Abstract
This study aimed to explore the neural development status of the visual system of children (around 8 years old) using contrast sensitivity. We achieved this by eliminating the influence of higher order aberrations (HOAs) with adaptive optics correction. We measured HOAs, modulation transfer functions (MTFs) and contrast sensitivity functions (CSFs) of six children and five adults with both corrected and uncorrected HOAs. We found that when HOAs were corrected, children and adults both showed improvements in MTF and CSF. However, the CSF of children was still lower than the adult level, indicating the difference in contrast sensitivity between groups cannot be explained by differences in optical factors. Further study showed that the difference between the groups also could not be explained by differences in non-visual factors. With these results we concluded that the neural systems underlying vision in children of around 8 years old are still immature in contrast sensitivity.
Similar content being viewed by others
Introduction
The contrast sensitivity function (CSF) shows the relationship between the scale of a stimulus and its minimum detectable contrast. Measuring the CSF is one of the most fundamental assessments of visual function. The shape of the CSF is jointly determined by the optical and neural components of the visual system1,2,3,4. Previous studies show worse contrast sensitivity in children and suggest this mainly stems from an immature neural system5,6,7. Children greater than 4 years old typically no longer have the refractive and accommodative errors that are common during infancy, therefore these optical factors will not affect the performance of these children6.
This old story needs to be updated however due to developments in our knowledge of the optical system, especially higher-order aberrations (HOAs)8 of the eye. HOAs are aberrations beside refractive errors (defocus and astigmatism), which cannot be measured or corrected by traditional methods9. Several studies have shown that HOAs vary among individuals10,11, ages12,13, pupil sizes14,15, ethnicities16,17, refractive errors18,19 and even time20. The hallmark effect of HOAs is that it reduces the resolution of images of the retina21. Contrast sensitivity has been demonstrated to increase when HOAs are corrected with an adaptive optics (AO) system in normal adults9,22,23 and in the elderly24. Larger values of HOAs have been found in children than in adults in several studies13,19,25, however their effects on the contrast sensitivity of children are still not fully understood.
Since HOAs vary among individuals and affect the measurement of contrast sensitivity, a precise comparison that eliminates the influence of HOAs is necessary for exploring the status of children's visual neural system in contrast sensitivity. In this study, we measured contrast sensitivities at different spatial frequencies in children of around 8 years old and in young adults under both monochromatic HOAs-corrected and HOAs-uncorrected conditions. Based on the measurements, we addressed one question: whether the neural component of the visual system of children of around 8 years old has reached an adult level in terms of contrast sensitivity? The reason for choosing children of around 8 years old is that previous studies have not reached an agreement on the developmental status of 8-year-old children in contrast sensitivity5,6,7,26,27,28,29,30,31.
We found that after HOAs were corrected both children and adults showed improvements in contrast sensitivity, while adults still exhibited a better performance than children. The worse performance of children may result from an immature visual neural system or simply non-visual factors (e.g., head movements, eye movements and inattention etc.). These non-visual factors would increase measured threshold32,33 and within-subject variability6,34,35. To reduce their influence, we conducted a 10-day training in children. After training, the within-subject variability of the CSF test in children decreased to the same level as adults, which suggested that these non-visual differences may have little influence on the CSF comparison between the two groups in the post-training measurement. In this condition, adults still exhibited a better performance than children. Our results indicate a substantial difference in spatial contrast sensitivity between children of around 8 years old and adults, which provides a new insight into the development of spatial vision in human.
Results
In general, subjects (all have normal vision without correction) had tests of contrast sensitivity function (CSF) conducted monocularly with a randomly chosen eye in the dark room on a real-time closed-loop adaptive optics (AO) visual stimulator system36. Two CSFs were tested on different days: one of them was measured with higher-order aberrations (HOAs) of the eye being corrected in real-time during the test (“with AO correction” in our figures) and the other one was measured with HOAs being uncorrected during the test (“without AO correction”). Optical aberrations were recorded during the CSF tests. Contrast sensitivity was defined as the reciprocal of contrast threshold for detecting sine-wave gratings at 79.3% correct.
Correction of optical aberrations
Figure 1 illustrates the average root mean square (RMS) value of Zernike coefficients up to 7th order with and without AO correction. Naturally, the children had larger HOAs (t(9) = 2.436, p = 0.038, two-tailed Independent Samples t-test) and third-order aberrations (t(9) = 3.157, p = 0.012) than the adults, but similar total aberrations (p > 0.05) and low-order aberrations (astigmatism and defocus) as adults (p > 0.05). The AO system worked well in correcting optical aberrations, which reduced the average RMS value of wavefront aberrations from 0.202 ± 0.016 μm (mean ± SE) to 0.085 ± 0.004 μm for the child group and from 0.187 ± 0.019 μm to 0.071 ± 0.002 μm for the adult group. Both reductions were significant (for children, t(5) = 6.634, p = 0.001; and for adults, t(4) = 6.209, p = 0.003).
Average root mean square (RMS) value of Zernike coefficients with and without adaptive optics (AO) correction.
Average RMS value of wavefront aberrations was provided up to 7th order. In all conditions, un-filled bars represent results that were measured without AO correction; filled bars represent results that were measured with AO correction; blue bars represent results of children (n = 6) and red bars represent results of adults (n = 5). Error bars represent standard errors of RMS value across subjects. ‘*’ represents p < 0.05 by a two-tailed Independent Samples t-test.
We also compared coma-like and spherical aberrations in the two age groups both with and without AO correction, as previous reports have shown that these aberrations could have a large influence on the contrast sensitivity function37,38,39. We found these aberrations were not significantly different between groups (p > 0.05) either with or without AO correction.
We calculated the horizontal (as the orientation of the sine-wave grating we used was vertical) modulation transfer function (MTF)40 to further quantify the improvement of the optical system by AO correction. The MTF indicates the ratio of image contrast on the retina to object contrast as a function of spatial frequency, which is a straightforward measure of the image quality on the retina41. The average MTFs with and without AO correction in the two age groups are presented in Figure 2a (to compare with the contrast sensitivity functions, we focused on modulation transfer factors of the seven spatial frequencies that were tested in the contrast sensitivity measurement). We found comparable MTFs in the two groups (F(1,9) = 0.064, p = 0.806) when HOAs were not corrected. AO correction significantly improved the MTFs in both the child (F(1,5) = 39.872, p = 0.001) and adult (F(1,4) = 26.798, p = 0.007) groups. After AO correction, the MTFs in the two groups still overlapped and had a small but significant difference (F(1,9) = 8.290, p = 0.018) due to small between-subject variance in MTF.
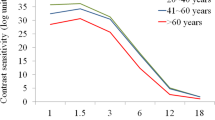
Effect of AO correction on Modulation transfer functions (MTFs) and Contrast sensitivity functions (CSFs) in the child and the adult groups.
(a) Modulation transfer factor with and without AO correction as a function of spatial frequency for the child and the adult groups. Modulation transfer factor of the eye denotes the contrast ratio of one particular spatial frequency that remains after the image comes through the eye; the x-axis shows spatial frequency (cycles/°). The black solid line represents the diffraction limit. Error bars indicate standard errors of the modulation transfer factors across subjects. (b) Contrast sensitivity with and without AO correction as a function of spatial frequency for the child and adult groups. Contrast sensitivity was defined as the reciprocal of contrast threshold for detecting sine-wave gratings at 79.3% correct. Error bars show standard errors of the contrast sensitivity across subjects.
Contrast sensitivity
The average contrast sensitivity functions (CSFs) with and without AO correction in the two age groups are plotted in Figure 2b . When HOAs were not corrected, the contrast sensitivity of the child group was significantly lower than that of the adult group (F(1,9) = 24.108, p = 0.001). The interaction between spatial frequency and age group was significant (F(6,54) = 18.641, p < 0.001): larger differences were shown at lower spatial frequencies. When HOAs were corrected, the CSFs were improved in both children (F(1,5) = 7.498, p = 0.041) and adults (but not significantly, F(1,4) = 3.235, p = 0.146). However, the CSFs of children were still worse than those of adults (F(1,9) = 15.761, p = 0.003), even worse than the performance of adults that were measured without AO correction (F(1,9) = 12.943, p = 0.006). Similar to the situation when HOAs were not corrected, the interaction between spatial frequency and age group was also significant when HOAs were corrected (F(6,54) = 8.921, p < 0.001), with larger differences found at lower spatial frequencies.
Performance of the children after 10 days of training
We have shown that our child subjects still exhibit lower contrast sensitivity than adults even when HOAs were corrected. The worse performance of children could result from immature neural circuits underlying spatial contrast sensitivity, but could also be due to the influence of non-visual factors (such as head movements, eye movements, inattention etc.). Since the non-visual factors have been shown to increase both measured threshold32,33 and within-subject variability6,34,35, we examined the extent of these non-visual effects by comparing the within-subject variability of the CSF test between the child and the adult groups. We found that the within-subject variability of the CSF test with AO correction (i.e., standard error of the reversal points of contrast in each staircase) was significantly larger in children than in adults ( Figure 3 , F(1,9) = 7.296, p = 0.024). To rule out the possible influence of non-visual factors on our measured thresholds we conducted 10 days of training for the children. A high spatial frequency, at which the contrast detection threshold was 0.4 in pre-training CSF test with AO correction, was selected as training frequency. The same task as CSF measurement (i.e., contrast detection task) was used for the training. Training successfully reduced the within-subject variability of the post-training CSF measurement in children such that it was no longer different from that in adults ( Figure 3 , F(1,9) < 0.001, p = 0.993). However, even though the contrast sensitivity of children with AO correction tended to improve after training ( Figure 4a , F(1,5) = 6.201, p = 0.055), it was still worse than that of adults ( Figure 4b , F(1,9) = 9.466, p = 0.013). The interaction between spatial frequency and age group was also significant (F(6,54) = 5.332, p < 0.001) and larger differences were found at lower spatial frequencies ( Figure 4b ).
Average within-subject variability of the CSF test of the untrained and trained children and untrained adults.
The within-subject variability in the CSF test was defined as the standard error of the reversal points (contrast in dB) in each staircase. The average within-subject variability of the CSF test was averaged across subjects and spatial frequencies. The blue bar shows results from the untrained children, the purple bar shows results from the trained children and the red bar shows results from the adults. ‘*’ represents p < 0.05 by a two-tailed Independent Samples t-test. Error bars represent standard errors of the variability of the CSF test across subjects.
Contrast sensitivity functions (CSFs) with AO correction in trained and untrained children and in untrained adults.
(a) CSFs with AO correction in the trained (‘ ’) and untrained (‘
’) and untrained (‘ ’) children. Error bars represent the standard error in contrast sensitivity across subjects. (b) CSFs with AO correction in the trained children (‘
’) children. Error bars represent the standard error in contrast sensitivity across subjects. (b) CSFs with AO correction in the trained children (‘ ’) and adults (‘
’) and adults (‘ ’). Error bars represent the standard error in contrast sensitivity across subjects. The solid lines are curve-fits with difference of Gaussian (DOG) functions.
’). Error bars represent the standard error in contrast sensitivity across subjects. The solid lines are curve-fits with difference of Gaussian (DOG) functions.
To further investigate the differences in contrast sensitivity between the trained children and the adults, we also conducted a curve-fitting approach to compare the CSFs between the two groups. We fitted the CSFs with difference of Gaussian (DOG) functions and used an F-test for nested models42 to compare a full model (where different DOG functions are needed to fit the two CSFs) with a reduced model (where the same DOG function is used to fit the two CSFs). The full model generated a quite good fit to our data (R2 = 99.6%), which was significantly better than the reduced model (F(3,4) = 17.521, p = 0.009). These results suggested that two different DOG functions were needed to fit CSFs of the trained children and the adults. The fitted results based on the full model are shown in Figure 4b as solid lines. Obviously, the CSF of children is lower than that of adults.
Discussion
We found that AO correction successfully improved optical quality by decreasing the wavefront aberrations and increasing the modulation transfer factor in both the child and the adult groups. AO correction also improved the CSF in both groups. Under the HOAs corrected condition, the contrast sensitivity of the child group was still significantly lower than that of the adult group. After intensive training, the within-subject variability of the CSF test with AO correction in children was reduced to a comparable level as in adults. Therefore in this condition the non-visual differences may have little influence on any differences between the two groups. However, the contrast sensitivity of the children in the trained condition was still worse than that of the adults. These results indicate an immaturity in the neural circuits underlying spatial contrast sensitivity in children.
One technical concern is the influence of optical factors such as fluctuation of accommodation43 and low order aberrations (defocus and astigmatism)1,44,45 on the measurement of the MTF and CSF. For the fluctuation of accommodation43, we corrected subjects' aberrations in real time during the whole CSF test. In order to reduce the low order aberrations' effects, all subjects we chose had normal vision without spectacles. The RMS value of the wavefront aberrations also showed that there was no difference between children and adults on the second order aberrations. On the other hand, we used a fixed defocus correction to correct chromatic aberration for both children and adults. It might or might not be the best position for all subjects, but from their subjective reports and their C-letter-test results we were confident that it was still in their clear position. Nevertheless, we should point that some potential uncorrected defocus might still exist and thus affect subjects' performance. However, such uncorrected defocus, if existed, was likely to have no notable effect on the comparison of CSFs between children and adults in the current study: Firstly, it has been shown that defocus has larger effect on visual performance with AO-correction than that without AO-correction41,46,47. Thus, if the uncorrected defocus affected children more, we would expect less benefit from AO correction in children, which was not the case in our study. Secondly, several studies have demonstrated that defocus mainly damage contrast sensitivity at higher spatial frequencies, but not at low spatial frequencies1,48,49. While when compared the CSF of children with adults, larger differences were found at lower spatial frequencies but not at higher frequencies.
Another technical concern is the influence of non-visual factors such as head movements, eye movements and inattention on the comparison between adults and children. Head movements and eye movements were carefully controlled in our study. During the test, subjects were instructed to keep stable with their heads on a chin rest to minimize the head movements. Subjects were also asked to maintain their fixation at the center of the screen to reduce the eye movements. On the other hand, stimulus was shown immediately after fixation and its presentation time was too short (i.e., 117 ms) for subjects to saccade50. Effects of inattention were reduced by asking subjects to take a break for 2–3 minutes after finishing the test in one block (typically lasted for about 5 minutes). Though we tried our best to minimize the influence of these non-visual factors, they still may have affected our comparison between children and adults, since the variability of our CSF test was significantly larger in children than in adults. To further diminish any such effects, we conducted a 10-day training protocol with a contrast detection task at a high spatial frequency. Training successfully reduced the variability of the CSF test of children to the same level as adults. In this condition therefore these non-visual factors may have a comparable influence on children and adults, however the contrast sensitivity of children was still worse than that of adults. It should be noted that former studies had shown that training with the contrast detection task would bring about perceptual learning in adult and child amblyopes51,52,53 and in normal adults under AO condition23. It is quite possible that our training in children also induced some perceptual learning effect, which in turn underestimated the difference of contrast sensitivities between children and adults. Therefore, we may confidently conclude that the contrast sensitivity of children is lower than that of adults.
Our data showed that HOAs were higher in children than adults, which is consistent with several previous reports13,19,25. Meanwhile, total aberrations and MTFs in the child and adult groups were similar before AO correction. The similarity of MTF in children and adults in the current study indicates similar optical quality in these two groups. This is consistent with a previous study which showed that the average MTF of children was slightly better than that of adults, but there was considerable overlap between MTFs for the two age groups54.
The aim of our study is to check whether the contrast sensitivity of the visual neural system in children of around 8 years old reaches the adult level. We showed that the CSF of children with AO correction was lower than that of adults, which can't be attributed to optical and non-visual factors. One notable phenomenon is that the peak contrast sensitivity of our subjects was not high even in adults (~100 in adults). Such peak contrast sensitivity is lower than that in some reports5,6,26,28,29, but also consistent with the magnitude in several other studies23,24,27,51. The variance of peak contrast sensitivity in different studies might come from different paradigms that were used in testing. For example, our study used a quite short exposure time of stimuli (117 ms) during the measurement, while most other studies presented stimuli for several seconds5,28 or with unlimited time26,29 in testing the contrast sensitivity of children and adults. Nevertheless, we believe that the magnitude of the peak contrast sensitivity couldn't limit the ability to detect the differences between children and adults in contrast sensitivity, as there were several studies that reported higher peak contrast sensitivity but still found less contrast sensitivity in children of around 8 years old than that of adults29,31.
Our data, therefore support the existence of immature neural circuits underlying spatial contrast sensitivity in children of around 8 years old55. This idea is consistent with several neurophysiological studies56,57,58. For instance, experiment on macaque monkeys suggested that the main factors limiting spatial contrast sensitivity in development are in the nervous system, not in the eye or photoreceptors57. Further study showed that the asynchronous changes also exist between the LGN cells and the behavioral performance58, indicating a later maturation in the visual cortex than in the LGN. The immature contrast sensitivity of our child subjects indicates the existence of a subsequent process of neural maturation in the visual system after the age of 8 years old.
Methods
Subjects
Six children (Mean age (± SD): 8.5 ± 0.55 years old; 2 males) and five adults (25 ± 2.00 years old; 3 males) with normal vision (Snellen acuity equal to or better than 20/20 in each eye without wearing any optical correction) participated in this experiment. All subjects were naïve to the purpose of the experiment. A written informed consent was obtained from each adult subject and each child's guardians/parents before the experiment. This study was approved by the Institutional Review Board of University of Science and Technology of China and the Institute of Optics and Electronics in the Chinese Academy of Sciences. The methods were carried out in accordance with the approved guidelines.
Apparatus
All the measures were conducted on a real-time closed-loop adaptive optics (AO) visual stimulator system36. The display had a pixel resolution of 800 × 600 and a frame refresh rate of 60 Hz. A special circuit was used to produce 14 bits of gray levels59.
Subjects viewed a 1.5° test field monocularly through a 4-mm artificial pupil in a dark room. Their pupils were naturally dilated after several minutes' adaptation in the dark room before the measurements were made. Their heads were fixed with a chin rest to rule out any influence of head movements during the test. They were instructed to focus on the center of the display when performing the task. Retinal illuminance, defined as the product of the luminance in the pupil plane (18.9 cd/m2) with the pupil area, was 237 Troland.
The minidisplay was fixed on a stepping motor for adjusting the potential focus shifts. Two experienced adult operators performed the adjustment of the stimulus position for a perfect focus for a Landolt C letter with all ocular aberrations corrected for several times. The position of the stepping motor was set to the average of these repetitions. In pilot study, we confirmed that all our subjects could clearly see the C letter (better than 20/20) at this position. Those who could not see clearly at this position were excluded in our experiment.
Aberrations calculation
Aberrations were calculated up to 35 Zernike coefficients (7th order). Root mean square (RMS) value of wavefront aberrations was calculated as the square root of the sum of the squares of the corresponding coefficients from each Zernike expansion. The modulation transfer function (MTF, describing the attenuation of contrast as a function of spatial frequency of the optical system) was calculated from the wavefront aberrations based on the standard Fourier optics theories60. Since vertical sine-wave gratings were used in our experiment, only the horizontal MTF was calculated. Contrast sensitivity was measured at seven spatial frequencies (0.9, 1.5, 3, 6, 12, 24 and 36 cycles/°).
Experiment design
Before the measurement, all subjects practiced for one hour in order to understand the experiment and familiarize themselves with the manipulations.
RMS values, MTFs and CSFs were then measured under both the HOAs-corrected (i.e. with AO correction) and HOAs-uncorrected (i.e. without AO correction) conditions. AO correction operated in real-time along the whole CSF measurement under the HOAs-corrected condition. CSF measurements in the two conditions were finished separately in two different days with counterbalanced orders in different individuals.
To further diminish the influence of non-visual factors, a 10-day training protocol was conducted after the measurements. A high spatial frequency, at which the contrast detection threshold was 0.4 in pre-training CSF test with AO correction, was selected as the training frequency. The same contrast detection task as was used in the CSF measurement was used for the training. After training, CSFs were re-measured under the HOAs-corrected condition using the same paradigm as the pre-training test.
Procedure
A two-interval forced-choice contrast detection task was used for both training and CSF measurements. The presentation sequence in each trial was as follows: a 267-ms fixation signaled by a brief sound, a 117-ms stimulus interval (such a short period of presentation was used to minimize the influence of eye movements50), a 500-ms blank, a 267-ms fixation signaled by a brief sound, a 117-ms second stimulus interval, a 500-ms blank and blank until response. A vertical sine-wave grating was randomly presented in one of the two stimulus intervals, while the other stimulus interval was blank. Subjects were instructed to answer which stimulus interval contained the signal by pressing a key. The next trial started immediately after the response. The contrast of the sine-wave grating was varied by a three-down one-up staircase procedure with a proportional step size of 10% to track the 79.3% correct value. For CSF measurement, each session consisted of 8 blocks with each containing 77 trials. Different spatial frequencies were randomly intermixed in each block. For training, one session consisted of 7 blocks with each containing 90 trials and a fixed spatial frequency (the frequency at which the contrast threshold was 0.4 in the pre-training CSF test with AO correction). Subjects were instructed to take a break for 2–3 minutes after finishing one block. The CSF measurement and training in each day lasted about 50 minutes in total.
Data analysis
The contrast detection threshold at each spatial frequency was calculated by averaging the contrast of the reversal points in the corresponding staircase.
The within-subject variability of the CSF test was defined as the standard error of the reversal points (contrast in dB) in each staircase. Contrast in dB was calculated as 20 × log10 (contrast). The average within-subject variability of the CSF test was averaged within-subject variability across subjects and spatial frequencies.
The differences of RMS values between the two age groups were compared using two-tailed Independent Samples t-test. The differences of MTF, CSF and variability between the two groups were compared using repeated-measures ANOVA with one within-subjects factor (spatial frequency) and one between-subjects factor (age group). The differences of RMS values with and without AO correction in each group were compared using two-tailed Paired Samples t-test. The differences of MTF or CSF with and without AO correction in each group were compared using repeated-measures ANOVA with two within-subjects factors (spatial frequency and AO correction).
The method of curve-fitting for CSFs is similar to a previously documented method in Huang et al.51. In particular, the average CSFs of children and adults were fitted with both a full model and a reduced model. For the full model, the CSF of child group was fitted with a difference of Gaussian (DOG) functions (G1-G2). The CSF of adult group was fitted with (G1-G2 + G3). The model assumed that the difference between the two age groups was a Gaussian function: G3. For the reduced model, the CSFs of children and adults were both fitted with a same function: G1-G2. This model assumed that there was no difference between the two groups. We used an F-test for nested models42 to compare these two models.

Where df1 = kfull − kreduced and df2 = N − kreduced − 1; k represents the number of parameters in each model and N is the number of data points. When p < 0.05, we chose the full model, if not, we chose the reduced model.
References
Campbell, F. W. & Green, D. G. Optical and Retinal Factors Affecting Visual Resolution. J Physiol-London 181, 576–593 (1965).
Michael, R., Guevara, O., de la Paz, M., de Toledo, J. A. & Barraquer, R. I. Neural contrast sensitivity calculated from measured total contrast sensitivity and modulation transfer function. Acta Ophthalmol. 89, 278–283 (2011).
Dressler, M. & Rassow, B. Neural contrast sensitivity measurements with a laser interference system for clinical and screening application. Invest Ophthalmol Vis Sci 21, 737–744 (1981).
Martinez-Roda, J. A. et al. Optical quality and intraocular scattering in a healthy young population. Clin Exp Optom 94, 223–229 (2011).
Benedek, G., Benedek, K., Keri, S. & Janaky, M. The scotopic low-frequency spatial contrast sensitivity develops in children between the ages of 5 and 14 years. Neurosci Lett 345, 161–164 (2003).
Ellemberg, D., Lewis, T. L., Liu, C. H. & Maurer, D. Development of spatial and temporal vision during childhood. Vision Res 39, 2325–2333 (1999).
Adams, R. J. & Courage, M. L. Using a single test to measure human contrast sensitivity from early childhood to maturity. Vision Res 42, 1205–1210 (2002).
Thibos, L. N., Applegate, R. A., Schwiegerling, J. T., Webb, R. & Taskforce, V. S. Standards for reporting the optical aberrations of eyes. Osa Trends Opt Photo 35, 232–244 (2000).
Yoon, G. Y. & Williams, D. R. Visual performance after correcting the monochromatic and chromatic aberrations of the eye. J Opt Soc Am A 19, 266–275 (2002).
Wang, L., Dai, E., Koch, D. D. & Nathoo, A. Optical aberrations of the human anterior cornea. J Cataract Refr Surg 29, 1514–1521 (2003).
Porter, J., Guirao, A., Cox, I. G. & Williams, D. R. Monochromatic aberrations of the human eye in a large population. JOSA A 18, 1793–1803 (2001).
Amano, S. et al. Age-related changes in corneal and ocular higher-order wavefront aberrations. Am J Ophthalmol 137, 988–992 (2004).
Brunette, I., Bueno, J. M., Parent, M., Hamam, H. & Simonet, P. Monochromatic aberrations as a function of age, from childhood to advanced age. Invest Ophth Vis Sci 44, 5438–5446 (2003).
Kasper, T., Buhren, J. & Kohnen, T. Intraindividual comparison of higher-order aberrations after implantation of aspherical and spherical intraocular lenses as a function of pupil diameter. J Cataract Refr Surg 32, 78–84 (2006).
Applegate, R. A., Donnelly, W. J., Marsack, J. D., Koenig, D. E. & Pesudovs, K. Three-dimensional relationship between high-order root-mean-square wavefront error, pupil diameter and aging. J Opt Soc Am A 24, 578–587 (2007).
Cervino, A., Hosking, S. L., Ferrer-Blasco, T., Montes-Mico, R. & Gonzalez-Meijome, J. M. A pilot study on the differences in wavefront aberrations between two ethnic groups of young generally myopic subjects. Ophthal Physl Opt 28, 532–537 (2008).
Lim, K. L. & Fam, H. B. Ethnic differences in higher-order aberrations: Spherical aberration in the South East Asian Chinese eye. J Cataract Refr Surg 35, 2144–2148 (2009).
Cheng, X., Bradley, A., Hong, X. & Thibos, L. N. Relationship between refractive error and monochromatic aberrations of the eye. Optom Vis Sci 80, 43–49 (2003).
He, J. C. et al. Wavefront aberrations in eyes of emmetropic and moderately myopic school children and young adults. Vision Res 42, 1063–1070 (2002).
Hofer, H., Artal, P., Singer, B., Aragon, J. L. & Williams, D. R. Dynamics of the eye's wave aberration. J Opt Soc Am A 18, 497–506 (2001).
Liang, J. Z. & Williams, D. R. Aberrations and retinal image quality of the normal human eye. J Opt Soc Am A 14, 2873–2883 (1997).
Williams, D. et al. Visual benefit of correcting higher order aberrations of the eye. J Refract Surg 16, S554–S559 (2000).
Zhou, J. et al. The eye limits the brain's learning potential. Sci. Rep 2, 364, 1–6 (2012).
Elliott, S. L. et al. Role of high-order aberrations in senescent changes in spatial vision. J Vis 9, 24, 1–16 (2009).
Li, T. et al. Relationship between ocular wavefront aberrations and refractive error in Chinese school children. Clin Exp Optom 95, 399–403 (2012).
Bradley, A. & Freeman, R. D. Contrast sensitivity in children. Vision Res 22, 953–959 (1982).
Beazley, L. D., Illingworth, D. J., Jahn, A. & Greer, D. V. Contrast Sensitivity in Children and Adults. Brit J Ophthalmol 64, 863–866 (1980).
Abramov, I. et al. Rocket-Ship Psychophysics - Assessing Visual Functioning in Young-Children. Invest Ophth Vis Sci 25, 1307–1315 (1984).
Gwiazda, J., Bauer, J., Thorn, F. & Held, R. Development of spatial contrast sensitivity from infancy to adulthood: Psychophysical data. Optom Vis Sci 74, 785–789 (1997).
Leat, S. J. & Wegmann, D. Clinical testing of contrast sensitivity in children: age-related norms and validity. Optom Vis Sci 81, 245–254 (2004).
Benedek, K. et al. Parallel development of contour integration and visual contrast sensitivity at low spatial frequencies. Neurosci Lett 472, 175–178 (2010).
Ling, S. & Carrasco, M. Sustained and transient covert attention enhance the signal via different contrast response functions. Vision Res 46, 1210–1220 (2006).
Carrasco, M., Penpeci-Talgar, C. & Eckstein, M. Spatial covert attention increases contrast sensitivity across the CSF: support for signal enhancement. Vision Res 40, 1203–1215 (2000).
Tschopp, C. et al. Automated visual field examination in children aged 5-8 years - Part II: Normative values. Vision Res 38, 2211–2218 (1998).
Wightman, F., Allen, P., Dolan, T., Kistler, D. & Jamieson, D. Temporal resolution in children. Child Dev 60, 611–624 (1989).
Li, S. M. et al. Effects of Monochromatic Aberration on Visual Acuity Using Adaptive Optics. Optom Vis Sci 86, 868–874 (2009).
Oshika, T., Okamoto, C., Samejima, T., Tokunaga, T. & Miyata, K. Contrast sensitivity function and ocular higher-order wavefront aberrations in normal human eyes. Ophthalmology 113, 1807–1812 (2006).
Oshika, T. et al. Influence of pupil diameter on the relation between ocular higher-order aberration and contrast sensitivity after laser in situ keratomileusis. Invest Ophth Vis Sci 47, 1334–1338 (2006).
Liu, T., Wang, Z. Q., Wang, Y., Mu, G. G. & Zhao, K. X. The study of the effects of higher-order aberrations on human contrast sensitivity with white-light retinal aerial image modulation (AIM). Optik 121, 1116–1122 (2010).
Campbell, F. W. & Gubisch, R. W. Optical quality of the human eye. J Physiol 186, 558–578 (1966).
Roorda, A. Adaptive optics for studying visual function: A comprehensive review. J Vis 11, 6,1–21 (2011).
Hays, W. L. Statistics. (Holt, Rinehart & Winston, 1988).
Anderson, H. A., Glasser, A., Manny, R. E. & Stuebing, K. K. Age-Related Changes in Accommodative Dynamics from Preschool to Adulthood. Invest Ophth Vis Sci 51, 614–622 (2010).
Atchison, D. A., Woods, R. L. & Bradley, A. Predicting the effects of optical defocus on human contrast sensitivity. J Opt Soc Am A 15, 2536–2544 (1998).
Apkarian, P., Tijssen, R., Spekreijse, H. & Regan, D. Origin of Notches in Csf - Optical or Neural. Invest Ophth Vis Sci 28, 607–612 (1987).
Nio, Y. K. et al. Spherical and irregular aberrations are important for the optimal performance of the human eye. Ophthal Physl Opt 22, 103–112 (2002).
Taylor, J., Charman, W. N., O'Donnell, C. & Radhakrishnan, H. Effect of target spatial frequency on accommodative response in myopes and emmetropes. J Vis 9, 16 1–14 (2009).
Nio, Y. K. et al. Age-related changes of defocus-specific contrast sensitivity in healthy subjects. Ophthal Physl Opt 20, 323–334 (2000).
Kay, C. D. & Morrison, J. D. A Quantitative Investigation into the Effects of Pupil Diameter and Defocus on Contrast Sensitivity for an Extended Range of Spatial-Frequencies in Natural and Homatropinized Eyes. Ophthal Physl Opt 7, 21–30 (1987).
Carpenter, R. H. S. Movements of the Eyes. 2nd edn, (London: Pion, 1988).
Huang, C. B., Zhou, Y. F. & Lu, Z. L. Broad bandwith of perceptual learning in the visual system of adults with anisometropic amplyopia. P Natl Acad Sci USA 105, 4068–4073 (2008).
Polat, U., Ma-Naim, T. & Spierer, A. Treatment of children with amblyopia by perceptual learning. Vision Res 49, 2599–2603 (2009).
Polat, U., Ma-Naim, T., Belkint, M. & Sagi, D. Improving vision in adult amblyopia by perceptual learning. P Natl Acad Sci USA 101, 6692–6697 (2004).
Carkeet, A., Leo, S. W., Khoo, B. K. & Eong, K. G. A. Modulation transfer functions in children: Pupil size dependence and meridional anisotropy. Invest Ophth Vis Sci 44, 3248–3256 (2003).
Leat, S. J., Yadav, N. K. & Irving, E. L. Development of Visual Acuity and Contrast Sensitivity in Children. J Optom 2, 19–26 (2009).
Jeon, S. T., Hamid, J., Maurer, D. & Lewis, T. L. Developmental changes during childhood in single-letter acuity and its crowding by surrounding contours. J Exp Child Psychol 107, 423–437 (2010).
Kiorpes, L., Tang, C., Hawken, M. J. & Movshon, J. A. Ideal observer analysis of the development of spatial contrast sensitivity in macaque monkeys. J Vis 3, 630–641 (2003).
Movshon, J. A., Kiorpes, L., Hawken, M. J. & Cavanaugh, J. R. Functional maturation of the Macaque's lateral geniculate nucleus. J Neurosci 25, 2712–2722 (2005).
Li, X. R., Lu, Z. L., Xu, P. J., Jin, J. Z. & Zhou, Y. F. Generating high gray-level resolution monochrome displays with conventional computer graphics cards and color monitors. J Neurosci Meth 130, 9–18 (2003).
Goodman, J. W. Introduction to Fourier optics. 2nd edn, (McGraw-Hill, 1996).
Acknowledgements
We thank Dr. Bo Liang and Dr. Tzvetomir Tzvetanov for critical comments. We also thank Dr. Alexander Baldwin for his very kind help in correcting our English. This research was supported by the National Natural Science Foundation of China (31230032 and 81261120562 to Y.-F.Z., 60808031 to Y.D. and 61205202 to H.Z.) and Frontier Research Foundation of Institute of Optics and Electronics: C09K006 (Y.D.).
Author information
Authors and Affiliations
Contributions
R.L., J.Z., Y.-D.Z. and Y.-F.Z. conceived the experiments. R.L., J.Z., H.Z. and Y.D., performed the experiments. R.L., J.Z. and H.Z. analyzed the data. R.L., J.Z., Y.T. and Y.-F.Z. interpreted the data and wrote the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Liu, R., Zhou, J., Zhao, H. et al. Immature visual neural system in children reflected by contrast sensitivity with adaptive optics correction. Sci Rep 4, 4687 (2014). https://doi.org/10.1038/srep04687
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04687
This article is cited by
-
Age-dependent favorable visual recovery despite significant retinal atrophy in pediatric MOGAD: how much retina do you really need to see well?
Journal of Neuroinflammation (2021)
-
Computational Imaging Prediction of Starburst-Effect Diffraction Spikes
Scientific Reports (2018)
-
Training to improve contrast sensitivity in amblyopia: correction of high-order aberrations
Scientific Reports (2016)
-
High-gain visual feedback exacerbates ankle movement variability in children
Experimental Brain Research (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.