Abstract
A technique for detecting brain injury at the bedside has great clinical value, but conventional imaging techniques (such as computed tomography [CT] and magnetic resonance imaging) are impractical. In this study, a novel method–the symmetrical channel electroencephalogram (EEG) signal analysis–was developed for this purpose. The study population consisted of 45 traumatic brain injury patients and 10 healthy controls. EEG signals in resting and stimulus states were acquired and approximate entropy (ApEn) and slow-wave coefficient were extracted to calculate the ratio values of ApEn and SWC for injured and uninjured areas. Statistical analyses showed that the ratio values for both ApEn and SWC between injured and uninjured brain areas differed significantly (P < 0.05) for both resting and name call stimulus states. A set of criteria (range of ratio values) to determine whether a brain area is injured or uninjured was proposed and its reliability was verified by statistical analyses and CT images.
Similar content being viewed by others
Introduction
Quantitative and topographic analysis of the human electroencephalogram (EEG) is a valuable method for evaluating brain function in healthy and diseased states, as those analyses could permit determination of scalp signal sources. For example, it has proven useful methods for mapping the seizure patient's “epileptogenic zone”1,2. It could also be used to characterize different brain pathological states, such as psychiatric and neurological disorders3. However, whether quantitative and spatial EEG analysis can be used as a technique for mapping injured brain areas has not been sufficiently explored4; recent studies that have attempted to resolve this issue5,6 and the current investigation is based on the conclusions from these reports.
Injured regions in the brain are typically identified using computed tomography (CT) or magnetic resonance imaging (MRI). However, compared with CT or MRI, the EEG method has advantages for its cost, high temporal resolution, flexibility, ease-of-use, performance and speed As such, using EEG at the bedside can be a powerful tool that provides dynamic information about brain functions7,8,9 and if applied to the assessment of brain injury, has great theoretical and clinical value10,11.
In this study, an EEG analysis method was developed that is refered to as symmetrical channel EEG signal analysis (SESA). The SESA method is based on hypothetical differences in EEG signal features between injured and uninjured brain areas (applicable for unilateral brain injuries, the most common brain injuries). If the difference is statistically significant, it could be used as an index to judge whether or not a brain area is injured. The SESA method is based on the characteristic anatomical and neurophysiological symmetry and functional offside symmetry of the brain12.
Previous studies have shown that EEG signals for corresponding regions in the cerebral hemispheres have a high degree of similarity in the resting state13,14. If a unilateral brain injury occurs, we can infer that this EEG similarity will decrease and differences will increase in the symmetrical cerebral hemisphere. In the active state, characteristic offside symmetry also increases EEG signal differences15. Indeed, recent developments in brain computer interfaces suggest that pattern recognition of imagined movements are based on the characteristic functional offside symmetry exhibited by the brain16,17,18. Therefore, it is feasible, both in theory and practice, to map brain injury areas using the SESA method.
We applied signal processing methods and statistical analyses to verify whether changes in EEG signal features (in the symmetrical cerebral hemisphere) are real and whether they can be used as a method for determining brain injury. A set of criteria (range of ratio values) to distinguish between injured and uninjured brain areas was also proposed based on SESA. To test whether the proposed criteria were reliable, statistical validation was carried out and the correspondence between diagnoses made based on SESA criteria and CT images was compared for five selected patients.
Results
The SESA method was developed as follows. First, two EEG signal features–approximate entropy (ApEn) and slow-wave coefficient (SWC) were extracted for all brain channels and the ratio (Cp value) of signals from injured and uninjured areas for these features was calculated. Statistical analyses of Cp values in population and individual channels were carried out and a range of ratio values was set as a standard for determining whether a brain area is injured or uninjured. Finally, the reliability of the SESA method was validated using CT images.
Statistical analysis of Cp values for total injured and uninjured channels
A statistical analysis was performed to evaluate differences in population Cp values for total injured and uninjured channels. The results are shown in Table 1.
Results from Table 1 indicated that:
-
1
For ApEn and SWC, in both the resting and active states, a P < 0.01 was observed for population mean Cp values, indicating that injured and uninjured brain channels differed significantly;
-
2
For the Cp value of ApEn, the value of the injured area during the call name stimulus was much smaller than the value in the resting state;
-
3
For the Cp value of SWC, the value of the injured area during the call name stimulus was much larger than the value in the resting state.
The Cp value for ApEn during the name call stimulus was smaller than in the resting state. As described in the Methods, in the resting state, the ApEn values for both the injured (e.g., Fp1) and symmetrical uninjured (e.g., Fp2) channels did not change significantly (i.e., Fp1/Fp2 remained constant); however, during the name call stimulus, the ApEn value for Fp2 increased but was unaltered for Fp1 due to injury, resulting in an overall decrease in Fp1/Fp2. Conversely, the Cp value for SWC during the name call stimulus was larger than in the resting state because Fp2 was unchanged while Fp1 increased.
Statistical analysis of Cp values for individual injured channels
An analysis of population Cp values for all injured and uninjured channels revealed differences in the Cp values for individual injured channels (Figure 1).
The results showed that:
-
1
For ApEn and SWC, in both resting and active states, there were statistically significant differences (P < 0.01) for all individual injured channels relative to uninjured channels;
-
2
The Cp value of ApEn for injured channels during the name call stimulus was smaller than in the resting state;
-
3
The Cp value of SWC for injured channels during the name call stimulus was larger than in the resting state (details in Appendix III);
An explanation for why the Cp value decreases for ApEn while increasing for SWC is given above (see Appendix I).
Criteria for assessing injured vs. uninjured brain channels
The above results indicated that there are significant differences in Cp values between injured and uninjured areas as well as individual channels. Thus, statistically determined differences served as a basis for establishing criteria that distinguished between injured and uninjured brain areas. These criteria (section 7 of Methods) and preliminary ranges of Cp values are given in Tables 2 and 3.
Verification of accuracy
Permutation testing was carried out to assess the accuracy of the established method. For example, the Cp values of ApEn for Fp1 or Fp2 with the name call stimulus comprised 31 cases; of these, 20 were randomly selected as a training group and the remaining 11 were the test group. The detailed procedure was as follows: the range of Cp values for the training and test groups were calculated using the above-described method; the reliability was assessed by determining the region of overlap between the Cp values of the training and test groups. This process was repeated for randomly selected training and test cases. After 50 iterations, the average accuracy was found to be 92.3%.
The calculation was repeated for the injured leads of F7 or F8, T3 or T4 and T5 or T6 and uninjured leads P3 or P4, C3 or C3, F3 or F4 and O1 or O2 (Figure 2). The results showed a high average accuracy, confirming that the method is reliable.
Calculation of probabilities of type I and II errors
The probability of occurrence of type I and II errors (incorrectly judging an uninjured area as injured one, or vice versa) was determined. The Cp values of ApEn for Fp1 or Fp2 is used as an example. Of 45 patients, 31 were injured at Fp1 or Fp2 and 14 were uninjured in these locations. Assuming that the 14 cases follow a normal distribution, Cp values of ApEn for Fp1 or Fp2 were calculated as for injured cases. The distribution of the ApEn Cp values for the two groups is shown in Figure 3. The probability of type I and II errors was calculated at the point at which the curves intersect and determined as the areas under the curve.
The calculated probabilities for all leads are shown in Table 4. Since the values were small, it can be concluded that the method is reliable.
Verification of the SESA method using CT images
To test the reliability of the proposed criteria, five additional patients were selected for whom diagnoses made based on the SESA method and CT images were compared. The Cp values from one patient are listed in Table 5. In this case, the exact location of the injured area is unknown. Calculating Cp values of the symmetric channel permitted determination of the injured areas. According to the diagnostic criteria, brain areas corresponding to channels with Cp values shown in bold italics in Table 5 (FP1/FP2 and F7/F8) were injured.
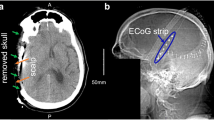
An example of results obtained using the SESA method and CT images for one patient is shown in Figure 4. A good correspondence was observed between the two methods.
Discussion
The SESA method was developed to map injured brain areas and its clinical utility was verified using theoretical and experimental methods. Although theoretical simulation studies have been conducted in which functional analyses of asymmetric EEG recordings have been applied towards clinical assessment19,20,21,22, our report investigates in practice the feasibility of using EEG signals for functional brain mapping. As an innovative brain mapping method, it has the potential to be used at the bedside in the clinic. Through further development, it could also serve as a powerful approach or portable imaging device for brain function analysis. Other fundamental work, including more analysis tools and more engineering, must be carried out to develop its practical use. We will specifically discuss some fundamental work required.
The scope of SESA applications
Brain injuries can have different causes, such as cerebral trauma and cerebral ischemia. From a topographical perspective, brain injuries can be classified as unilateral injuries, bilateral injuries, dispersed injuries and whole brain injuries. The SESA method is especially suitable for unilateral brain injuries, as it is based on hypothetical EEG signal differences between asymmetrical signals of brain injury and symmetrical signals for uninjured areas.
In the clinic most brain injuries are unilateral; however, whether SESA is suitable for analyzing other kinds of brain injuries requires further investigation. To this end, a method for multi-area neural mass modeling based on EEG and MEG signals has been developed23. We suggest that other methods, such as regional symmetrical EEG signal analysis, might also have the potential for applications to unilateral and whole brain injuries.
In addition, our research sample included patients with severe disorders of consciousness. Compared with brain injury patients with normal consciousness, these patients' EEG signals are relatively stable24,25 and thus used for this study. Further research will be performed to experimentally test and verify whether this method is suitable for assessing brain injuries associated with normal consciousness.
Establishment of diagnostic criteria using patient data
A range of ratio values defined for each channel is proposed as a standard for determining whether or not a brain area is injured. Although the method was validated by statistical analyses and was proven reliable when applied to the diagnosis of five patients, for routine use in practice (i.e., as an adjuvant clinical diagnostic standard) the accuracy should be confirmed statistically in a larger patient cohort. The present study population included only 45 patients and it is expected that through cooperative efforts between hospitals, large amounts of data can be obtained that will allow verification of the reliability of this method.
Selection of EEG signal features
As many EEG signal features can be used as EEG parameters, the selection of suitable EEG characteristics as parameters is also important for the SESA method. A good EEG parameter represents brain injury states well and has values that can be easily calculated. The ApEn and a defined EEG frequency domain (SWC) were chosen as EEG parameters. Characteristics of nonlinear EEG dynamics include ApEn, sample and permutation entropies and Lempel-Ziv complexity26,27,28. Future research will test different features to determine the best for various applications. In terms of EEG frequency domain features, the ratio of the slow wave is a good EEG feature for patients with severe disorders of consciousness. Whether SWC is a good EEG feature for patients with normal consciousness also requires further experimental investigation.
The spatial resolution of SESA mapping
We used a 40-channel EEG acquisition instrument to acquire EEG signals, using 16 channels for the actual scalp electrodes. Applying high-density EEG signal recording instruments (with 128 or 256 channels) will greatly increase the spatial resolution of injured areas, facilitating studies that are both clinically relevant and provide basic functional information by revealing the topography of injured brain areas. However, signal interference from the various leads presents a technical limitation that must be addressed in order to maximize the benefits of improved spatial resolution.
In conclusion, we innovated a SESA method to determine the injured areas of the brain. Future studies will focus on the development of specific data analysis software and additional clinical verification, which will allow SESA to be used as an adjuvant brain mapping technique along with conventional imaging methods.
Methods
All experiments were carried out in accordance to the approved by the Wu Jing Hospital Institutional Review Board in Hangzhou, Zhejiang, China; and informed written consent was obtained from the families of all patients.
Subjects
Subjects in this paper were placed in patient or control groups. All patients had unilateral brain injuries with severe disorders of consciousness, including minimally conscious and vegetative states29. Patients with brain injury but who exhibited a normal state of consciousness were not included in the present study, but have been examined in other studies30 and will be the focus of future research. The cases in these experiments met the inclusion criteria and exclusion criteria detailed in Appendix I. The 45 patients were collected from inpatients received by the Rehabilitation Center of Zhejiang Wujing Hospital from September 2011 to October 2012. There were 25 cases with left brain trauma and 20 cases with right brain trauma (detailed information is listed in Appendix II). The control group consists of 10 volunteers (6 males and 4 females ranging in age from 20 to 24 years of age) from Hangzhou Dianzi University. All of the control patients were healthy, with no mental disease or brain trauma. The average time between injury and study participation is at least 3 months; the range of time is from 3 months to 6 months.
EEG acquisition and noise reduction
The EEG acquisition instrument with 40 channels (NuoCheng, Shanghai, China) had 16 channels used to record EEG signals. Scalp electrodes were placed according to the international standard 10/20 system31,32 and the electrode impedance was ≤5 KΩ and the sampling frequency was 256 HZ, A1 and A2 were the reference electrodes and Fpz was the grounding electrode.
• EEG signal acquisition
EEG signals in two brain states were recorded, one under the resting state and the other under the call-name stimulus state. The two states were recorded to determine which brain state's EEG signals were better for extracting features for the ratio calculation (further details in the Discussion section).
For recording in the resting state, 10 min sustainable EEG signals were acquired from each subject. For recording the in call-name stimulus state33, the acquisition process is shown in Figure 5. There was a 12 s call-name stimulus recording, during which a selected patient's family member would call the patient's name three consecutive times. When the third call ended, data collecting continued for 2 min before ending.
• EEG noise reduction
Unstable data caused by external interference (such as EMG interference caused by sudden movements of patient limbs or EOG interference when patients blink during the acquisition process) was manually removed. The toolbox-IIR filter in EEGLAB was processed to remove interference caused by the 50 Hz power frequency.
Extraction of EEG features
Two EEG features were chosen as parameters to compute ratios of symmetrical channels for injured and uninjured brain areas (and channels). One ratio is Approximate Entropy (ApEn)34 and the other is a defined EEG feature termed the slow wave coefficient (SWC). The extraction processes of the two features are described below.
• Slow wave coefficient (SWC) calculation
EEG rhythm is considered an overall indicator of the excitability of the central nervous system. In general, a lower frequency of slow waves corresponds to a greater degree of abnormality in the corresponding areas35. thus, brain damage causes an increase in the slow wave and decrease in the fast wave band in the EEG power spectrum36.As such, SWC provides a good index for the identification of damaged brain areas.
Since the EEG power spectrum is typically divided into six bands, i.e., δ (1.0–4.0 Hz), θ (4.1–8.0 Hz), α1 (8.1–10.0 Hz), α2 (10.1–13.0 Hz), β1 (13.1–17.5 Hz) and β2 (17.6–30.0 Hz), SWC was defined as:

In this formula, α = α1 + α2, β = β1 + β2.
Calculation of SWC was as follows:
-
1
2 min EEG signals of each subject in the resting state and 12 s in call-name stimulus state were intercepted;
-
2
The power spectrum of each band was extracted by Fourier transform;
-
3
By using the above-mentioned definition of SWC, values of each channel for every subject were obtained.
• Approximate Entropy (ApEn) calculation
Approximate Entropy (ApEn) is a nonlinear dynamic character used to describe the complexity and regularity of signals to measure the irregularity of a time series37,38. Its physical significance is the probability of generating a new model of a time series when the dimension changes. The larger probability indicates the sequence is more complex, leading to a greater ApEn. Recent research has shown that nonlinear analysis can directly monitor and measure the suppression level of the cerebral cortex in real-time. For patients with severe disorders of consciousness, the higher entropy indicates the patient is more conscious; thus ApEn is another index that reflects damaged brain areas.
For the calculation of ApEn a time window was put on each channel of EEG data and 2 s (N = 512) chosen as the window time, then one sampling point was used as a window shift to calculate the ApEn of each channel. After that, the average ApEn from all time windows was taken as the ApEn value. Details of the computing method are provided elsewhere34.
Grouping of symmetrical channels
For the grouping of symmetric channels, the side and area of injury in each patient were identified from CT or MRI images by a clinician. Channels corresponding to injured areas were referred to as injured channels (with the offside channel designated as the symmetric uninjured channel; see Fig. 3). Channels for the whole brain were then divided into injured and uninjured areas (shown as the areas above and below the red line in Fig. 3, respectively), with ratios values of symmetric channels calculated as [injured channels vs. symmetric uninjured channels] and [uninjured channels vs. symmetric uninjured channels], respectively. (as shown the area under the red line in Figure 6).
Computation of ratio values
As the ratio values are critical for the SESA method (according to our proposed hypothesis) the ratio values of EEG features between injured and uninjured brain areas as well as channels were calculated. For ApEn and SWC, the calculations were as follows: for injured areas, the feature (ApEn or SWC) of the injured channel was divided by the feature of the symmetric uninjured channel; for uninjured areas, the feature of the uninjured channel was divided by the feature of the symmetric uninjured channel.
Statistical analysis of ratio values in population and individual channels
-
1
After ratio values were acquired, differences in ratio values for population (i.e., total injured vs. uninjured channels) and individual injured channels (i.e., injured individual F1 vs. total uninjured channels) were evaluated. For convenience, Cp was used instead of ratio values. The analysis of Cp values in population channels was performed as follows:
-
2
Computation of the mean ratio for all injured area channels;
-
3
Computation of the mean ratio for all uninjured channels;
-
4
Placement of 25 means (cases) in a column and one-way ANOVA of the ratio between injured and uninjured channels.
The analysis of Cp values in individual injured channels was performed as follows:
-
1
The totals for individual injured channels were calculated as 20 FP1 or FP2, 18 F7 or F8, 17 T3 or T4, 5 T5 or T6;
-
2
The mean ratio for all individual injured channels was calculated;
-
3
The mean ratio for all individual uninjured channels was calculated;
-
4
One-way ANOVA was performed for injured and uninjured channel groups.
Establishing criteria for diagnosing an injured area
If the statistical analysis of ratio values revealed significant differences in population and individual channels between injured and uninjured brain areas, a determination of whether a brain injury had occurred was made based on a range of ratio values.
SPASS analyses were first used to determine whether the Cp values of all channels follow a normal distribution. By applying statistical theory, preliminary criteria for judgment were derived. For an injured channel (e.g., Fp1 or Fp2), the ratio of 20 patients were taken as sample values to estimate the range of the ratio for corresponding injured brain areas (for other injured channels, the computing methods were the same). For an uninjured channel (e.g., F3–F4, C3–C4, P3–P4, O1–O2), the ratios of 25 patients were regarded as sample values to estimate the range of the ratio for corresponding uninjured brain areas. More details about the computing methods are in Appendix III.
References
Brodbeck, V. et al. Electroencephalographic source imaging: a prospective study of 152 operated epileptic patients. Brain 134, 2887–2897 (2011).
Sperli, F. et al. EEG source imaging in paediatric epilepsy surgery: a new perspective in presurgical workup. Epilepsia 47, 981–990 (2006).
Saletu, B., Anderer, P., Saletu-Zyhlarz, G. M. & Pascual-Marqui, R. D. EEG mapping and low-resolution brain electromagnetic tomography (LORETA) in diagnosis and therapy of psychiatric disorders: evidence for a key-lock principle. Clin. EEG Neurosci. 36, 108–115 (2005).
Michel, C. M. & Murray, M. M. Towards the utilization of EEG as a brain imaging tool. NeuroImage 61, 371–385 (2012).
Irimia, A., Goh, S. Y. M., Torgerson, C. M. et al. Electroencephalographic inverse localization of brain activity in acute traumatic brain injury as a guide to surgery, monitoring and treatment. Clin. Neurol. Neurosur. 115, 2159–2165(2013).
Irimia, A., Matthew Goh, S. Y., Torgerson, C. M. et al. Forward and inverse electroencephalographic modeling in health and in acute traumatic brain injury. Clin. Neurophysiol. 124, 2129–2145 (2013).
Cruse, D. et al. Bedside detection of awareness in the vegetative state: a cohort study. Lacent 378, 2088–2094 (2011).
Goldfine, A. M. Victor, J. D., Conte, M. M., Bardin, J. C. & Schiff, N. D. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin. Neurophysiol. 122, 2157–2168 (2011).
Tzovara, A., Murray, M. M., Michel, C. M. & De Lucia, M. A tutorial review of electrical neuroimaging from group-average to single-trial event-related potentials. Dev. Neuropsychol. 37, 518–544 (2012).
Erika, Pa. Focus on Mapping the Brain. Nat. Methods 10, 481 (2013).
Murray, M. M., De Lucia, M., Brunet, D. & Michel, C. M. [Chapter 2] Brain Signal Analysis [21–54] (Cambridge, MA, 2009).
Mazzola, G., Wieser, H.-G., Brunner, V. & Muzzulini, D. A symmetry-oriented mathematical model of classical counterpoint and related neurophysiological investigations by depth EEG. Comput. Math. Appl. 17, 539–594 (1989).
Bodner, M. et al. Detecting symmetry patterns in EEG data: A new method of analysis. Clin.EEG Neurosci. 30, 143–150 (1999).
Bodner, M., Zhou, Y. D., Shaw, G. L. & Fuster, J. M. Symmetric temporal patterns in cortical spike trains during performance of a short-term memory task. Neurol Res. 19, 509–514 (1997).
Kwon, H., Cho, J. & Lee, E. EEG asymmetry analysis of the left and right brain activities during simple versus complex arithmetic learning. J. Neurother. 13, 109–116 (2009).
Yvonne, H. Real movement vs. motor imagery in healthy subjects. Int. J. Psychophysio. 87, 35–41 (2013).
Wu, W. et al. Classifying Single-Trial EEG During Motor Imagery by Iterative Spatio-Spectral Patterns Learning (ISSPL). IEEE Trans Biomed Eng 55, 1733–1743 (2008).
Bai, O., Lin, P., Huang, D., Fei, D.-Y. & Floeter, M. K. Towards a user-friendly brain-computer interface: initial tests in ALS and PLS patients. Clin Neurophysiol. 121, 1293–1303 (2010).
Fastenrath, M., Friston, K. J. & Kiebel, S. J. Dynamical causal modelling for M/EEG: spatial and temporal symmetry constraints. Neuroimage 44, 154–63 (2009).
Jurriaan, M. P., Meritxell, T.-F., Michel, J. A. M., van Putten & Tobias, L. Behavioral measures and EEG monitoring using the Brain Symmetry Index during the Wada test in children. Epilepsy Behav. 23, 247–253 (2012).
Athina, T. The timing of exploratory decision-making revealed by single-trial topographic EEGanalyses. NeuroImage 60, 1959–69 (2012).
van Putten Michel, J. A. M. et al. A brain symmetry index (BSI) for online EEG monitoring in carotid endarterectomy. Clin. Neurophysiol. 115, 51189–1194 (2004).
Abbas, B.-F. & Hamid, S.-Z. Multi-area neural mass modeling of EEG and MEG signals. NeuroImage 52, 793–811 (2010).
Husain, A. M. Electroencephalographic assessment of coma. J. Clin. Neurophysiol. 23, 208–220 (2006).
Andrew, M. G., Jonathan, D. V., Mary, M. C., Jonathan, C. B. & nNicholas, D. S. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clin.Neurophysiol. 11, 2157–2168 (2011).
Abásolo, D. et al. Entropy analysis of the EEG background activity in Alzheimer's disease patients. Physiol. Meas. 27, 241 (2006).
Ranannd, P., Nampoori, V. P. N. & Sreenivasan, R. Complexity quantification of dense array EEG using sample entropy analysis. J. Integr. Neurosci. 3, 343–358 (2004).
Abásolo, D. et al. Analysis of EEG background activity in Alzheimer's disease patients with Lempel–Ziv complexity and central tendency measure. Med. Eng. Phys. 28, 315–322 (2006).
Kotchoubey, B. et al. Information processing in severe disorders of consciousness: egetative state and minimally conscious state. Clin. Neurophysiol. 116, 2441–2453 (2005).
Gosseries, O., Schnakers, C., Ledoux, D. et al. Automated EEG entropy measurements in coma, vegetative state/unresponsive wakefulness syndrome and minimally conscious state. Funct. Neurol. 26, 25–30 (2011).
Okamoto, M. et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 21, 99–111 (2004).
Herwig, U., Satrapi, P. & Schönfeldt-Lecuona, C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 16, 95–99 (2003).
Di, H. B., Yu, S. M., Weng, X. C. et al. Cerebral response to patient's own name in the vegetative and minimally conscious states. Neurology 68, 895–899 (2007).
Pincus, S. Approximate entropy (ApEn) as a complexity measure. Chaos 5, 110–117 (1995).
Adler, G. & Brassen, S. Short-term rivastigmine treatment reduces EEG slow-wave power in Alzheimer patients. Neuropsychobiology 43, 273–276 (2001).
Leon-Carrion, J. et al. Brain function in the minimally conscious state: a quantitative neurophysiological study. Clin. Neurophysiol. 119, 1506–1514 (2008).
Richman, J. S. & Moorman, J. R. Physiological time-series analysis using approximate entropy and sample entropy. AM. J. Physiol-Heart C. 278, H2039–H2049 (2000).
Pincus, S. M. Approximate entropy as a measure of system complexity. P. Natl. Acad. Sci. 88, 2297–2301 (1991).
Acknowledgements
Y.Y. would like to acknowledge the financial supports provided by Natural Science Foundation of China (Grant No. 30770685). L.L.H. acknowledges the financial support provided by the Grant 2013CB329502 of the National Key Basic Research Program of the Chinese Ministry of Science and Technology.
Author information
Authors and Affiliations
Contributions
Y.Y. and L.Y. conceived the project and experiment designed; L.Y. and L.X.P. wrote the manuscript draft; Y.Y., L.X.P. and L.L.H. co-revise the manuscript; L.X.P., Z.D.K. and Y.W.W. processed data and prepared the figures; L.J.Q. and L.X.P. collected patients. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Yi, L., Xiao-ping, L., Xian-hong, L. et al. Mapping Brain Injury with Symmetrical-channels' EEG Signal Analysis – A Pilot Study. Sci Rep 4, 5023 (2014). https://doi.org/10.1038/srep05023
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05023
This article is cited by
-
Responses of Patients with Disorders of Consciousness to Habit Stimulation: A Quantitative EEG Study
Neuroscience Bulletin (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.