and BamHI and ligated to generate pK18mob-PH (Figure 2b). To construct the hspB deletion strain (P. putida P-HSP), plasmid pK18mob-PH was transferred into strain S16 and triparental filter mating was performed as previously described23. Integrants were isolated on M9 minimal medium plates containing 50 ng l−1 kanamycin and verified by analysis of PCR products using the primers mob-F (5′-cggctcgtataatgtgtgga-3′) and hspB-R (5′-ctacagaaaggtttccatagt-3′) (Figure 2b).
To construct the hspB-complemented strain, hspB, including the promoter region, was amplified from the genomic DNA of strain S16 using the primers C-F (5′-ccgGAATTCgcaccgatgactacatcagttttga-3′) and C-R (5′-ccgCTCGAGctacagaaaggtttccatagtctct-3′). The 1,332-bp fragment was purified and inserted into the EcoRI and XhoI sites of the E. coli-P. putida shuttle vector pME603235, generating recombinant plasmid pME-hspB. Plasmid pME-hspB was then transformed into strain P. putida P-HSP (P. putida S16 hspB::pK18mob) to produce the hspB-complemented strain (P. putida S16 hspB::pK18mob (pME-hspB)).
Catalyst preparation and transformation optimization
For whole-cell preparation, 1% (v/v) pre-cultures of P. putida P-HSP were added to 1 l of fresh LB medium in a 2.5-l conical flask (containing 1 g l−1 nicotine and 50 ng l−1 kanamycin) and incubated at 30°C with shaking at 200 rpm for 10 h until growth reached the late-exponential phase. Cells were collected by centrifugation at 8,000 rpm for 5 min and then washed twice with 0.9% NaCl. Biotransformation reactions were performed in 250-ml conical flasks containing 50 ml of the reaction mixtures with shaking at 120 rpm. The reaction conditions were optimized by testing the following parameters: pH, 6.0–10.0; temperatures, 16°C–44°C; nicotine concentrations, 1.5 g l−1–10 g l−1; and cell concentrations, 1.7 g DCW l−1–13.6 g DCW l−1.
Batch and fed-batch transformation
Large-scale production of HSP was carried out in a 2.5-l conical flask containing 500 ml of the reaction mixture under optimal conditions. The resting cells of wild P. putida S16 were prepared in the same way as P. putida P-HSP and both strains were used for the biotransformation of nicotine. A cell-free system and heat-killed cells were used as control under the same conditions. For batch transformation, the biocatalyst was collected by centrifugation after the reaction and reused in the next batch. For fed-batch transformation in the conical flask, crude tobacco-waste extract and nicotine were directly fed at the desired time. Each sample was analyzed in triplicate and the mean value was used for further calculations. In the multi-enzyme steps reaction that forms HSP, one molecule of nicotine produces one molecule of HSP (Figure 2a). The yield was defined as the ratio between the molar concentrations of the consumed nicotine and the produced HSP (mol/mol).
Analytical methods
The activity of HSP hydroxylase (HspB) was assayed according to previous reports23. The OD620nm was determined using a UV-2550 spectrophotometer (Shimadzu, Kyoto, Japan). The concentrations of nicotine and HSP were measured by high-performance liquid chromatography (HPLC) (Agilent, Santa Clara, CA, USA) using an Eclipse XDB-C18 column (5 μm 4.6 × 150 mm, Keystone Scientific, Bellefonte, PA, USA). Briefly, the sample was analyzed with a mobile phase of 12% (v/v) methanol and 88% (v/v) 1 mM H2SO4 at a flow rate of 0.5 ml min−1. Liquid chromatography-mass spectrometry (LC-MS) was performed on an Agilent 6230 TOF-MS equipped with electrospray ionization sources. DCW was calculated from the optical density (OD620 nm) with a linear correlation factor (1 OD620nm = 0.56 g DCW l−1).
References
Schmid, A. et al. Industrial biocatalysis today and tomorrow. Nature 409, 258–268 (2001).
Rathbone, D. A. & Bruce, N. C. Microbial transformation of alkaloids. Curr. Opin. Microbiol. 5, 274–281 (2002).
Forster-Carneiro, T., Berni, M., Dorileo, I. & Rostagno, M. Biorefinery study of availability of agriculture residues and wastes for integrated biorefineries in Brazil. Resour. Conserv. Recy. 77, 78–88 (2013).
Wang, J. H. et al. Bioaugmentation of activated sludge with Acinetobacter sp. TW enhances nicotine degradation in a synthetic tobacco wastewater treatment system. Bioresour. Technol. 142, 445–453 (2013).
Nanohar, B. & Sridharan, S. Extraction of nicotine from tobacco waste. Chem. Eng. World 22, 89–91 (1987).
Civilini, M., Domenis, C., Sebastianutto, N. & de Bertoldi, M. Nicotine decontamination of tobacco agro-industrial waste and its degradation by micro-organisms. Waste Manage. Res. 15, 349–358 (1997).
Petersen, M. & Kiener, A. Biocatalysis. Green Chem. 1, 99–106 (1999).
Hill, M. D. Recent strategies for the synthesis of pyridine derivatives. Chemistry (Weinheim an der Bergstrasse, Germany) 16, 12052–12062 (2010).
Andersson, H., Almqvist, F. & Olsson, R. Synthesis of 2-substituted pyridines via a regiospecific alkylation, alkynylation and arylation of pyridine N-oxides. Org. Lett. 9, 1335–1337 (2007).
Liu, C., Luo, J., Xu, L. & Huo, Z. Synthesis of 2-substituted pyridines from pyridine N-oxides. Arkivoc. 1, 154–174 (2013).
Grether-Beck, S. et al. Structural analysis and molybdenum-dependent expression of the pAO1-encoded nicotine dehydrogenase genes of Arthrobacter nicotinovorans. Mol. Microbiol. 13, 929–936 (1994).
Tang, H. et al. Systematic unraveling of the unsolved pathway of nicotine degradation in pseudomonas. PLoS Genet. 9, e1003923 (2013).
Youssif, S. Recent trends in the chemistry of pyridine N-oxides. Arkivoc 1, 242–268 (2001).
Balzarini, J., Stevens, M., De Clercq, E., Schols, D. & Pannecouque, C. Pyridine N-oxide derivatives: unusual anti-HIV compounds with multiple mechanisms of antiviral action. J. Antimicrob. Chemother. 55, 135–138 (2005).
Goetz, A. E. & Garg, N. K. Regioselective reactions of 3,4-pyridynes enabled by the aryne distortion model. Nat. Chem. 5, 54–60 (2013).
Brandsch, R. Microbiology and biochemistry of nicotine degradation. Appl. Microbiol. Biotechnol. 69, 493–498 (2006).
Tang, H. et al. Genomic analysis of Pseudomonas putida: genes in a genome island are crucial for nicotine degradation. Sci. Rep. 2, 377 (2012).
Ma, Y. et al. Isolation, transposon mutagenesis and characterization of the novel nicotine-degrading strain Shinella sp. HZN7. Appl. Microbiol. Biotechnol. (2013).
Ishige, T., Honda, K. & Shimizu, S. Whole organism biocatalysis. Curr. Opin. Chem. Biol. 2005, 9, 174–180.
Xue, R. & Woodley, J. M. Process technology for multi-enzymatic reaction systems. Bioresour. Technol. 115, 183–195 (2012).
Roduit, J.-P., Wellig, A. & Kiener, A. Renewable functionalized pyridines derived from microbial metabolites of the alkaloid (S)-nicotine. Heterocycles 45, 1687–1702 (1997).
Wang, S. N. et al. “Green” route to 6-hydroxy-3-succinoyl-pyridine from (S)-nicotine of tobacco waste by whole cells of a Pseudomonas sp. Environ. Sci. Technol. 39, 6877–6880 (2005).
Tang, H. et al. A novel NADH-dependent and FAD-containing hydroxylase is crucial for nicotine degradation by Pseudomonas putida. J. Biol. Chem. 286, 39179–39187 (2011).
Scriven, E. F. & Murugan, R. Pyridine and pyridine derivatives. Kirk-Othmer Encyclopedia of Chemical Technology 4th edn, Vol. 20 (John Wiley & Sons, Inc.: Hoboken, NJ, 2005).
O'Hagan, D. Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids. Nat. Prod. Rep. 17, 435–446 (2000).
Bleeker, E. A. et al. Toxicity of azaarenes. Rev. Environ. Contam. Toxicol. 173, 39–83. (2002).
Xu, P., Yu, B., Li, F. L., Cai, X. F. & Ma, C. Q. Microbial degradation of sulfur, nitrogen and oxygen heterocycles. Trends Microbiol. 14, 398–405 (2006).
Yoshida, T. & Nagasawa, T. Enzymatic functionalization of aromatic N-heterocycles: hydroxylation and carboxylation. J. Biosci. Bioeng. 89, 111–118 (2000).
Cordell, G. A., Quinn-Beattie, M. L. & Farnsworth, N. R. The potential of alkaloids in drug discovery. Phytother. Res. 15, 183–205 (2001).
Parshikov, I. A., Netrusov, A. I. & Sutherland, J. B. Microbial transformation of azaarenes and potential uses in pharmaceutical synthesis. Appl. Microbiol. Biotechnol. 95, 871–889 (2012).
Shaw, N. M., Robins, K. T. & Kiener, A. Lonza: 20 years of biotransformations. Adv. Synth. Catal. 345, 425–435 (2003).
Angel, T. E. et al. Mass spectrometry-based proteomics: existing capabilities and future directions. Chem. Soc. Rev. 41, 3912–3928 (2012).
Murphy, A. C. Metabolic engineering is key to a sustainable chemical industry. Nat. Prod. Rep. 28, 1406–1425 (2011).
Wang, S. N. et al. Biodegradation and detoxification of nicotine in tobacco solid waste by a Pseudomonas sp. Biotechnol. Lett. 26, 1493–1496 (2004).
Heeb, S. et al. Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Mol. Plant-Microbe Interact. 13, 232–237 (2000).
Hurh, B., Ohshima, M., Yamane, T. & Nagasawa, T. Microbial production of 6-hydroxynicotinic acid, an important building block for the synthesis of modern insecticides. J. Fermentat. Bioeng. 77, 382–385 (1994).
Nakano, H. et al. Purification, characterization and gene cloning of 6-hydroxynicotinate 3-monooxygenase from Pseudomonas fluorescens TN5. Eur. J. Biochem. 260, 120–126 (1999).
Ueda, M. & Sashida, R. Microbial production of 2-hydroxynicotinic acid from nicotinic acid by intact cells of MCI3289. J. Mol. Catal. 4, 199–204 (1998).
Nagasawa, T., Mathew, C. D., Mauger, J. & Yamada, H. Nitrile hydratase-catalyzed production of nicotinamide from 3-cyanopyridine in Rhodococcus rhodochrous J1. Appl. Environ. Microbiol. 54, 1766–1769 (1988).
Mathew, C. D., Nagasawa, T., Kobayashi, M. & Yamada, H. Nitrilase-catalyzed production of nicotinic acid from 3-cyanopyridine in Rhodococcus rhodochrous J1. Appl. Environ. Microbiol. 54, 1030–1032 (1988).
Wieser, M., Heinzmann, K. & Kiener, A. Bioconversion of 2-cyanopyrazine to 5-hydroxypyrazine-2-carboxylic acid with Agrobacterium sp. DSM 6336. Appl. Microbiol. Biotechnol. 48, 174–176 (1997).
Acknowledgements
The work was supported in part by the National Basic Research Program of China (grant no. 2011CBA00800) and by the Chinese National Natural Science Foundation (grant nos. 31230002, 31270154 and 31121064). We also acknowledge the “Shanghai Rising-Star Program” (grant no. 13QA1401700).
Author information
Authors and Affiliations
Contributions
P.X., H.Y. and H.T. conceived and designed the project. P.X. and H.T. contributed reagents and materials. H.Y. analyzed data. H.Y. and P.X. wrote the manuscript. All authors have read and approved.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Yu, H., Tang, H. & Xu, P. Green strategy from waste to value-added-chemical production: efficient biosynthesis of 6-hydroxy-3-succinoyl-pyridine by an engineered biocatalyst. Sci Rep 4, 5397 (2014). https://doi.org/10.1038/srep05397
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05397
This article is cited by
-
Nicotine metabolism pathway in bacteria: mechanism, modification, and application
Applied Microbiology and Biotechnology (2022)
-
Transcriptome analysis of genes and metabolic pathways associated with nicotine degradation in Aspergillus oryzae 112822
BMC Genomics (2019)
-
Microbial Degradation of Nicotinamide by a Strain Alcaligenes sp. P156
Scientific Reports (2019)
-
2-Hydroxy-4-(3′-oxo-3′H-benzofuran-2′-yliden)but-2-enoic acid biosynthesis from dibenzofuran using lateral dioxygenation in a Pseudomonas putida strain B6-2 (DSM 28064)
Bioresources and Bioprocessing (2018)
-
Proteomics based analysis of the nicotine catabolism in Paenarthrobacter nicotinovorans pAO1
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.







 ; nicotine,
; nicotine,  .
.