Abstract
The clinical use of cisplatin was severely limited by its associated nephrotoxicity. In this study, we investigated whether the pseudoginsenoside F11 had protective effects against cisplatin-induced nephrotoxicity. To clarify it, one in vivo model of cisplatin-induced acute renal failure was performed. The results showed that pretreatment with F11 reduced cisplatin-elevated blood urea nitrogen and creatinine levels, as well as ameliorated the histophathological damage. Further studies showed that F11 could suppress P53 activation, inverse the ratio of Bax/Bcl2 and the anti-oxidative and free radical levels induced by cisplatin, which in turn inhibited tubular cell apoptosis. Importantly, F11 enhanced rather than inhibited the anti-tumor activity of cispaltin in murine melanoma and Lewis lung cancer xenograft tumor models. Our findings suggested that administering F11 with cisplatin might alleviate the associated nephrotoxicity without compromising its therapeutic efficiency. This finding provides a novel potential strategy in the clinical treatment of cancer.
Similar content being viewed by others
Introduction
Cisplatin (CDDP) is a chemotherapeutic drug to be used in several standard regimens for a variety of malignant tumors, such as non-small cell lung cancer, bladder, cervical, ovarian cancer, testicular cancer et al1,2. Although CDDP has been a mainstay for chemotherapy, its usefulness is often restricted by the following side-effects, especially the dose-dependent nephrotoxicity, which negatively affects patients' quality of life3,4,5. Although the hydration therapy has been proved as an effective renal-protective strategy, a certain percentage of patients still suffers from CDDP-induced nephrotoxicity5. Thus, new compounds, which could reduce this side effect while maintaining and/or enhancing its anti-tumor activity, would be of great clinical benefit.
Considerable research has focused on elucidating the mechanisms of nephrotoxicity induced by CDDP as well as on finding reasonable ways to prevent its development and progression. To date, the exact cellular and molecular mechanisms of CDDP-induced nephrotoxicity remain unclear, although the oxidative stress had been recognized as one of the key factors5,6,7. CDDP is freely filtered by the glomerulus and taken up by renal tubular cells, which might increase the production of reactive oxygen species (ROS) by disrupting the respiratory chain7. Given their broadly reactive nature, ROS could target and modify multiple cellular targets, such as lipids, proteins and DNA7,8. The resulting cellular stress could then induce cellular apoptosis by activating the p53 and/or initiating the mitochondrial cell death pathways9,10. Several antioxidants and oxygen radical scavengers, such as cilastatin, edarabone and sildenafil, had been reported to protect against such damage11,12,13. Novel antioxidants might therefore be potential candidates for preventing and treating CDDP-induced nephrotoxicity.
Ginseng (Panax ginseng) had been used medicinally for thousands of years in China and was already shown to improve general and physical fatigue symptoms in cancer patients14,15. There were substantial evidences to show that ginseng had a wide range of pharmacological actions, including neuroprotective, cardioprotective, antioxidant and anticancer properties, in which the active ingredient were primarily thought to the ginsenosides14. Recently, we reported that Rh2 and ocotillol, two ingredients in the gingeng, attenuated doxorubicin-induced cardiotoxicology via their antioxidant capabilities16,17. Here we reported that F1118, one pseudoginsenoside, attenuated the nephrotoxicity induced by CDDP without affecting its anti-tumor efficacy.
Results
F11 attenuated CDDP-induced renal injury
The biomarkers Blood urea nitrogen (BUN) and creatinine (Cre) were used to evaluate renal damage19. A dramatic increase in plasma BUN and Cre levels was observed 5 days after CDDP administration (Fig 1, p = 0.0001 and 0.0057 respectively, compared with that in control group). Pretreated with F11 at the dosages of 10 mg/kg, however, could significantly reduce the increase in plasma BUN and Cre levels (p = 0.0269 and 0.0025 respectively, compared with that in CDDP group). F11 at the dose of 10 mg/kg alone had no observable effect on the levels of BUN and Cre.
F11 ameliorated CDDP-induced histopathological damage
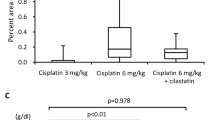
The animals in control and F11 alone groups were observed with normal kidney architecture (Fig. 2A and 2B). CDDP treatment produced obvious structure damage, such as tubular degeneration, swelling, extensive epithelial vacuolization and luminal ectasia (Fig. 2C), in which the histological injury score was dramatic higher (Fig. 2E, p = 0.0032, compared with that in control group). Pretreated with F11 markedly attenuated the histopathological changes and significantly reduced the injury score (Fig 2D and 2E, p = 0.0045, compared with that in CDDP group).
Effects of the pseudoginsenoside F11 on kidney morphology in cisplatin-injected rats.
The kidneys were conducted for H.E staining after treatment and light microscope observations were carried out. The injury in renal tubules was blindly assessed based on a 4 - point scale and the results were expressed as means ± SD (n = 8). The black arrows indicated the representative morphological changes: tubular degeneration, swelling, extensive epithelial vacuolization and luminal ectasia (400× magnifications). *: p<0.05, compared with control group; #: p<0.05, compared with CDDP group.
F11 reduced CDDP-induced tubular cell apoptosis
Apoptosis played an important role in renal damage7. To explore the effect of F11 on CDDP-induced apoptosis, TUNEL staining was conducted. The kidneys of control and F11 only group were observed with quite low ratios of apoptotic cells, which were elevated dramatically in CDDP treated tissue (Fig. 3, p = 0.0013, compared with that in control group). Pretreatment with F11 significantly decreased the number of TUNEL positive staining cells (Fig. 3, p = 0.0231, compared with that in CDDP group). These findings were supported by the western blot analyses, in which pretreated F11 dramatically decreased the protein levels of cl-caspase 3 and cl-caspase 9 (Fig. 4C).
Effects of the pseudoginsenoside F11 on tubular cell apoptosis in cisplatin-injected rats.
The animals were treated as indicated and the fixed kidney tissues were performed for TUNEL staining. The slides were scanned by a confocal fluorescence microscope (A) and around 1000 cells (DAPI staining cells) were chosen randomly to quantify the number of apoptotic cells in renal tubules (B). The red arrows indicated the TUNEL-positive cells. *: p<0.05, compared with control group; #: p<0.05, compared with CDDP group.
F11 suppressed P53 activation and inversed CDDP-induced Bax/Bcl2 ratio
To explore the effects of F11 on cisplatin induced-apoptosis, western-blot analyses was performed to detect the expression levels of P53, Bcl2 and Bax in kidney tissue. CDDP treatment increased the expression of P53 and Bax and decreased Bcl2 expression (Fig. 4A and 4B). Conversely, pretreatment with F11 decreased the expression of P53 and Bax and increased the expression of Bcl2 (Fig 4). F11 at the dose of 10 mg/kg alone had no obvious effect on the expressions of P53, Bax or Bcl2.
F11 attenuated CDDP-induced oxidative stress
As oxidative stress played an important role in CDDP-induced nephrotoxicity5, the effects of F11 treatment on the content of GSH-px, SOD and LPO in kidney tissues were investigated. After CDDP administration, GSH-px and SOD levels were observed with significantly decrease (Fig. 5A and 5B, p = 0.0046 and 0.0281 respectively, compared with that in control group), while LPO level was noted with dramatic increased (Fig. 5C, p = 0.0491, compared with that in control group). Conversely, pretreatment with F11 significantly elevated GSH-px and SOD levels and decreased the LPO level (p = 0.0001, 0.04395 and 0.02259 respectively, compared with that in CDDP group). There was no difference in the levels of LPO, SOD and GSH-px between the F11 alone and control groups.
Effects of the pseudoginsenoside F11 on GSH-px, SOD and LPO levels in cisplatin-injected rats.
The animals were treated as indication and the levels of GSH-px, SOD and LPO were detected. All data were expressed as means ± SD (n = 8). *: p<0.05, compared with control group; #: p<0.05, compared with CDDP group.
F11 did not attenuate the in vivo anti-tumor activity of CDDP
To evaluate the effect of F11 on the anti-tumor activity of CDDP, two murine tumor models were used. CDDP, at a dose of 3 mg/kg, inhibited tumor growth in both the B16 melanoma and Lewis lung cancer xenograft models (Fig. 6). Co-treatment with F11 at the dose of 10 mg/kg did not attenuate, but rather seemed to augment the growth inhibiting properties of CDDP to a certain degree. No obvious anti-tumor effect of F11 alone was observed.
Effects of the pseudoginsenoside F11 on the anti-tumor activity of cisplatin against B16 melanoma and Lewis lung cancer xenograft tumors in C57BL/6 mice.
The C57BL/6 mice were transplanted with Lewis lung cancer (A) or B16 melanoma (B) tumors and treated as shown. Following treatment, the mice were sacrificed and the tumors were peeled off and weighed. All data were expressed as means ± SD (n = 10). *: p<0.05, compared with control group.
Discussion
The defining limitation of CDDP-based chemotherapy was its associated nephrotoxicity, which was caused by oxidative stress and tubular cell apoptosis6,7,20. Therefore, novel antioxidants and/or apoptosis inhibitors, that could protect the kidney from CDDP damage without compromising its anti-tumor activity, could be of great use in treating certain cancers. In this study, we demonstrated for the first time the protective activity of the pseudoginsenoside F11against CDDP-induced nephrotoxicity without affecting its anticancer properties.
There was strong evidence that CDDP-induced renal injury was caused by the accumulated exposure of the drug in the tubules8, in which the glomerular filtration rate was reduced and followed by an increase of BUN and Cre levels19. Other labs had established well proven rat models, in which CDDP was administrated with a single intraperitoneally (i.p) injection at 5~10 mg/kg20. In the current study, such animals were given one dosage of CDDP (6 mg/kg) and renal function parameters, such as BUN and Cre levels, as well as morphology characteristics, were observed. There were obvious pathological changes 5 days after injection, which were all attenuated by F11 co-treatment. Thus, F11 might be a potential protector against the renal damage induced by CDDP.
The toxic ROS induced by CDDP, which could cause oxidative stress damage, had been well documented to play an important role in the development of renal damage5,20. In agreement with the previous reports, CDDP administration in this study resulted in remarkable decrease in GSH-px and SOD, two important antioxidants in renal tissue21. In addition, LPO, a common indicator of oxidative stress22, was increased. F11, as one free radical scavenger, at a dose of 10 mg/kg, could reverse, in part or completely, the deterioration of the unbalanced anti-oxidative and free radical. This finding was consistent with our previous reports that one derivation of F11 could restore the activities of SOD and GSH-Px and decrease the production of lipid peroxides in the mouse cortex23. Our findings were consistent with other reports, in which several anti-oxidants, such as cilastatin, edarabone and lycopene, also exerted protective activity against CDDP-induced renal damage6,12,24.
There was growing evidence that apoptosis played a predominant role in CDDP-induced nephrotoxicity. The cellular damage caused by oxidative stress could activate the P53 and alter the physiological ratio of Bcl2 and Bax7,8,20. The activation of P53, as well as an unbalanced mitochondrial state, could activate caspase 3 and induce tubular cells undergoing apoptosis, which were easily detected by TUNEL staining. In this study, CDDP treatment did increase the protein levels of P53 and Bax, as well as decrease the Bcl2 expression. Thess effects were mitigated by co-treatment with F11 at a dosage of 10 mg/kg. The number of TUNEL positive cells and the expression of apoptosis protein markers cl-caspase 3 and cl-caspase 925, were decreased (Fig. 3 and Fig. 4C), which indicated pretreatment with F11 decreased CDDP-induced renal tubular cell apoptosis.
The last and the most important question we addressed was whether F11 attenuated the anti-tumor activity of CDDP. To this end, two murine models were established and the tumor mass were used as an index of anti-tumor activity. The data showed that F11 did not inhibit the anti-tumor activity of CDDP, but actually enhanced its potency to a certain degree (Fig. 6, p = 0.12 and p = 0.57 for B16 and Lewis lung cancer respectively, compared with that in CDDP group). The action of F11 on the toxicity of CDDP was distinct from its anti-tumor activity, although the exact mechanism of this was still unknown. One possible interpretation was that this occurred in a cell/tissue-dependence manner. Understanding the conditions surrounding this may further benefit its clinical use. This interesting finding, which has also been observed with several other compounds, such as cilastatin11, carvedilol26 and ginsenoside Rh216, supported that further investigation into the role of F11 as a protector against CDDP-induced nephrotoxicity was warranted.
In summary, this study provided first evidence that F11 was protective against CDDP-induced nephrotoxicity. It appears to support the maintenance of endogenous anti-oxidants levels and inhibit tubular cell apoptosis. Importantly, F11 did not reduce the anti-tumor activity of CDDP and therefore might be a promising adjuvant therapy in cancer patients receiving with CDDP-based treatment.
Methods
Chemicals
F11 was isolated from Panax ginseng and had the molecular formula C42H72O14 with MW 801.03. The purity was detected by HPLC to be 99.7%. For in vivo study, F11 and CDDP (Qilu Pharm, Jinnan, China) were dissolved in 1% carboxymethycellulose sodium (CMCS) and 0.9% sodium chloride solutions as proposed dose, respectively.
Animals
Male SD rats (160–180 g) and male C57BL/6 mice (18–22 g) were obtained from Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (Beijing, China). The animals were housed in a light and temperature-controlled room (21~22°C, humidity 60~65%) and kept on a standard diet and water. All of the experimental protocols were approved by the Experimental Animal Research Committee of Yantai University.
In vivo rat model of CDDP-induced nephrotoxicity
Male SD rats were randomly assigned to 4 groups (n = 8). The control group was given one doses of 0.9% NaCl i.p. and 9 doses of CMC gavage (P.O) daily. The CDDP group was administered one dose of CDDP at 6 mg/kg and total 9 doses of CMC P.O. daily. Based on the finding in the preliminary study (data not shown), F11 at 10 mg/kg was the optimal dosage. So, the pretreated groups received total 9 doses of F11 at 10 mg/kg daily with the first administration 72 h before CDDP injection. Twenty four hours after the final dose, the animals were anesthetized with sodium pentobarbitone (50 mg/kg) i.p. and sacrificed by carbon dioxide asphyxiation. Plasma and kidneys were collected for assays.
Renal function detection
Blood was collected in heparinized tubes and the plasma separated by centrifugation at 3,500 rpm for 10 min. BUN and Cre levels were detected by commercial kits supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Antioxidants and lipid peroxidation measurement
The kidney were homogenized in 0.9% NaCl and centrifuged at 12,000 rpm for 20 min at 4°C. The supernatants were separated and glutathione peroxidase (GSH-px), lipid peroxides (LPO) and superoxide dismutase (SOD) levels determined using commercial kits (Nanjing Jiancheng Bioengineering Institute) following the product instruction as described previously16. Total protein concentrations were determined using a BCA kit from Beyotime Institute of Biotechnology (Hangzhou, China) and used for normalization.
Histological analysis
Kidney tissues were fixed in 4% neutral buffered paraformaldehyde and embedded in paraffin. Tissue sections (4 μm) were stained with hematoxylin and eosin (HE) staining and the morphology analyzed blindly by two pathologist using Nikon ECLIPSE50i microscope (Chiyoda, Japan). The percentage of damaged in renal tubules was evaluated by one pathologist on a 4 - point scale: 0 = no damage, 1 = 0–20%, 2 = 20%–50%, 3 = 50%–70%, 4 = more than 70%. The mean score was calculated by counting ten different fields for each group.
TUNEL staining
Cellular apoptosis was assessed by TUNEL staining using the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) as previously described27. Briefly, following dewaxing and hydration, the tissues were permeabilized and incubated with 50 μl of reaction mixture containing the labeling enzyme and the TMR green labeled-dUTP at 37°C for 1 h. After extensive washing, the tissues were counterstained with DAPI and observed under a confocal fluorescence microscope (Leica, Osaka, Japan). For quantitation, at least 1000 cells (DAPI staining cells) were chosen randomly and the number of TUNEL-positive cells in renal tubules was recorded.
Immunoblotting assay
Kidney samples were lysed in RAPI buffer and centrifuged at 12,000 rpm for 20 min at 4°C. The supernatants, containing total proteins, were electrophoresed on SDS–polyacrylamide gels and the proteins were transferred to a PVDF membrane. After blocking, the membrane was incubated with primary antibodies to P53 (Santa Cruz Biotechnology Inc, Texas, USA), cl-caspase 3, cl-caspase 9 and Bax, Bcl2 and β-actin (Cell Signaling Technology, Massachusetts, USA) overnight. The membranes were washed with TBS-0.05% Tween 20 for 5 min thrice, incubated with secondary antibodies and then visualized with an enhanced chemiluminescence detection kit (GE Healthcare Bio-Sciences, Pittsburgh, USA). The film was developed and the scanned images were shown.
Xenograft tumor model in mice
C57BL/6 mice were used to establish xenograft tumors of Lewis lung cancer and melanoma (B16) as previously reported28. In this experiment, tumors were isolated from donor mice and implanted at the dorsum of the recipients by subcutaneous injection (S.C.). The day after implantation, the animals were randomized into 4 groups each containing 10 animals: the control group was given one doses of 0.9% NaCl i.p. and 10 doses of CMC P.O daily; the CDDP group was administrated one dose of CDDP at 3 mg/kg and total 10 doses of CMC P.O daily; the pretreated groups received total 10 doses of F11 at 10 mg/kg daily with the first administration 72 h before CDDP injection; the F11 only group was given one doses of 0.9% NaCl i.p and 10 doses of F11P.O. daily. At the end of treatment, the mice were sacrificed and the tumor were peeled off and weighted.
Data analysis and statistics
The results were presented as mean ± SD. Comparisons between more than 2 groups were performed by analysis of variance (one way ANOVA), then Student t test were performed. P <0.05 was used as the level of statistical significance unless indicated otherwise.
References
Boulikas, T. & Vougiouka, M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol Rep 10, 1663–1682 (2003).
Bose, R. N. Biomolecular targets for platinum antitumor drugs. Mini Rev Med Chem 2, 103–111 (2002).
Arany, I. & Safirstein, R. L. Cisplatin nephrotoxicity. Semin Nephrol 23, 460–464 (2003).
Perazella, M. A. & Moeckel, G. W. Nephrotoxicity from chemotherapeutic agents: clinical manifestations, pathobiology and prevention/therapy. Semin Nephrol 30, 570–581 (2010).
Yao, X., Panichpisal, K., Kurtzman, N. & Nugent, K. Cisplatin nephrotoxicity: a review. Am J Med Sci 334, 115–124 (2007).
Atessahin, A., Yilmaz, S., Karahan, I., Ceribasi, A. O. & Karaoglu, A. Effects of lycopene against cisplatin-induced nephrotoxicity and oxidative stress in rats. Toxicology 212, 116–123 (2005).
Pabla, N. & Dong, Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73, 994–1007 (2008).
Kannan, K. & Jain, S. K. Oxidative stress and apoptosis. Pathophysiology 7, 153–163 (2000).
Florea, A. M. & Busselberg, D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel) 3, 1351–1371 (2011).
Miyaji, T., Kato, A., Yasuda, H., Fujigaki, Y. & Hishida, A. Role of the increase in p21 in cisplatin-induced acute renal failure in rats. J Am Soc Nephrol 12, 900–908 (2001).
Humanes, B. et al. Cilastatin protects against cisplatin-induced nephrotoxicity without compromising its anticancer efficiency in rats. Kidney Int 82, 652–663 (2012).
Satoh, M. et al. A novel free radical scavenger, edarabone, protects against cisplatin-induced acute renal damage in vitro and in vivo. J Pharmacol Exp Ther 305, 1183–1190 (2003).
Lee, K. W. et al. Sildenafil attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Toxicology 257, 137–143 (2009).
Attele, A. S., Wu, J. A. & Yuan, C. S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 58, 1685–1693 (1999).
Sood, A. & Moynihan, T. J. Cancer-related fatigue: an update. Curr Oncol Rep 7, 277–282 (2005).
Wang, H. et al. Cardioprotective Effects of 20(S)-Ginsenoside Rh2 against Doxorubicin-Induced Cardiotoxicity In Vitro and In Vivo. Evid Based Complement Alternat Med 2012, 506214 (2012).
Fu, X. et al. Protective effect of ocotillol against doxorubicininduced acute and chronic cardiac injury. Mol Med Rep 9, 360–364 (2014).
Chen, R. J., Chung, T. Y., Li, F. Y., Lin, N. H. & Tzen, J. T. Effect of sugar positions in ginsenosides and their inhibitory potency on Na+/K+-ATPase activity. Acta Pharmacol Sin 30, 61–69 (2009).
Edelstein, C. L. Biomarkers of acute kidney injury. Adv Chronic Kidney Dis 15, 222–234 (2008).
Ravi, R., Somani, S. M. & Rybak, L. P. Mechanism of cisplatin ototoxicity: antioxidant system. Pharmacol Toxicol 76, 386–394 (1995).
Cetin, R. et al. Cisplatin impairs antioxidant system and causes oxidation in rat kidney tissues: possible protective roles of natural antioxidant foods. J Appl Toxicol 26, 42–46 (2006).
Yousef, M. I., Saad, A. A. & El-Shennawy, L. K. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol 47, 1176–1183 (2009).
Yu, C., Fu, F., Yu, X., Han, B. & Zhu, M. Cardioprotective effect of ocotillol, a derivate of pseudoginsenoside F11, on myocardial injury induced by isoproterenol in rats. Arzneimittelforschung 57, 568–572 (2007).
Camano, S. et al. Cilastatin attenuates cisplatin-induced proximal tubular cell damage. J Pharmacol Exp Ther 334, 419–429 (2010).
Silke, J. et al. The anti-apoptotic activity of XIAP is retained upon mutation of both the caspase 3- and caspase 9-interacting sites. J Cell Biol 157, 115–124 (2002).
Carvalho Rodrigues, M. A., Silva Faria, M. C., Santos, N. A., Gobe, G. C. & dos Santos, A. C. Carvedilol efficiently protects kidneys without affecting the antitumor efficacy of cisplatin in mice. Chem Biol Interact 206, 90–99 (2013).
Wang, H., Ma, X., Ren, S., Buolamwini, J. K. & Yan, C. A small-molecule inhibitor of MDMX activates p53 and induces apoptosis. Mol Cancer Ther 10, 69–79 (2011).
Huang, D. et al. Anti-tumor activity of a 3-oxo derivative of oleanolic acid. Cancer Lett 233, 289–296 (2006).
Acknowledgements
This study was supported by Taishan Scholar Project, Shandong Province Higher Educational Science and Technology Program (J12LM53), Startup Project of Doctor scientific research in Yantai University (YX11B31) and National Natural Science Foundation of China (No. 81202038). We thank Yuan Fu from School of Psychology in University of Nottingham (Nottingham, UK) for critical reading of this paper.
Author information
Authors and Affiliations
Contributions
H.W. and F.F. designed the research and wrote the manuscript; H.W. and L.K. performed the majority of the experiments; J.Z., G.L., G.Y. and F.Z. supported several experiments. X.C. and J.T. contributed materials and revised the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wang, H., Kong, L., Zhang, J. et al. The pseudoginsenoside F11 ameliorates cisplatin-induced nephrotoxicity without compromising its anti-tumor activity in vivo. Sci Rep 4, 4986 (2014). https://doi.org/10.1038/srep04986
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04986
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.