Abstract
Significant efforts have been paid to exploring the fundamental properties of topological insulators (TIs) in recent years. However, the investigation of TIs as functional materials for practical device applications is still quite limited. In this work, electronic sensors based on Bi2Te3 nanoplates were fabricated and the sensing performance was investigated. On exposure to different surrounding environments, significant changes in the conducting properties were observed by direct electrical measurements. These results suggest that nanostructured TIs hold great potential for sensing applications.
Similar content being viewed by others
Introduction
Topological insulators (TIs) are materials with gapped insulating bulks and metallic conducting surfaces1,2. The strong spin-orbit coupling in TIs reduces the probability of the electrons being backscattered by surface defects, which lowers the surface resistivity and leads to the formation of topologically protected surface states3,4. This special spin helical Dirac transport regime makes TIs promising for a number of novel applications such as spintronics and quantum computing4,5,6. The recently discovered three-dimensional (3D) TIs such as Bi2Se3 and Bi2Te3 which are referred to as the second generation of TIs open up new possibilities for device applications2,7,8. This is because these 3D TIs are composed by layered structures in which the adjacent layers are bonded together by weak van der Waals forces, thereby enabling mass production by both top-down mechanical exfoliation9,10 and bottom-up chemical synthesis11,12,13,14. But nowadays the substantial bulk carrier density is still a major challenge, hence enhancing the surface conduction is critical. Making TIs into nanostructures is a simple but effective strategy because the large surface to volume ratios of nano-scaled materials can maximize the surface contribution to the total conduction15,16. So nanostructured TIs may have wider potential applications than bulk single crystals due to the reduced bulk conduction15,17,18.
Device applications do require detailed investigation on the electric and dielectric properties of the material under different conditions, as the practical operating environment can be quite diversified. The chemical doping effect of gas molecules on TIs has drawn much attention because of its potential applications in tuning the transport properties of TI based devices. It has been demonstrated that the energy position of the Dirac point (ED) in TIs can be tuned by dosing with certain gas molecules4,19,20. Reducing gases such as H2 and CO serve as n-type doping agents20 while oxidizing ones such as O2 and NO2 serve as p-type doping agents4. All these experimental evidences are obtained using non-transport, surface sensitive techniques such as angle resolved photoemission spectroscopy (ARPES) and scanning tunneling microscopy (STM)21. However, these techniques are difficult to be used in portable applications because of the large size and high cost of the equipment. Therefore, investigating the material properties by direct electrical measurement is of fundamental importance for device development and innovation.
In this work, single-crystalline Bi2Te3 nanoplates (NPs) are synthesized through a solvothermal route. The crystal structure and growth orientation are analyzed by high resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SEAD) patterns. We have performed two-terminal measurements for the Bi2Te3 nanostructures in different surroundings with the purpose of inspecting whether they are sensitive to the external changes. Interestingly, exposing to different chemical environment dramatically influences the conduction and polarization properties of this material. What's more, we show that these parameters can be reversibly tuned, demonstrating that Bi2Te3 may find use as multifunctional sensing materials.
Results
The hexagonal Bi2Te3 NPs were synthesized by a solvothermal method at 180°C22. The XRD patterns of the as grown Bi2Te3 NPs are shown in Fig. S1, all of the detected peaks are indexed to the rhombohedral Bi2Te3 crystal system (space group: R-3m) with hexagonal cells (JCPDS card No.089-2009). Fig. 1a and b show the typical low-magnification scanning electron microscopy (SEM) images of the as-grown Bi2Te3 NPs. They display regular hexagonal shapes with edge lengths ranging from 300 to 450 nm. An enlarged SEM image of a representative individual Bi2Te3 NP is shown in the inset of Fig. 1b, where a hexagonal-shaped NP can be clearly identified. The maximum diagonal length which is twice the length of the edge is 708 nm and the interior angle is 120°. A typical transmission electron microscopy (TEM) image is shown in Fig. 1c in which the NPs exhibit flat surfaces and sharp edges. A defective NP with one ragged edge was chosen for element mapping by energy dispersive X-ray (EDX) spectroscopy on a scanning transmission electron microscope (STEM). The element maps of the overlapped areas show higher color intensities while that of the other areas are uniform. So the ripple-like patterns in the upper image are most likely resulted from bend of the NP but not overlap or thickness variation. The crystal structure of the as prepared Bi2Te3 NPs was investigated by high-resolution transmission electron microscopy (HRTEM) and SAED patterns. The single-crystalline nature is revealed by the continuous lattice fringes oriented in the same direction in Fig. 1e and the sharp diffraction pattern in Fig. 1g. Bulk Bi2Te3 has a hexagonal primitive cell with parameters a = 4.384 Å and c = 30.45 Å at room temperature23. The d-spacing of 0.22 nm is in good agreement with the { } interplaner spacing of Bi2Te3. The diffraction pattern indicates that <
} interplaner spacing of Bi2Te3. The diffraction pattern indicates that < > is the dominate growth direction and the six side surfaces of the NP belong to the {
> is the dominate growth direction and the six side surfaces of the NP belong to the { } family of crystallographic planes. The top and bottom surfaces are identified as the {0001} facets, which is also the mainly exposed facet. This result is consistent with the layered structure of Bi2Te3 in which the quintuple layer (QL) [Te1-Bi-Te2-Bi-Te1] is the fundamental building block as shown in Fig. 1f. It is considered that the bonding within one QL is covalent while that between adjacent QLs is the van der Waals forces and <0001> is the slowest growth direction23.
} family of crystallographic planes. The top and bottom surfaces are identified as the {0001} facets, which is also the mainly exposed facet. This result is consistent with the layered structure of Bi2Te3 in which the quintuple layer (QL) [Te1-Bi-Te2-Bi-Te1] is the fundamental building block as shown in Fig. 1f. It is considered that the bonding within one QL is covalent while that between adjacent QLs is the van der Waals forces and <0001> is the slowest growth direction23.
Synthesis and characterizations of the hexagonal single-crystalline Bi2Te3 NPs exposed with {0001} facets.
(a, b) Scanning electron microscopy images of the as-grown NPs. The inset in (b) is a representative individual NP. (c) TEM image of the as-grown NPs. (d) TEM image of a defective Bi2Te3 NP (top) and its element maps (bottom). (e) HRTEM image of the Bi2Te3 NP in (d) taken along the [0001] direction, revealing a high quality crystalline structure. The inset is the corresponding fast Fourier transform (FFT) image. (f) Side (left) and top (right) views of the crystal structure of Bi2Te3. A quintuple layer (Te1-Bi-Te2-Bi-Te1) is indicated by a double-headed arrow. (g) Selected area diffraction pattern taken along the [0001] direction.
We performed both two-probe and four-probe measurements on the device and the contact resistance between the probe and the Au electrode was found to be negligible (Fig. S2 in supporting information). So two-probe measurements were conducted for the Bi2Te3 NPs under controlled environments in a metal shielded chamber (Fig. 2a and b) using a semiconductor parameter analyzer (Agilent 4156C with 1 fA current measurement resolution). A schematic of the measurment configuration is sketched in Fig. 2c and a typical photograph of the interdigital electrode is shown in Fig. 2d. The device showed nearly symmetric and linear current-voltage (I–V) characteristics, as shown in Fig. 2e. The conductance (I/V) of the device increased from 5.7 to 10.2 pS as air pressure increased from 10 to 1 × 103 Pa. Afterwards, the chamber was evacuated until the pressure was less than 1 × 10−6 torr and then refilled with high purity nitrogen (99.999%, Linde Gas Co. Ltd.). The above operation was repeated 3 times to drive away the residual air. Under the same pressure of 1 × 103 Pa, the conductance decreased from 10.2 pS in air to 0.67 pS in pure N2. Similar results were obtained as the pressure was set to 10 or 100 Pa, that the conductance of the device in air was over 10 times larger than that in N2 under the same pressure. This result indicates that the conducting property of the Bi2Te3 NPs is sensitive to the chemical content of the surrounding gas atmospheres.
Measurement configuration and electrical test results under different gas environments.
(a) A photograph showing the two-probe configuration. (b) Enlarged image of the selected area in (a). (c) A simplified schematic of the testing method (applying a variable voltage on the device with the current being monitored by a high resolution source monitor unit). (d) A photograph of the interdigital electrode. (e) I–V curves of the device under different gas environments.
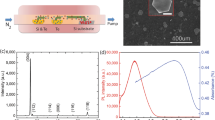
A series of electrical measurements were performed on the device with an impedance analyzer (Agilent 4294A) under increasing relative humidity (RH). The relationships between the impedance (Z) and the corresponding phase angle of the device and RH are shown in Fig. 3(a,b) obtained under a 500 mV alternating voltage. In the same ambient conditions, the impedance decreased with increasing frequency, indicating that the device acted as a capactive load. The impedance changed from 100 kΩ to about 3 kΩ as the RH varied from 11% to 98% at 10 kHz, exhibiting a good sensitivity.
Humidity sensing properties of the sensor.
(a,b,c,d) The dependence of impedance, phase, capacitance and resistance of the sensor on relative humidity measured at various frequencies. (e,f) Transient response of the impedance and capacitance from 43% RH to the corresponding humidities measured at 1 kHz.
In order to explain the enhanced conductivity under high RH, we consider two possible mechansims that affect the transport properties of the Bi2Te3 NPs. At relatively low humidities, the change is ascribed to the band bending effect induced by the adsorption of water molecules, which has been experimentally discovered independently using ARPES by several other research groups19,20. The band bending shifts the Dirac point into the occupied states and increases the charge carrier densities at the surface, which results in the decreased impedance. But as the water molecule adsorption progresses, a continuous water layer will be formed at the surface. At this point, proton conduction will play a role in enhancing the total conductivity of the material24,25, which can be written as:

Where l denotes the liquid phase, x and x′ indicate the different spatial positions. There we neglect the contribution of hydroxide (OH−) because its mobility is much smaller than that of the protons26. The relationships between the capacitance and resistance of the device and RH were also measured and shown in Fig. 3(c,d). The results were obtained using the Cs-Rs measurement mode in which the sensor was treated as a two terminal device under test (DUT) composed by a resistor and a capacitor in series. In contrast to the resistance, the capacitance increases with increasing RH. This is because the capacitance reflects the dielectric property (or polarization property) of the Bi2Te3 NPs. Since water molecule is highly polar, the accumulation of water on the surface of the Bi2Te3 film increases the dielectric coefficient, which accounts for the increased capacitance. The transient responding dynamics of impedance and capacitance of the device are shown in Fig. 3(e,f). The response times (the time for the device to attain 90% of the final value) from 43% RH to 75%, 85% and 98% RHs are all less than 10 s. The transient responding characteristics at low RHs are also shown in Fig. S3. The impedance and capacitance change curves as the RH decreased step by step from 98% to 11% are shown in Fig. S4, showing the device can be used as a full range RH sensor.
To gain further information about the surrounding sensitive properties of the sensors. Ammonia was used as the probe molecule for its explicit n-doping characteristics, which had been widely used to explore the sensing properties of carbon nanotubes27,28, graphene29,30 and molybdenum disulfide31,32. The transient response of the sensor to NH3 (0.05% to 0.4% in air) is shown in Fig. 4a. The response time τres and recovery time τrec are defined as the time required for the sensor to attain 90% of the maximum conductance change after a sudden change in the ammonia concentration. And the gas response is defined as R = Ra/Rg, where Ra and Rg are the resistances of the sensor in air and in the target gas, respectively.
Ammonia sensing characteristics of the sensor.
(a) Response plots show conductance change of the Bi2Te3 NPs versus time with a bias voltage of 5 V at various concentrations (in vol%). (b) Sensor response as a function of NH3 concentration. The black line indicates the fitted line. (c) Response and recovery times of the sensor at different NH3 concentrations. (d) The response curves versus time of three model sensors made from the Bi2Te3 NPs to 0.4 vol% ammonia in air. The void IDE was also measured for comparison.
As shown in Fig. 4b, the response exhibits an almost linear dependence on the NH3 concentration. At relatively low NH3 concentrations, the surface coverage is expected to be much less than a monolayer and the interaction between absorbed NH3 molecules is negligible so it can be described by the Langmuir adsorption model. The fractional coverage θ depends on the ammonia concentration  by means of the binding energy Eb33,34:
by means of the binding energy Eb33,34:

where α = exp(Eb/kBT), in which kB is the Boltzmann's constant and T the absolute temperature. Assuming the number of NH3 gas molecules per unit area under a monolayer coverage is ρ and the charge transfer per NH3 molecule to Bi2Te3 is δ. The number of excess electrons per unit area on the surface of Bi2Te3 is given by Δn = ρθδ. The increased conductance can be expressed as35:

Under low ammonia concentrations,  , so that ΔGs exhibits good linear dependence on NH3 concentration. However, this is only a very simplified model to describe the relationship between the change of conductance and gas concentration. As the concentration increases, both the repulsive interactions between the absorbed NH3 molecules and the change in the carrier mobility can't be neglected19,33,34,35. The ultrafast response speed of this model sensor at room temperature is striking. The response time is 1.2 s at 0.05 vol% and becomes less than 1 s as the concentration is higher than 0.1 vol%, which is much faster than most metal oxides based NH3 sensors, even those working at very high temperatures of 300–600°C36. Another interesting thing is that the response time of this sensor is much shorter than its recovery time as shown in Fig. 4c. The long recovery time indicates that NH3 molecules are strongly bound to the Bi2Te3 surface and desorb very slowly. Both response time and recovery time have a tendency to decrease as the concentration of NH3 increases, which can be explained by the higher adsorption/desorption rate resulted from the larger concentration gradients. In addition, we have fabricated another two sensors based on the Bi2Te3 NPs to test the repeatability of this phenomenon. As shown in Fig. 4d, the three sensors named SN-1, SN-2 and SN-3 all exhibit fast and high response to ammonia but the void IDE doesn't show any response.
, so that ΔGs exhibits good linear dependence on NH3 concentration. However, this is only a very simplified model to describe the relationship between the change of conductance and gas concentration. As the concentration increases, both the repulsive interactions between the absorbed NH3 molecules and the change in the carrier mobility can't be neglected19,33,34,35. The ultrafast response speed of this model sensor at room temperature is striking. The response time is 1.2 s at 0.05 vol% and becomes less than 1 s as the concentration is higher than 0.1 vol%, which is much faster than most metal oxides based NH3 sensors, even those working at very high temperatures of 300–600°C36. Another interesting thing is that the response time of this sensor is much shorter than its recovery time as shown in Fig. 4c. The long recovery time indicates that NH3 molecules are strongly bound to the Bi2Te3 surface and desorb very slowly. Both response time and recovery time have a tendency to decrease as the concentration of NH3 increases, which can be explained by the higher adsorption/desorption rate resulted from the larger concentration gradients. In addition, we have fabricated another two sensors based on the Bi2Te3 NPs to test the repeatability of this phenomenon. As shown in Fig. 4d, the three sensors named SN-1, SN-2 and SN-3 all exhibit fast and high response to ammonia but the void IDE doesn't show any response.
Discussion
We have shown that the electric and dielectric properties of the Bi2Te3 NPs could be significantly changed by the outside environments. The high, fast and reversible response indicates a high possibility and potential of this material for chemical sensing applications. Considering the function of an electronic chemical sensor is to convert a surface molecular binding event into an electric signal, TIs seem to be ideal materials for this task. This is because the conducting channels of an ideal TI exist on the surface which are directly and totally exposed to outside environment. Although the exploration of TIs for sensing is just beginning and the practical application is still quite limited, the intrinsic merits make the technological future of TIs bright. Together with the fast advances in material science and manufacturing technology, multifunctional high performance sensors based on TIs can be highly expected.
Methods
Synthesis of Single-crystalline Bi2Te3 NPs
The synthesis of Bi2Te3 NPs was achieved by a solvothermal method. Typically BiCl3 (1.0 mmol, 0.315 g), Na2TeO3 (1.53 mmol, 0.34 g), NaOH (15 mmol, 0.6 g) and polyvinylpyrrolidone (PVP, 0.5 g) were added to ethylene glycol (EG, 36 mL) under magnetic stirring. After 1 h of stirring, the homogeneous solution was transferred to a 50 mL Teflon-lined stainless autoclave which was then sealed and heated to 180°C and maintained at this temperature for 36 h. After cooling down to room temperature, the resulting product was collected by centrifugation and washed with distilled water and ethanol each for 3 times.
Device Fabrication
Alumina ceramic was used as the substrate for its high dielectric strength and excellent chemical stability. The IDEs composed of 10 nm of titanium and 80 nm of gold with a line-width of 80 μm were fabricated by sputtering. The devices were fabricated by dropping the Bi2Te3 NPs dispersion onto the pre-cleaned interdigital electrodes.
Device Characterizations and Gas Sensing Measurements
The current-voltage (I–V) tests were conducted in a four-probe microscopy cryostat (JANIS ST-500) equipped with a turbo-molecular pump. The chamber pressure could be adjusted by a controlled valve. Electrical measurements were performed on a semiconductor parameter analyzer (Agilent E4156C, 1 fA/2 uV resolution). The impedance and capacitance of the device were obtained with an impedance analyzer (Agilent 4294A, 40 Hz to 110 MHz). Open and short calibration were performed with user defined kit before the measurement to ensure accuracy. The ammonia sensing test was carried out on a ZhongKe NS-4003 smart sensor analyzer under a constant voltage of 5 V.
References
Moore, J. Topological Insulators the Next Generation. Nat Phys 5, 378–380 (2009).
Chen, Y. L. et al. Experimental Realization of a Three-Dimensional Topological Insulator, Bi2Te3 . Science 325, 178–181 (2009).
Cha, J. J. & Cui, Y. Topological Insulators the Surface Surfaces. Nature nanotechnology 7, 85–86 (2012).
Hsieh, D. et al. A tunable topological insulator in the spin helical Dirac transport regime. Nature 460, 1101–1105 (2009).
Zhao, Y. H., Hu, Y. B., Liu, L., Zhu, Y. & Guo, H. Helical States of Topological Insulator Bi2Se3. Nano Lett 11, 2088–2091 (2011).
Arakane, T. et al. Tunable Dirac cone in the topological insulator Bi2-xSbxTe3-ySey. Nat Commun 3 (2012).
Zhang, H. J. et al. Topological insulators in Bi2Se3, Bi2Te3 and Sb2Te3 with a single Dirac cone on the surface. Nat Phys 5, 438–442 (2009).
Fu, L., Kane, C. L. & Mele, E. J. Topological insulators in three dimensions. Phys Rev Lett 98, 106803 (2007).
Hong, S. S. et al. Ultrathin Topological Insulator Bi2Se3 Nanoribbons Exfoliated by Atomic Force Microscopy. Nano Lett 10, 3118–3122 (2010).
Goyal, V., Teweldebrhan, D. & Balandin, A. A. Mechanically-exfoliated stacks of thin films of Bi2Te3 topological insulators with enhanced thermoelectric performance. Appl Phys Lett 97 (2010).
Li, Y. Y. et al. Intrinsic Topological Insulator Bi2Te3 Thin Films on Si and Their Thickness Limit. Adv Mater 22, 4002–4007 (2010).
Hong, S. S., Cha, J. J., Kong, D. & Cui, Y. Ultra-low carrier concentration and surface-dominant transport in antimony-doped Bi(2)Se(3) topological insulator nanoribbons. Nat Commun 3, 757 (2012).
Kong, D. S. & Cui, Y. Opportunities in chemistry and materials science for topological insulators and their nanostructures. Nat Chem 3, 845–849 (2011).
Chen, X., Ma, X. C., He, K., Jia, J. F. & Xue, Q. K. Molecular Beam Epitaxial Growth of Topological Insulators. Adv Mater 23, 1162–1165 (2011).
Zhang, H. B. et al. Magnetoresistance switch effect of a Sn-doped Bi2Te3 topological insulator. Adv Mater 24, 132–136 (2012).
Zhang, H. B., Yu, H. L. & Yang, G. W. Experimental evidence of the nanoscaled topological metallic surface state of Bi2Te3 and Sb2Te3 films. Epl-Europhys Lett 95, 56002 (2011).
Cha, J. J., Koski, K. J. & Cui, Y. Topological insulator nanostructures. Phys Status Solidi-R 7, 15–25 (2013).
Xiu, F. X. et al. Manipulating surface states in topological insulator nanoribbons. Nature nanotechnology 6, 216–221 (2011).
Benia, H. M., Lin, C., Kernm, K. & Ast, C. R. Reactive chemical doping of the Bi2Se3 topological insulator. Phys Rev Lett 107, 177602 (2011).
Zhou, B. et al. Controlling the carriers of topological insulators by bulk and surface doping. Semicond Sci Tech 27, 124002 (2012).
Hasan, M. Z. & Kane, C. L. Colloquium: Topological insulators. Rev Mod Phys 82, 3045–3067 (2010).
Zhang, Y., Hu, L. P., Zhu, T. J., Xie, J. & Zhao, X. B. High yield Bi2Te3 single crystal nanosheets with uniform morphology via a solvothermal synthesis. Cryst Growth Des 13, 645–651 (2013).
Lu, W., Ding, Y., Chen, Y., Wang, Z. L. & Fang, J. Bismuth telluride hexagonal nanoplatelets and their two-step epitaxial growth. J Am Chem Soc 127, 10112–10116 (2005).
Wan, Q. et al. Positive temperature coefficient resistance and humidity sensing properties of Cd-doped ZnO nanowires. Appl Phys Lett 84, 3085–3087 (2004).
Yamamoto, S. et al. In situ x-ray photoelectron spectroscopy studies of water on metals and oxides at ambient conditions. J Phys-Condens Mat 20, 184025 (2008).
Gregori, G., Shirpour, M. & Maier, J. Proton Conduction in Dense and Porous Nanocrystalline Ceria Thin Films. Adv Funct Mater 23, 5861–5867 (2013).
Kong, J. et al. Nanotube molecular wires as chemical sensors. Science 287, 622–625 (2000).
Modi, A., Koratkar, N., Lass, E., Wei, B. Q. & Ajayan, P. M. Miniaturized gas ionization sensors using carbon nanotubes. Nature 424, 171–174 (2003).
Schedin, F. et al. Detection of individual gas molecules adsorbed on graphene. Nat Mater 6, 652–655 (2007).
Fowler, J. D. et al. Practical Chemical Sensors from Chemically Derived Graphene. Acs Nano 3, 301–306 (2009).
Lee, K., Gatensby, R., McEvoy, N., Hallam, T. & Duesberg, G. S. High Performance Sensors Based on Molybdenum Disulfide Thin Films. Adv Mater 25, 6699–6702 (2013).
Late, D. J. et al. Sensing Behavior of Atomically Thin-Layered MoS2 Transistors. Acs Nano 7, 4879–4891 (2013).
Romero, H. E. et al. Adsorption of ammonia on graphene. Nanotechnology 20, 245501 (2009).
Gautam, M. & Jayatissa, A. H. Graphene based field effect transistor for the detection of ammonia. J Appl Phys 112, 064304 (2012).
Checkelsky, J. G., Hor, Y. S., Cava, R. J. & Ong, N. P. Bulk Band Gap and Surface State Conduction Observed in Voltage-Tuned Crystals of the Topological Insulator Bi2Se3 . Phys Rev Lett 106, 196801 (2011).
Timmer, B., Olthuis, W. & Berg, A. v. d. Ammonia sensors and their applications—a review. Sens Actuators B Chem 107, 666–677 (2005).
Acknowledgements
The authors would like to thank Prof. Qi-Kun Xue from Tsinghua University for helpful discussions about the device application of topological insulators. This work was financially supported by the National Basic Research Program of China (Grant No. 2007CB310500) and the National Science Foundation of China (Grant No. 61376073).
Author information
Authors and Affiliations
Contributions
B.L., Q.L. and T.W. conceived the experiments. D.W. and L.W. carried out material synthesis. D.C and H.L. carried out material characterizations. Y.L. carried out the device fabrication. B.L. Y.W. and W.X. implemented electrical measurements and analysis. All authors discussed the results and contributed to writing of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Surrounding Sensitive Electronic Properties of Bi2Te3 Nanoplates-Potential Sensing Application of Topological Insulators
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported license. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Liu, B., Xie, W., Li, H. et al. Surrounding Sensitive Electronic Properties of Bi2Te3 Nanoplates—Potential Sensing Applications of Topological Insulators. Sci Rep 4, 4639 (2014). https://doi.org/10.1038/srep04639
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04639
This article is cited by
-
Investigation on the structural and magnetic properties of MxBi2–xTe3 (M = Gd, Fe, Cr) (x = 0, 1) using colloidal hot-injection method
Bulletin of Materials Science (2022)
-
Effect of carbon co-doping on magnetic properties of transition and rare-earth metal-doped bismuth telluride
Bulletin of Materials Science (2022)
-
Nanoscopic diffusion of water on a topological insulator
Nature Communications (2020)
-
Band Structure of Topological Insulator BiSbTe1.25Se1.75
Scientific Reports (2017)
-
PVP-Assisted Solvothermal Synthesis of High-Yielded Bi2Te3 Hexagonal Nanoplates: Application in Passively Q-Switched Fiber Laser
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.