Abstract
Ocean acidification driven by rising levels of CO2 impairs calcification, threatening coral reef growth. Predicting how corals respond to CO2 requires a better understanding of how calcification is controlled. Here we show how spatial variations in the pH of the internal calcifying fluid (pHcf) in coral (Stylophora pistillata) colonies correlates with differential sensitivity of calcification to acidification. Coral apexes had the highest pHcf and experienced the smallest changes in pHcf in response to acidification. Lateral growth was associated with lower pHcf and greater changes with acidification. Calcification showed a pattern similar to pHcf, with lateral growth being more strongly affected by acidification than apical. Regulation of pHcf is therefore spatially variable within a coral and critical to determining the sensitivity of calcification to ocean acidification.
Similar content being viewed by others
Introduction
The seawater environment that marine organisms inhabit is changing due to rising atmospheric CO2, which is causing a decline in seawater pH or ‘ocean acidification’1. Declining seawater pH and the resultant decrease in the calcium carbonate saturation state are expected to lead to reduced calcification rates for a wide range of calcifying organisms2,3. This is because bio-calcification, which generally occurs in an internal semi-isolated environment created and controlled by the organism, is nevertheless influenced by the external environment4,5,6,7,8.
Among the organisms expected to be affected by ocean acidification are scleractinian corals. The effects of acidification on coral calcification have been extensively studied9,10,11,12,13,14,15,16,17, with a wide range of responses being observed18,19. Such variability likely reflects the degree to which corals are able to control the chemistry at the site of calcification. One of the mechanisms by which corals are thought to facilitate calcification is through up-regulation of the pH of the calcifying fluid (pHcf)19,20,21. An increase in pH is generally linked to an increase in the aragonite saturation state due to HCO3− converting to CO32− at elevated pH, thus favoring the precipitation of skeletal aragonite (CaCO3). The aragonite saturation state, represented by ΩAragonite, is defined as:

where KSP is the solubility of aragonite.
The decline in calcification rate often observed with ocean acidification has been suggested to be linked to a decline in pH in the calcifying fluid induced by lower pH in the external seawater environment19,22,23. Understanding how the pH at the site of calcification changes in response to changes in the external seawater environment, both in the modern ocean and over evolutionary timescales, represents a critical step in predicting how calcifying organisms will fare under future conditions of ocean acidification.
In addition to the importance of pHcf for the calcification process itself, understanding how pHcf relates to seawater pH is also of critical importance for the development of seawater pH proxies. Boron is of particular interest in this regard as it exists in seawater in one of two forms – borate or boric acid. The relative proportion of borate versus boric acid is determined by pH. Further there is a large (~27‰) difference in the isotopic composition of borate relative to boric acid, so the isotopic composition of borate is a function of pH24. Boron in the coral skeleton is thought to be derived almost entirely from borate present in the calcifying fluid25 and boron in the calcifying fluid is thought to come directly from seawater, which has a known boron isotopic composition26. Thus measurements of the isotopic composition of boron in corals can be used to calculate pHcf6,27. Since pHcf is related to seawater pH28 and the boron isotopic composition of the coral skeleton6,29, boron isotopes can be used to calculate seawater pH, thus allowing the estimation of seawater pH for periods prior to instrumental records.
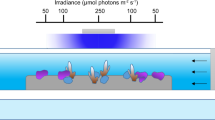
Here we investigate the sensitivity of corals to ocean acidification at intra-colony spatial scales by measuring pHcf and calcification rates in different regions within coral colonies exposed to reduced seawater pH. Branch tips (apexes) from colonies of the tropical coral Stylophora pistillata were attached to glass cover-slips and allowed to grow out laterally across the cover-slip (basal-lateral growth) and to extend the original branch tip (apical growth) (Fig. 1a). Measurements of pHcf were made in different regions of coral colonies using analytical methods suited to each region. Confocal microscopy with the pH indicator SNARF-1 was used to measure pHcf during the early stages of basal-lateral growth at the growing edge of the colonies where skeletal material is limited, thus facilitating observations with confocal microscopy. Boron isotope measurements were made on skeletal calcium carbonate samples from two different regions: 1) the lateral region (Fig. 1a), which included the material from the growing edge where confocal microscopy was undertaken, as well as the more mature skeletal regions overlying the material initially formed at the growing edge; and 2) the upwardly growing tips of the apexes, or apical region (Fig. 1a). The apical region is the region in which most growth normally occurs.
Regions sampled for pHcf measurements and growth rates of different regions.
(a) Sampling locations for boron isotope samples and SNARF based measurements. (b) Relative growth rates for lateral expansion28 and apical extension, values are expressed relative to the mean rate at ambient seawater pH for the given measurement period. Symbols are means, error bars are standard deviation.
Results
Corals were maintained in seawater with varying degrees of acidification (Supplementary Table S1) ranging from near ambient, pHT = 7.94 (pCO2 = 550 μatm), to pHT = 7.17 (pCO2 = 4140 μatm, used to achieve undersaturation with respect to aragonite). Corals produced new skeleton under the full range of pH treatments. However lateral growth rates (measured as changes in skeletal area) declined progressively with decreasing seawater pH (Fig. 1b) and growth was significantly reduced at pHT = 7.16 relative to ambient conditions (F3,32 = 5.81, p<0.01)28. In contrast to lateral growth, the apical extension of branches showed no significant change (F3,52 = 1.1, p = 0.36) in response to acidification (Fig. 1b).
Measurements of δ11B showed a consistent decline in δ11B values associated with a decline in seawater pHT (Fig. 2a). For all regions, although pHcf was invariably elevated relative to seawater pHT (Fig. 2b), pHcf decreased with a decline in seawater pHT (Fig. 2b). The decline in pHcf was smaller than the corresponding pHT change in the seawater, thus the difference between seawater pHT and pHcf (ΔpH6) increased as seawater pHT declined (Fig. 2b and Supplementary Fig. S1). Although measurements of both apical and lateral growth showed the same general pattern, lateral growth generally had lower pHcf values than apical growth and the difference between lateral and apical regions increased as pHT declined (Fig. 2b). Apexes thus had smaller changes in pHcf in response to acidification than adjacent lateral skeletal growth. For the growing edge, pHcf estimates were even lower and showed the largest declines in pHcf in response to acidification.
Measured δ11B and pHcf estimates for different regions of the coral plotted against seawater pHT.
(a) δ11B values measured in apical (black) and lateral (grey) skeletal regions plotted against average seawater pH. Symbols are average measurements of a given sample, error bars are standard error. (b) Calcifying fluid pH estimates plotted against average seawater pH (aquarium pHT for boron isotope based estimates, perfusion chamber pHT for SNARF based estimates). Symbols are weighted means, error bars are standard deviation, with n = 3 time-points. Symbols for apical and lateral measurements are offset on the x-axis for clarity. Solid lines are weighted regression fits to the data, dotted lines are 95% confidence intervals for the regressions. For reference, the 1:1 line is shown as a dashed line, this is where pHcf = seawater pHT. All pH values are on the total scale (pHT).
Discussion
Our estimates of the effects of ocean acidification on the extension of branch tips (apical growth) are similar to those of other studies on S. pistillata colonies9,30,31, being fairly stable over a wide range of pHT conditions (Fig. 1b). In contrast, extension of the lateral part of the colonies (lateral growth) was more strongly affected by ocean acidification, similar to other studies that have examined lateral growth in S. pistillata30. Boron isotope-based estimates of pHcf exhibited patterns similar to calcification data. Lateral growth, which showed the greatest decline in calcification in response to acidification also showed a greater decline in pHcf in response to acidification than adjacent apical growth. Thus spatial differences in the regulation of pHcf may account for changes in calcification, consistent with the IpHRAC19 model in which internal pHcf regulation controls abiotic calcification rates.
Variation in the ability of the overlying tissue layer to control the pH at the site of calcification, as suggested by pHcf estimates (Fig. 2), may be linked to a number of differences existing between the apically and basal laterally growing regions32,33. The growth of these two regions is fundamentally different: apical growth occurs in a largely unrestricted environment whereas basal lateral growth occurs at an interface between the coral and a substrate. Thus the growing edge (initial basal lateral growth) faces a number of potential challenges not generally encountered by the rest of the coral tissue, including competing with other organisms for substrate and isolating new substrates from the surrounding environment to allow crystal growth to occur. The devotion of resources to compete for substrate could limit the energy available for calcification and in-turn reduce the ability of the tissue to up-regulate pH when faced with a more acidified environment. Or the isolation of new substrates from the surrounding environment may not be as complete as the isolation of existing skeletal regions, thus allowing higher rates of seawater ingress resulting in a more pronounced effect of acidification. Regardless of the underlying mechanism(s), pHcf and thus calcification, was more strongly affected by acidification in the basal, laterally growing regions than the apical growth. Thus stages of coral growth that involve extension over new substrates are likely to be more strongly affected by ocean acidification. This may be particularly relevant to the larval-stage of coral growth when all calcification is occurring on a new substrate, to coral fragments that must cement themselves to a new substrate, as well as to damaged corals attempting to re-grow over exposed skeleton; all represent growth stages in which lateral growth over a substrate plays an important role. These stages are therefore least likely to be able to maintain pHcf under acidified conditions and thus are likely to be more adversely affected by ocean acidification.
Since calcification within a coral differs spatially in its sensitivity to ocean acidification and that variations in pHcf appear to correspond to these differences in calcification, pHcf may help to predict how calcification will respond to ocean acidification. Measurements of pHcf thus represent an important tool for identifying stages of coral growth (e.g. colonization of new substrates) and particular species that will most likely be adversely affected by ocean acidification. Measurements of pHcf using boron isotopes can allow pHcf to be estimated over long time scales and allow variations in biologically controlled pH up-regulation to be linked to events (e.g. bleaching, storms, etc) in the natural environment which may further impact the ability of corals to regulate pHcf. Collectively such data can help to better predict how corals will respond to the range of conditions they face.
Methods
See the supplemental material.
References
Caldeira, K. & Wickett, M. E. Anthropogenic carbon and ocean pH. Nature 425, 365 (2003).
Langdon, C. & Atkinson, M. J. Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J. Geophys. Res. 110, C09S07, 10.1029/2004JC002576 (2005).
Ries, J. B., Cohen, A. L. & McCorkle, D. C. Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134 (2009).
Comeau, S., Edmunds, P. J., Spindel, N. B. & Carpenter, R. C. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceanogr. 58, 388–398 (2013).
Tambutté, S. et al. Coral biomineralization: From the gene to the environment. J Exp Mar Biol Ecol 408, 58–78 (2011).
Trotter, J. et al. Quantifying the pH ‘vital effect’ in the temperate zooxanthellate coral Cladocora caespitosa: Validation of the boron seawater pH proxy. Earth Planet Sci Lett 303, 163–173 (2011).
Gagnon, A. C. Coral calcification feels the acid. Proc Natl Acad Sci U S A 110, 1567–1568 (2013).
Allison, N. & Finch, A. A. δ11B, Sr, Mg and B in a modern Porites coral: the relationship between calcification site pH and skeletal chemistry. Geochim Cosmochim Acta 74, 1790–1800 (2010).
Reynaud, S. et al. Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Global Change Biol. 9, 1660–1668 (2003).
Ohde, S. & Hossain, M. M. M. Effect of CaCO3 (aragonite) saturation state of seawater on calcification of Porites coral. Geochem J 38, 613–621 (2004).
Albright, R., Mason, B. & Langdon, C. Effect of aragonite saturation state on settlement and post-settlement growth of Porites astreoides larvae. Coral Reefs 27, 485–490, 10.1007/s00338-008-0392-5 (2008).
Chauvin, A., Denis, V. & Cuet, P. Is the response of coral calcification to seawater acidification related to nutrient loading? Coral Reefs 30, 911–923, 10.1007/s00338-011-0786-7 (2011).
Edmunds, P. J., Brown, D. & Moriarty, V. Interactive effects of ocean acidification and temperature on two scleractinian corals from Moorea, French Polynesia. Global Change Biol 18, 2173–2183, 10.1111/j.1365-2486.2012.02695.x (2012).
Holcomb, M., Cohen, A. L. & McCorkle, D. C. An investigation of the calcification response of the scleractinian coral Astrangia poculata to elevated pCO2 and the effects of nutrients, zooxanthellae and gender. Biogeosciences 9, 29–39, 10.5194/bg-9-29-2012 (2012).
Drenkard, E. J. et al. Calcification by juvenile corals under heterotrophy and elevated CO2. Coral Reefs 32, 727–735, 10.1007/s00338-013-1021-5 (2013).
Schoepf, V. et al. Coral Energy Reserves and Calcification in a High-CO2 World at Two Temperatures. PloS one 8, e75049, 10.1371/journal.pone.0075049 (2013).
Takahashi, A. & Kurihara, H. Ocean acidification does not affect the physiology of the tropical coral Acropora digitifera during a 5-week experiment. Coral Reefs 32, 305–314, 10.1007/s00338-012-0979-8 (2013).
Holcomb, M., McCorkle, D. C. & Cohen, A. L. Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786). J. Exp. Mar. Biol. Ecol. 386, 27-33 (2010).
McCulloch, M., Falter, J., Trotter, J. & Montagna, P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nature Clim. Change 2, 623–627 (2012).
Kawaguti, S. & Sakumoto, D. The effect of light on the calcium deposition of corals. Bull. Oceanograpaical Inst. Taiwan 4, 65–70 (1948).
Cohen, A. L. & McConnaughey, T. A. Geochemical perspectives on coral mineralization. Reviews in Mineralogy Geochem: Biomineralization 54, 151–187 (2003).
Cohen, A. L. & Holcomb, M. Why corals care about ocean acidification. Oceanography 22, 118–127 (2009).
Ries, J. B. A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim Cosmochim Acta 75, 4053–4064 (2011).
Klochko, K., Kaufman, A. J., Yao, W., Byrne, R. H. & Tossell, J. A. Experimental measurement of boron isotope fractionation in seawater. Earth Planet Sci Lett 248, 276–285, 10.1016/j.epsl.2006.05.034 (2006).
Hemming, N. G. & Hanson, G. N. Boron isotopic composition and concentration in modern marine carbonates. Geochim Cosmochim Acta 56, 537–543, 10.1016/0016-7037(92)90151-8 (1992).
Foster, G. L., Pogge von Strandmann, P. A. E. & Rae, J. W. B. Boron and magnesium isotopic composition of seawater. Geochemistry, Geophysics, Geosystems 11, n/a-n/a, 10.1029/2010gc003201 (2010).
Zeebe, R. E. & Wolf-Gladrow, D. CO2 in seawater: equilibrium, kinetics, isotopes. (Elsevier 2001).
Venn, A. A. et al. Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc Natl Acad Sci U S A 110, 1634–1639 (2013).
McCulloch, M. et al. Resilience of cold-water scleractinian corals to ocean acidification: Boron isotopic systematics of pH and saturation state up-regulation. Geochim Cosmochim Acta 87, 21–34, 10.1016/j.gca.2012.03.027 (2012).
Krief, S. et al. Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim Cosmochim Acta 74, 4988–5001 (2010).
Cohen, S. & Fine, M. Measuring gross and net calcification of a reef coral under ocean acidification conditions: methodological considerations. Biogeosciences Discuss. 9, 8241 (2012). 10.5194/bgd-9-8241-2012.
Raz-Bahat, M., Erez, J. & Rinkevich, B. In vivo light-microscopic documentation for primary calcification processes in the hermatypic coral Stylophora pistillata. Cell Tissue Res 325, 361–368 (2006).
Gladfelter, E. Skeletal development in Acropora palmata (Lamarck 1816): a scanning electron microscope (SEM) comparison demonstrating similar mechanisms of skeletal extension in axial versus encrusting growth. Coral Reefs 26, 883–892 (2007).
Acknowledgements
We thank K. Rankenburg, L. Georgio, C. Godinot, N. Segonds, N. Techer, E. Elia and D. Desgré for assistance. Research conducted at the Centre Scientifique de Monaco was funded by the government of the Principality of Monaco. Research conducted at the University of Western Australia was supported by the Australian Research Council (ARC) Centre of Excellence for Coral Reef Studies. M.H. was supported by a NSF International Research Fellowship at CSM and by an ARC Super Science Fellowship at UWA. M.M. was supported by a Western Australian Premiers Fellowship and an ARC Laureate Fellowship.
Author information
Authors and Affiliations
Contributions
M.H., A.A.V., E.T., S.T., D.A., J.T. and M.M. designed the experiments. M.H., A.A.V. and E.T. performed research. M.H., A.A.V., E.T., S.T., D.A., J.T. and M.M. were involved in the preparation of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Holcomb, M., Venn, A., Tambutté, E. et al. Coral calcifying fluid pH dictates response to ocean acidification. Sci Rep 4, 5207 (2014). https://doi.org/10.1038/srep05207
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05207
This article is cited by
-
Is the Coral Triangle’s future shown in a Pliocene reef gap?
Coral Reefs (2023)
-
Coral calcification mechanisms in a warming ocean and the interactive effects of temperature and light
Communications Earth & Environment (2022)
-
Mapping coral calcification strategies from in situ boron isotope and trace element measurements of the tropical coral Siderastrea siderea
Scientific Reports (2021)
-
Inorganic carbon fluxes and perturbations by ocean acidification estimated using a data-constrained, process-based model of coral physiology
Marine Biology (2021)
-
Responses of coral gastrovascular cavity pH during light and dark incubations to reduced seawater pH suggest species-specific responses to the effects of ocean acidification on calcification
Coral Reefs (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.