Abstract
Hydrogen sulfide (H2S) is an endogenously produced gaseous signalling molecule with multiple biological functions. In order to visualize and quantify the endogenous in situ production of H2S in living cells, here we developed two new sulphide ratiometric probes (SR400 and SR550) based on fluorescence resonance energy transfer (FRET) strategy for live capture of H2S. The FRET-based probes show excellent selectivity toward H2S in a high thiol background under physiological buffer. The probe can be used to in situ visualize cysteine-dependent H2S production in a chiral-sensitive manner in living cells. The ratiometric imaging studies indicated that D-Cys induces more H2S production than that of L-Cys in mitochondria of human embryonic kidney 293 cells (HEK293). The cysteine mimics propargylglycine (PPG) has also been found to inhibit the cysteine-dependent endogenous H2S production in a chiral-sensitive manner in living cells. D-PPG inhibited D-Cys-dependent H2S production more efficiently than L-PPG, while, L-PPG inhibited L-Cys-dependent H2S production more efficiently than D-PPG. Our bioimaging studies support Kimura's discovery of H2S production from D-cysteine in mammalian cells and further highlight the potential of D-cysteine and its derivatives as an alternative strategy for classical H2S-releasing drugs.
Similar content being viewed by others
Introduction
Hydrogen sulfide (H2S) is an important endogenous signalling molecule along with nitric oxide (NO) and carbon monoxide (CO)1,2,3. H2S has also been demonstrated to exert protective effects such as preservation of mitochondrial function, protection of neurons from oxidative stress, inhibition of apoptosis, intervention of neuronal transmission, regulation of inflammation, stimulation of angiogenesis, reduce blood pressure, etc4,5,6,7. The deregulation of endogenous H2S is correlated with the symptoms of Alzheimer's disease, Down syndrome, diabetes and liver cirrhosis8,9,10. Kimura et al. indicated that H2S is enzymatic generated from L-cysteine (L-Cys) and its derivatives in vivo7,11. Recently, they further reported a novel possible pathway for the production of H2S from D-cysteine in mammalian cells12. As new biogenesis and biological functions of H2S continue to emerge12,13,14,15, new biocompatible tools to selectively visualize cellular H2S in real-time at its site of release are urgently needed11.
Fluorescent probes could be used for H2S detection in a non-invasive manner in living systems13,14,15,16,17. The probe-based tools have been successfully used to image endogenous H2S production from thiol substrates including L-Cys and glutathione (GSH)13 or by vascular endothelial growth factor stimulation17. Inspired by these work13,17, we are interested to image endogenous H2S production of all possible pathways in living cells semi-quantitatively or quantitatively, based on ratiometric probes rather than intensity-based probes, in view of that ratiometric measurements can cancel out variability caused by uneven loading and distribution of fluorescence probes in cells18.
In order to visualize and quantify the endogenous in situ production of H2S in living cells, here we have developed new ratiometric probes based on fluorescence resonance energy transfer (FRET) for live capture of H2S. The newly developed ratiometric probes revealed the chiral-sensitive cysteine-dependent H2S production and regulation in living cells.
Results
Design and synthesis of ratiometric fluorescent probes
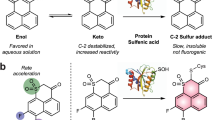
A strategy for ratiometric detection of H2S could be based on modulating FRET change in a two-fluorophore cassette, which contains a FRET donor, a reaction-site-containing FRET acceptor and a rigid spacer between donor and acceptor (Figure 1a). FRET can occur after chemical reaction on the reaction-site of the FRET acceptor. To test the general applicability of the FRET-based strategy, we employed two FRET pairs (coumarin-rhodamine and sulforhodamine-naphthorhodamine) for construction of FRET-based probes.
Chang14 and Wang15 took advantage of the unique reduction of an azide moiety by H2S in the probe design. To this end, we employed azido group as reaction groups on the FRET acceptor. As shown in Figure 1b, the double azide groups in FRET-based probes force the acceptor to adopt a closed and non-fluorescent lactone form and only FRET donor emission can be observed. Upon selective reaction of azido group with H2S to generate the open and fluorescent moiety, excitation of donor should result in increased acceptor emission and decrease in donor emission by FRET. Furthermore, the density function theory (DFT) calculations showed that the energy difference of HOMO and LUMO for coumarin moiety is lower than that of azido-capped rhodamine moiety, but significantly higher than that of rhodamine moiety (see SI), suggesting that FRET could occur between coumarin and rhodamine rather than azido-capped rhodamine.
Probes SR400 and SR550 could be conveniently prepared by coupling reactions of FRET donor (4), piperazyl linker and azido-capped FRET-acceptor (6) (Fig. 2, S1). The structural characterization of the probes was confirmed by 1H NMR, 13C NMR and HR-MS (see SI).
Fluorescent measurements of the probes
Following chemical synthesis and characterization, we tested the fluorescence response of the reaction between FRET-based probes and H2S (using Na2S as an equivalent) in simulated physiological conditions (PBS buffer, pH 7.4). As expected, the optical properties of the FRET-based probes are dominated by the FRET-donor fluorophore (Fig. 3). The fluorescent quantum yields for SR400 and SR550 are 0.18 and 0.08, respectively. The molar extinction coefficients at the excitation wavelength for SR400 and SR550 are 9800 and 7000 M−1cm−1, respectively. Upon treatment with H2S, significant FRET-acceptor fluorescence from SR400 or SR550 could be observed upon excitation under FRET-donor wavelength. At the same time, the FRET-donor fluorescence decreases, implying that FRET occurs from the donor to the acceptor.
FRET-based probes react with sulfide to give ratiometric fluorescence response.
(a) Fluorescence spectra of SR400 (2 μM) (black line) and its reaction with Na2S (1 mM) (red line) for 1 h in PBS buffer (20 mM, pH 7.4) with excitation at 400 nm. (b) Emission ratio I525 nm/I465 nm of SR400 (2 μM) in PBS (pH 7.4) in the presence of Na2S (1 mM) and the marked biological thiols (Cys, 1 mM or GSH, 5 mM). The reaction was performed at 25°C for 1 h. (c) Fluorescence spectra of SR550 (5 μM) (black line) and its reaction with Na2S (2 mM) (red line) for 2 h in PBS buffer (20 mM, pH 7.4) with excitation at 550 nm. (d) Emission ratio I680 nm/I590 nm of SR550 (2 μM) in PBS (pH 7.4) in the presence of Na2S (1 mM) and the marked biological thiols (Cys, 1 mM or GSH, 5 mM). The reaction was performed at 25°C for 1 h.
A major challenge for H2S detection in biological systems is to develop a highly selective probe that exhibits notably distinctive response to H2S over other cellular molecules including high concentration of biothiols. To investigate the selectivity of FRET-based probes, various biologically relevant species were incubated with probes in PBS buffer to test their fluorescence response. These biologically relevant species include reactive sulfur species (GSH, L-Cys and SO32−), reactive oxygen species (H2O2, OCl−), other cations and anions. The ratio of donor-to-acceptor emission intensities (I525 nm/I465 nm and I675 nm/I590 nm) upon excitation at donor was recorded (Fig. 3, S5). The results showed that only H2S caused a large change in the fluorescence intensity ratio, implying that our FRET probes are highly selective for H2S. Furthermore, SR400 was pH-insensitive over a biologically relevant pH range (pH 5.9–8.5) (Fig. S6). In our biomaging experiments, the addition of both probe and Na2S resulted in significant increase of FRET-acceptor fluorescence compared with the probe-only treated cells (Fig. S7). Therefore, the new FRET-based ratiometric probes can be used to detect intracellular H2S.
Imaging of endogenous H2S production and inhibition
To test if our developed FRET-based probes could detect endogenous production of H2S from living cells, we selected human embryonic kidney 293 cells (HEK293), because endogenous H2S can be produced in the cells19,20. Upon treatment of the cell with 200 μM L-Cys or GSH, an enhanced fluorescence responses after 30 min incubation in FRET-acceptor channel (SR400) was observed (Fig. S8). The triggered fluorescence by SR400 could be attributed to enzymatic generation of endogenous H2S from L-Cys or GSH in living cells13. In order to accurately analyze the endogenous production of H2S in living cells, ratiometric imaging was used.
As shown in Figure 4a–e, the good morphology of cells via bright field transmission images suggested the good biocompatibility of the probe. From the ratiometric signal, the production of endogenous H2S from D-Cys is much stronger than that from L-Cys (Fig. S9). The ratiometric fluorescence images for 10 and 100 μM D-Cys pre-incubation (Fig. 4b, 4c) give similar intensity, implying that the endogenous production of H2S under the low concentration D-Cys is already efficient. While for L-Cys pre-stimulation, the ratiometric images show higher intensity for higher L-Cys concentrations (Fig. 4d, 4e). The Cys-stimulation cells were further co-labelled with Mito-tracker red (a well-known mitochondria specific dye) and SR400. The fluorescent co-localization was examined by the merged images of both dyes (Fig. S10, S11). The Pearson coefficient of D-Cys and L-Cys pre-stimulated cells are 0.795 and 0.681, respectively (Fig. S12). These results revealed that Cys-dependent H2S production occurred majorly in mitochondria.
Cysteine-dependent H2S production/inhibition in living cells can be in situ visualized by the ratiometric probe SR400.
(a) Pseudo-colored ratiometric imaging of HEK293 cells loaded with 1 μM SR400 probe for 30 min. The up and down pictures show fluorescence and bright field transmission images, respectively. (b,c) Cells were pre-stimulated with 10 or 100 μM D-Cys for 30 min, then incubated with 1 μM probe for 30 min. (d,e) Cells were pre-stimulated with 10 or 100 μM L-Cys for 30 min, then incubated with 1 μM probe for 30 min. (f) Cells were incubated with DL-PPG (50 mg L−1) for 20 min first, then incubated with 1 μM probe for 30 min. (g) Cells were incubated with DL-PPG (50 mg L−1) for 20 min first, followed by incubation with 10 μM D-Cys for 30 min and then incubated with 1 μM probe for 30 min. (h) Cells were incubated with DL-PPG (50 mg L−1) for 20 min, followed by incubation with 10 μM L-Cys for 30 min and then incubated with 1 μM probe for 30 min. Scale bar represents 50 μm for all images. (i) Schematic representation of bioimaging results indicates that D-Cys is more efficient to stimulate production of H2S in mitochondria (using bold arrow) than that of L-Cys and both endogenous H2S-production pathways can be inhibited by DL-PPG.
The inhibition experiments were performed by addition of DL-propargylglycine (DL-PPG), an analogue of cysteine as a known inhibitor for CBS and CSE21. The results of ratiometric fluorescence analysis showed the suppression of endogenous H2S production, confirmed the enzyme inactivation by the inhibitor. Both endogenous H2S-production pathways from D-Cys and L-Cys can be inhibited by the inhibitor (Fig. 3g, 3h, S13). We further performed the inhibition of endogenous H2S production from chiral-pure PPG (L-PPG and D-PPG) and found that the inhibition is chiral-sensitive. D-PPG inhibited D-Cys-dependent H2S production more efficiently than L-PPG (Fig. S14), while, L-PPG inhibited L-Cys-dependent H2S production more efficiently than D-PPG (Fig. S15).
Discussion
Efficient methods for selective and sensitive detection of H2S in living systems are urgently required for better understanding its biological functions. The intensity-based fluorescence probes may suffer from multiple factors to influence the accurate analysis of H2S production and regulation in living cells. However, the ratiometric fluorescence probes provide built-in correction for environmental effects to improve the accuracy of bioimaging analysis22,23,24,25,26,27. The reported sulfide ratiometric probes are mainly based on two strategies: 1) a tuneable electronic system, after reaction with H2S, results in a spectroscopic alteration such as blue or red shift in emission. This method may suffer from the relative closeness of the two emission wavelengths from the probe change22,23,24. 2) the interruption of a large conjugate system by H2S addition can produce ratiometric probes with relatively large Stokes shifts. However, this method can only turn-on the signal at the shorter emission wavelength25,26,27. If we employ FRET effect as an alternative strategy for ratiometric H2S probes, 1) the turn-on signal can be at longer emission wavelength (FRET acceptor emission) and 2) the FRET pairs could be chosen flexibly for facile control of two emission wavelengths at specific wavelength ranges. As a result, the photophysical properties of FRET-based ratiometric probes can be tuned on as expected. To our knowledge, no small-molecule ratiometric fluorescent probe based on FRET effect has been reported so far for H2S detection28. In this work, two new FRET-based ratiometric probes were successfully developed as efficient chemical tools for H2S detection in vivo.
Kimura has proposed a D-Cys-dependent H2S biogenesis as a possible new pathway for H2S production in living mammalian cells12. Our newly developed FRET-based ratiometric probes have been tested to visualize this new H2S biogenesis in situ in living cells. Based on our confocal experiments, D-Cys stimulated the cells to produce higher concentration H2S in short time than that of L-Cys, supporting Kimura's hypothesis that D-Cys may be immediately metabolized to produce H2S in vivo12. Our co-localization experiments based on co-staining the cells with Mito-tracker red and H2S-reactive SR400 implied that both L-Cys- and D-Cys-dependent H2S production majorly occurs in mitochondria. Furthermore, we firstly find that PPG could inhibit the endogenous H2S production in a chiral-sensitive manner in living cells. D-PPG inhibited D-Cys-dependent H2S production more efficiently than L-PPG, while, L-PPG inhibited L-Cys-dependent H2S production more efficiently than D-PPG. These results imply that chiral-pure PPG inhibitors could be extremely useful chemical-tools in understanding H2S biology and in probing the roles of H2S in several human diseases.
Compounds that can induce the production of endogenous H2S or act as H2S precursors are considered as potential drugs to protect specific cells from oxidative stress or ischaemia-reperfusion injury3,29. D-Cys may may not be a good drug in some tissues, as D-Cys-dependent H2S production in living cells is very fast, as reveal in this work. However, the derivatives of D-Cys, which can be digested slowly in vivo to produce D-Cys, may act as a new kind of H2S sustained release drugs.
Methods
Synthesis of probes
Detailed description of the synthesis of probes can be found in the Supporting Information. The compounds were characterized by high-resolution mass spectra, 1H and 13C NMR.
Spectroscopic analysis of the probe
Spectroscopic measurements were performed in PBS (20 mM, pH 7.4) buffer. Compounds were dissolved into DMF to prepare the stock solutions with a concentration of 10.0 to 1.0 mM. The UV-visible spectra were recorded on a CARY 100 Bio (Varian, USA). Fluorescence study was carried out using Varian Cary Eclipse spectrophotometer. All measurements were performed in a 3 mL cuvette with 2 mL solution. For SR400, samples were excited at 400 nm with excitation and emission slit widths of 5 nm. For SR550, samples were excited at 550 nm with excitation and emission slit widths of 10 nm and 20 nm, respectively.
Cell culture
HEK-293 and HeLa cells were cultured at 37°C, 5% CO2 in DMEM/HIGH GLUCOSE (GIBICO) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin and 4 mM L-glutamine. The cells were maintained in exponential growth and then seeded in glass-bottom 35 mm plate at the density about 2 × 104/well. Cells were passaged every 2–3 days and used between passages 3 and 10.
Confocal Imaging Experiments
Cells were imaged on a confocal microscope (Olympus FV1000 UPLSAPO40X) with a 40× objective lens. All images were analyzed with Olympus FV1000-ASW. For living cell imaging, cells were first treated with FRET-based probes at 37°C for 30 min and then incubated with Na2S (50 and 200 μM) for another 30 min. Control cells were only treated with probe at 37°C for 30 min. For double-excitation imaging, emission was collected at blue channel (425–475 nm) and green channel (500–600 nm) with 405 nm and 488 nm excitation, respectively, for SR400; red channel (550–600 nm) and near-infrared (NIR) channel (640–740 nm) with 543 nm and 633 nm excitation, respectively, for SR550.
Thiol stimulation and inhibition experiments
HEK293 cells were incubated with thiols (GSH or Cys) for 30 min at 37°C and 5% CO2, washed and then incubated with SR400 for another 30 min. The media was replaced by PBS and cells were imaged immediately after media exchange. For inhibition experiments, cells were pre-incubated with PPG for 20 min and then with Cys for 30 min without media exchange. After washing and incubation with SR400 as descript above, cells were used for imaging immediately. For ratiometric imaging, emission was collected at blue channel (425–475 nm) and green channel (500–600 nm) with only 405 nm excitation.
Co-localization
HEK293 cells were incubated with D-Cys or L-Cys (100 μM) for 30 min, washed and with SR400 (1 μM) for 30 min. The probe-stained cells were further co-stained with Mito-tracker red (0.2 μM) for 20 min. The media was replaced by PBS and cells were imaged with green channel (500–600 nm, excitation at 488 nm) and red channel (600–700 nm, excitation at 543 nm).
References
Abe, K. & Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 16, 1066–1071 (1996).
Wang, R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal. 5, 493–501 (2003).
Li, L., Rose, P. & Moore, P. K. Hydrogen sulfide and cell signaling. Annu. Rev. Pharmacol. Toxicol. 51, 169–187 (2011).
Yang, G. et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322, 587–590 (2008).
Kimura, Y. & Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 18, 1165–1167 (2004).
Elrod, J. W. et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 104, 15560–15565 (2007).
Kimura, H. Hydrogen sulfide: its production, release and functions. Amino Acides 41, 113–121 (2011).
Li, L. & Moore, P. K. Putative biological roles of hydrogen sulfide in health and disease: a breath of not so fresh air? Trends Pharmacol. Sci. 29, 84–90 (2008).
Szabó, C. Hydrogen sulfide and its therapeutic potential. Nat. Rev. Drug Discov. 6, 917–935 (2007).
Eto, K., Asada, T., Arima, K., Makifuchi, T. & Kimura, H. Biochem. Biophys. Res. Commun. 293, 1485–1488 (2002).
Kimura, H. Hydrogen sulfide: its production and fuctions. Exp. Physiol. 96, 833–835 (2011).
Shibuya, N. et al. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 4, 1366–1372 (2013).
Qian, Y. et al. Selective fluorescent probes for live-cell monitoring of sulphide. Nat. Commun. 2, 495–502 (2011).
Lippert, A. R., New, E. J. & Chang, C. J. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J. Am. Chem. Soc. 133, 10078–10080 (2011).
Peng, H. et al. A fluorescent probe for fast and quantitative detection of hydrogen sulfide in blood. Angew. Chem. Int. Ed. 50, 9672–9675 (2011).
Peng, H., Chen, W., Burroughs, S. & Wang, B. Recent advances in fluorescent probes for the detection of hydrogen sulfide. Curr. Org. Chem. 17, 641–653 (2013).
Lin, V. S., Lippert, A. R. & Chang, C. J. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc. Natl. Acad. Sci. USA 110, 7131–7135 (2013).
Ueno, T. & Nagano, T. Fluorescent probes for sensing and imaging. Nat. Methods 8, 642–645 (2011).
Telezhkin, V. et al. Hydrogen sulfide inhibits human BKCa channels. Adv. Exp. Med. Biol. 648, 65–72 (2009).
Sekiguchia, F. et al. Endogenous and exogenous hydrogen sulfide facilitates T-type calcium channel currents in Cav3.2-expressing HEK293 cells. Biochem. Biophys. Res. Comm. 445, 225–229 (2014).
Zhao, W. M., Zhang, J., Lu, Y. J. & Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 20, 6008–6016 (2001).
Yu, F. et al. An ICT-based strategy to a colorimetic and ratiometric fluorescence probe for hydrogen sulfide in living cells. Chem. Commun. 48, 2852–2854 (2012).
Wan, Q., Song, Y., Li, Z., Gao, X. & Ma, H. In vivo monitoring of hydrogen sulfide using a cresyl violet-based ratiometric fluorescence probe. Chem. Commun. 49, 502–504 (2013).
Bae, S. K. et al. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in Parkinson's disease gene knockout astrocytes. J. Am. Chem. Soc. 135, 9915–9923 (2013).
Liu, J. et al. A ratiometric fluorescent probe for biological signaling molecule H2S: fast response and high selectivity. Chem. Eur. J. 19, 4717–4722 (2013).
Wang, X. et al. A near-infrared ratiometric fluorescent probe for rapid and highly sensitive imaging of endogenous hydrogen sulfide in living cells. Chem. Sci. 4, 2551–2556 (2013).
Chen, Y. et al. A ratiometric fluorescent probe for rapid detection of hydrogen sulfide in mitochondria. Angew. Chem. Int. Ed. 52, 1688–1691 (2013).
Wei, C., Wei, L., Xi, Z. & Yi, L. A FRET-based fluorescent probe for imaging H2S in living cells. Tetrahedron Lett. 54, 6937–6939 (2013).
Kamat, P. K. et al. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience 252, 302–319 (2013).
Acknowledgements
This work was supported by the MOST (2010CB126102), NSFC (21332004), SRF for ROCS (SEM), Public Hatching Platform for Recruited Talents of Beijing University of Chemical Technology.
Author information
Authors and Affiliations
Contributions
L.Y. and Z.X. conceived the idea, directed the work and wrote the paper. L.W. and L.Y. designed experiments. F.S., C.W. and L.Y. performed the organic synthesis. L.W. and L.Y. performed the cell-based imaging. B.W. provided the molecular calculation data. All authors contributed to data analysis.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
FRET ratiometric probes reveal the chiral-sensitive cysteine-dependent H2S production and regulation in living cells
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images in this article are included in the article's Creative Commons license, unless indicated otherwise in the image credit; if the image is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the image. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wei, L., Yi, L., Song, F. et al. FRET ratiometric probes reveal the chiral-sensitive cysteine-dependent H2S production and regulation in living cells. Sci Rep 4, 4521 (2014). https://doi.org/10.1038/srep04521
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04521
This article is cited by
-
A new fluorescent probe for colorimetric and ratiometric detection of sulfur dioxide derivatives in liver cancer cells
Scientific Reports (2017)
-
Structural effects of naphthalimide-based fluorescent sensor for hydrogen sulfide and imaging in live zebrafish
Scientific Reports (2016)
-
A Redox-Nucleophilic Dual-Reactable Probe for Highly Selective and Sensitive Detection of H2S: Synthesis, Spectra and Bioimaging
Scientific Reports (2016)
-
A Versatile Multiple Target Detection System Based on DNA Nano-assembled Linear FRET Arrays
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.