Abstract
A novel sodium N-fatty acyl amino acid (SFAAA) surfactant was synthesized using pupa oil and pupa protein hydrolysates (PPH) from a waste product of the silk industry. The aliphatic acids from pupa oil were modified into N-fatty acyl chlorides by thionyl chloride (SOCl2). SFAAA was synthesized using acyl chlorides and PPH. GC-MS analysis showed fatty acids from pupa oil consist mainly of unsaturated linolenic and linoleic acids and saturated palmitic and stearic acids. SFAAA had a low critical micelle concentration, great efficiency in lowering surface tension and strong adsorption at an air/water interface. SFAAA had a high emulsifying power, as well as a high foaming power. The emulsifying power of PPH and SFAAA in an oil/water emulsion was better with ethyl acetate as the oil phase compared to n-hexane. The environment-friendly surfactant made entirely from silkworm pupae could promote sustainable development of the silk industry.
Similar content being viewed by others
Introduction
Recently, widespread environmental problems have become of great concern to producers and consumers, motivating the search for products that have biodegradability and security and are green1. Surfactants are consumed worldwide in large quantities every day and their characteristics have become almost as important to the consumer as their functional performance. The preparation of surfactant compounds relies on naturally occurring renewable feedstock and synthesis via ecologically acceptable technology2. N-Fatty acyl amino acid surfactants, which are one of the preferred choices for food, pharmaceutical and cosmetic applications, can be prepared by chemical methods using renewable raw materials, including amino acids and vegetable oils and were recognized as soon as they were discovered early in the last century3,4,5,6,7,8. A large variety of amino acid/peptide structures and fatty acid chains can vary in their structure, length and number of carbon atoms, which explains their wide structural diversity and different physicochemical and biological properties9,10,11.
Pupae of the silkworm Bombyx mori are an extremely valuable natural resource rich in protein, pupa oil, chitin and other ingredients, among which pupa oil accounts for ~23% and the protein accounts for ~65% of the whole pupa. Large quantities of pupa are turned out owing to the overproduction of silk and silk-based products in China and large amounts are used as dry feed in China. A small amount of the silkworm pupae is used as a functional food to treat adult diseases, including diabetes and high blood pressure and to boost the immune system12,13,14. It has been shown that the utilization of pupal extract can lead to the development of new products, such as antioxidants, cosmetics and anti-aging therapies15. It was reported recently that fermented pupa powder has a protective effect in alcohol-induced hepatotoxicity in a rat model16. There are few studies in the literature, however, of silkworm pupae as raw materials for chemical industries.
The high cost of raw materials, including fatty acids and amino acids, have become a major factor restricting development of the surfactant industry. Combination of amino acids/peptides (hydrophilic groups) and fatty acids (hydrophobic groups) can form amphiphilic structures and has produced molecules with a high level of surface activity17,18,19. Some studies demonstrated the possibility of producing the mixed-chain surfactants known to have superior performance compared to single-chain surfactants20. The fatty acids in vegetable oil are mixed-chain compounds and there is a tendency for researchers to choose vegetable oils for surfactant synthesis. For example, soybean oil was reacted with l-lysine to produce the N-ε-acyl-lysines21. Further, it was reported that the utility of soybean (Glycinemax), corn (maize Zea mays) and olive oils (Olea sp.) as substrates for the synthesis of O-acyl homoserines22 and the use of palm oil (Elaeis sp.) fractions as the raw materials for the synthesis of amino acid surfactants was investigated23. The pupae obtained from the native industrial byproducts should be readily available at relatively low cost.
The highly efficient use of pupae has long been a problem that needed to be solved for sericulture enterprise. This study was aimed at utilizing silkworm pupae as raw materials for the production of sodium N-fatty acyl amino acid (SFAAA) surfactants (e.g. raw materials as the waste products of industrial production of silk from B. mori pupae). Such progress is essential for growth in the use of silkworm pupae as renewable material, promoting the development of sericulture and reaping additional economic benefits; however, the silkworm pupae are very far from being exploited fully. Interdisciplinary collaboration involving chemistry, biochemistry and agriculture is needed to extend the uses of silkworm pupae available as waste products of the silk industry.
Results
Synthesis of SFAAA surfactants
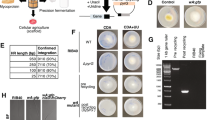
SFAAAs were synthesized as follows. (a) Fatty acids are prepared from pupa oil then modified into N-fatty acyl chlorides by treatment with SOCl2. Pupa proteins were hydrolyzed into a mixture of amino acids. (b) SFAAA was synthesized via the acyl chlorides and the mixture, followed by purification and neutralization with alcoholic sodium hydroxide. The yield of SFAAA was about 65% and a flow chart of the procedure is shown in Figure 1.
Composition of pupa oil
In order to determine the composition of the fatty acids extracted from silkworm pupae, they were derivatized with methanol into fatty acid methyl esters (FAME) as described24. The FAME solution was stable for more than t2 weeks. The composition of fatty acids present in pupa oil was measured on a GC-MS (Agilent J&W scientific, Folsom, CA) and the GC-MS apparatus was linked to a PC running software designed for data acquisition and processing24. Figure 2 shows a chromatogram of the FMAE derived from silkworm pupa oil. Table 1 gives the composition of fatty acids extracted from silkworm pupae and their relative contents.
GC chromatogram of FAME from pupa oil.
The FAME was analyzed by passage through an HP-5MS capillary column (5% phenyl methylsilox: 30 m × 250 μm i.d., film thickness 0.25 μm; Agilent; J&W Scientific, Folsom, CA). A 1 μL sample was injected in split mode (split/column flow ratio 20:1). The column head pressure of the carrier gas (nitrogen) was 200 kPa at the initial oven temperature; flow rate, 1 mL min−1; injection temperature, 280°C; oven temperature 40°C (6 min) increasing to 300°C over a period of 26 min. It was held at this temperature for 6 min. The output from the GC column entered into the ionization chamber of the mass spectrometer via an interface tube maintained at 250°C. Mass spectrometry (ESI, 70 eV, ion source temperature 200°C) was done in full scan mode. Quadrupole scan monitoring was at m/z 50 ~ 780.
Six fatty acid components were identified (Table 1). Both saturated and unsaturated fatty acids were found, including a very high proportion of unsaturated fatty acids, especially linolenic acid (~63.59%) with linoleic acid (~4.12%), stearic acid (~6.26%) and palmitic acid (~25.33%). Other fatty acids (palmitoleic acid ~ 0.17% and heptadecanoic acid ~ 0.09%) constituted <1% of the total fatty acids.
Amino acid composition of PPH and SFAAA
The amino acid composition of synthetic samples and stock materials were determined with an L-8900 High-Speed Amino Acid Analyzer (Hitachi Co. Ltd., Japan) as described31. Table 2 gives the amino acid composition of pupae protein hydrolyzates (PPH) and SFAAA. PPH consists of 17 amino acids, mainly Asp (16.87 mol%), Glu (17.55 mol%), Gly (8.08 mol%) and Ala (8.02 mol%). The SFAAA consists of 16 amino acids, mainly Gly (20.23 mol%), Asp (16.31 mol%), Glu (15.25 mol%) and Ala (7.53 mol%). The glycine content was higher in SFAAA compared to PPH. Among the other essential amino acids found, there was no significant difference between the amounts of PPH and SFAAA. They may be related to the side groups of other amino acids that are larger than glycine, thereby hindering their acylation.
FT-IR analysis
The FT-IR spectra of the pupa oil, fatty acids derived from pupa oil, the PPH and SFAAA surfactant synthesized from silkworm pupae are shown in Figure 3. The FT-IR spectrum of pupa oil was consistent with the structures of a mixture of triglycerides (Figure 3a). A typical ester carbonyl stretch was observed at 1742 cm−1. The peak at 3006 cm−1 (C = C) confirmed the presence of unsaturated carbon atoms, while peaks at 2925 and 2851 cm−1 of significant intensity were characteristic (-CH2 and -CH3 stretching bands) of the presence of long-chain alkyl groups. The formation of the free fatty acids was confirmed by the results shown in Figure 3b. The FT-IR spectrum showed an intense carbonyl stretch at 1711 cm−1 that corresponded to the free carboxylic acid, which replaced the original ester carbonyl stretch of the starting material at 1742 cm−1. The intense signals centered on 1172 cm−1 (C–O stretch) associated with the glyceryl moiety had disappeared. Besides, a very broad O–H stretch associated with the carboxyl group was noted between 3500 and 2500 cm−1. Formation of the acid chlorides was confirmed by the presence of intense carbonyl absorption at 1800 cm−1 and the absence of the broad O–H band of the acid in the region of 3500–2500 cm−1 (Figure 4). Figure 3d shows the FT-IR spectra of the PPH. Two typical peaks were observed at 1645 cm−1 and 1550 cm−1, which were attributed to the amide Ι and amide ΙΙ. Formation of an SFAAA surfactant was confirmed by the presence of the peaks at 1410, 1620 and 1600 cm−1 in Figure 3c, which are attributed to the C–N, C = O and COO− groups, respectively. Besides, it shows an intense C-H stretch at 2925 cm−1 and 2851 cm−1 that corresponds to the long fatty acid chain.
Physicochemical properties of PPH and SFAAA
Amino acids may be divided into polar and non-polar amino acids according to the polarity of the amino acid side chain; in other words, amino acids are ideal natural surfactants and have been used in the cosmetics industry25. In this work, the surface properties of the PPH had been measured.
The surface tension of PPH and SFAAA were measured using Wilhelmy tensiometry and the results of surface tension measurements are shown in Figure 5, where we can see the surface tension of PPH and SFAAA decreases with increases of their concentration. This is typical behavior of surfactants in solution and is widely used to determine purity and critical micelle concentration (CMC) for a surfactant, because the amphiphilic structure leads the surfactant molecules to concentrate at the surface and, thus, reduce the surface tension. However, when the concentration of a surfactant reaches a certain point, the interface is fully occupied by surfactant molecules and, thus, cannot accommodate extra molecules, which is a sign of the formation of micelles in solution. At this moment, the surface tension of the aqueous solutions does not decrease further, which is taken as CMC. The CMC value was determined by measuring the surface tension of a surfactant as a function of concentration. In addition to CMC, physicochemical properties such as surface tension, Γmax, surface excess concentration at CMC; Amin, occupied area per molecule at the CMC and foam height were measured and the results are given in Table 3. As expected, the SFAAA had lower CMC and greater efficiency in lowering the surface tension compared to the PPH and possessed better foaming capacity. The emulsifying power of the PPH and SFAAA in oil/water (O/W) emulsion is illustrated by Figure 6, which shows the emulsifying power of the PPH and SFAAA in O/W emulsion was greater with ethyl acetate as the oil phase compared to benzene and the emulsifying power of the SFAAA was far greater compared to the PPH, indicating PPH and SFAAA were better for emulsification in the polar organic solvent.
Discussion
Vast quantities of silkworm pupae are produced with the over-production of silk products in China. However, the uses for silkworm pupal material is seriously restricted by its offensive smell, which is difficult for humans to accept. There are many procedures that can be used to remove the unpleasant odor26 but the high cost seriously limits their industrial use. Large amounts of silkworm pupae are used as a substantially dry feed in China. Owing to the rich content of unsaturated fatty acids, a small amount of pupae is used commonly as a health food to treat human adult diseases, including diabetes and high blood pressure and to boost the immune system12,13,14. The types of fatty acids present in pupae vary between species; the content of linolenic acid is highest in any variety in which it is necessary for the maintenance of growth27. It had been shown to be a potential inhibitor of aycloxygenase-2 (COX-2) catalyzed prostaglandin biosynthesis28. Some research proved vegetable oil can be used as feedstock for the synthesis of surfactants. However, the higher costs of raw materials, including vegetable oils and amino acids, gradually became a main factor restricting the development of the surfactant industry. The fatty acid chains vary in structure, length and number of carbon atoms. These facts can explain the wide structural diversity and different physicochemical and biological properties of the fatty acid chains9,10,11. The possibility of producing mixed-chain surfactants known to have superior performance over single-chain-length surfactants has been demonstrated20. The silkworm pupa is rich in fatty acids, which can be used as the raw materials for surfactant synthesis; nevertheless, the pupa oil is not used for the synthesis of surfactants. To date, the amino acids produced from pupa proteins have been used for surfactant synthesis29. Industrial pupal waste is a readily available and relatively inexpensive renewable material. In this study, the fatty acids and the proteins extracted from silkworm pupae have been developed into an environment-friendly N-acyl amino acid surfactant.
As is well known, amino acids are natural surfactants, which have been used directly in the cosmetics industry. However, they are not ideal surfactants, however, because they have poor surface properties. The data obtained from this study indicated SFAAA displayed a good surface property when the PPH was conjugated with the fatty acids from the pupa oil. The surfactant prepared entirely from silkworm pupae contains almost no odor. It would be a good choice, therefore, for developing pupae into a surfactant rather than food in the future. Generally, such research efforts could lead to an effective conversion from a cheap, renewable and readily available feedstock into a novel daily chemical product with a high value.
Methods
Preparation of fatty acids
Fresh silkworm pupae were heated in an oven at 80°C for 48 h and then pulverized in a grinder. The dry powder (1 kg) was added to an extraction vessel (20 L), followed by the addition of hexane (4 L) and the mixture was stirred at 40°C for 3 h. At this point, the color of the extraction solution was orange. The organic solvent was recovered by a rotary evaporator and, finally, 230 g of pupa oil (~23% of the dry pupa powder) was obtained. A 200 g sample of the pupa oil was placed into 800 mL of a hot (65°C) ethanolic solution of 2 M NaOH and stirred until the formation of a uniform solution, which was mixed with hot saturated sodium chloride to separate the sodium fatty acid from by-products (such as glycerol). The sodium fatty acid was dissolved in water and adjusted to pH 2 by dropwise addition of concentrated HCl with constant stirring; then it was rinsed with distilled water to remove excess HCl and the fatty acids were collected with ethyl acetate (800 mL); finally, the organic solvent was removed and the extracts were dried with anhydrous Na2SO4. The product was obtained as a waxy solid (190 g, different pupa oils had different yields) with a melting point of 30–37°C.
Preparation of fatty acyl chlorides
The fatty acids derived from pupa oil were converted to the corresponding fatty acyl chlorides essentially as described30 but with slight modification. A 150 g sample of crude fatty acids was dissolved in dichloromethane (DCM, Sigma-Aldrich) and the mixture was stirred mechanically at 15°C in a three-neck flask fitted with a gas scrubber (N2); 75 mL of thionyl chloride (SOCl2, Sigma-Aldrich) was added dropwise over a period of 25 min under reflux conditions; a catalytic amount of the N,N-dimethyl formamide (DMF, Sigma-Aldrich) was added to the flask. After the addition was complete, the mixture was heated gradually to 65°C for 2 h. Considerable darkening of the mixture was noted as the reaction progressed. Upon evaporation of the excess thionyl chloride, the product obtained was a dark brown and fuming liquid. Excess SOCl2 was removed under reduced pressure on a rotary evaporator at 40°C. The product (~140 g, different cocoon varieties had different yields) was used directly in the next step without further purification.
Hydrolysis of pupa proteins
The defatted pupa proteins (100 g) were hydrolyzed at 125°C (0.235 MPa) with 1 L of 1.5 M HCl for 30 min and the hydrolyzates were purified as described31. The resulting lyophilized powder (96 g) was used for synthesis of the SFAAA surfactant.
Preparation of SFAAA
The PPH (50 g) was dissolved in water (20%, w/v), neutralized with aqueous sodium hydroxide (2 M) and adjusted to pH 8–9. The prepared samples were placed into a four-necked flask equipped with an electronic stirrer and a programmable temperature controller (BILON-W-2003T, Tianjin Bilon Lab Equipment Co., Ltd, Tianjin, P. R. China). About 250 mL of acetone was added to the reactor and 50 mL of fatty acyl chloride added dropwise, then sodium hydroxide (2 M) was added to the mixture while maintaining pH ~ 9. The reaction took place at room temperature for 4 h, by which time it was reddish brown and viscous. The reaction mixture was adjusted to pH 1–2 by adding 2 M HCl to induce precipitation after completion of the reaction; then it was filtered, the reddish brown precipitate (N-fatty acyl amino acid) was collected and washed several times with hot water and petroleum ether sequentially; finally, it was dissolved in ethyl alcohol to obtain a red product and the pH of the reaction mixture was adjusted to 7–8 by the addition of alcoholic sodium hydroxide (2 M) with stirring at room temperature for ~30 min. The organic solvent in the reaction mixture was recovered and the product was dissolved in water to obtain the reddish brown product (SFAAA)31. The procedure for the synthesis of an SFAAA surfactant is shown in Figure 7.
FT-IR analysis
The FT-IR spectra of PPH and SFAAA were obtained with a Nicolet 6700 series spectrometer (Thermo, USA) as described31. The spectra were read within the wave-number range between 4000 and 530 cm−1 for each sample in triplicate with a spectral resolution of 4 cm−1. The pupa oil, fatty acids and N-fatty acyl chloride were detected by pipetting 1 μL of those onto the attenuated total reflectance (ATR) crystal at 25 ± 0.1°C maintained with a DC-50-K10 temperature control unit (Thermo, USA). The number of scans per spectrum (n = 64 for solid PPH and SFAAA samples; n = 32 for liquid pupa oil, fatty acids and fatty acyl chloride) was selected according to a sufficient signal-to-noise ratio (main signals/baseline) and a reasonable read time.
Measurements of aqueous solution behavior of the PPH and SFAAA
The surface tension was determined by the Wilhelmy plate method at 25 ± 0.1°C32 using a DCAT21 completely automatic surface tensiometer (Dataphysics, Germany). CMC was assessed at the breakpoint of the linear portions of the plot of surface tension against the logarithm of the PPH and SFAAA concentration. CMC was obtained from the breakpoint of γ – lnc curves (i.e. the minimum concentration of sample solution at which the sample molecules are transformed from unassociated molecules to micelles). The surface tension at this intersection point was named the surface tension at CMC (γCMC). For each concentration, the surface tension was measured in triplicate at intervals of 60 s after 150 s stirring and then averages were calculated.
Γmax taken as the moles of surfactant molecules in the saturated solution of the surface portion is larger than the interior in the unit area before the formation of surfactant micelles. Thus, the arrangement of the surfactant molecules at the air–water interface can be reflected by the maximum surface excess concentration; and Amin, the area occupied by a single surfactant molecule at the air/water interface; both of them can be calculated by applying the following Gibbs adsorption isotherm equation33:

where γ is the surface tension in mN m−1, C is the surfactant concentration and dγ/dlnc is the slope from the plot of γ versus lnc (Figure 4), R is the gas constant (8.31 J mol−1 K−1) and T is the absolute temperature. The occupied area per molecule at the CMC (Amin) was obtained from the saturated adsorption; that is, the Γmax is the surface excess concentration at the CMC:

where NA is Avogadro's number.
Foaming power: foaming properties were determined by the Ross–Miles method according to the international standard ISO696 at 50°C. Foaming production was measured as the height of the foam produced initially; the foam height was measured after 5 min to assess the stability.
Emulsifying power: the performances of the PPH and SFAAA in an O/W emulsion were tested using n-hexane and ethyl acetate as the oil phase, respectively. Equal volumes of samples (0.5 g/L) and organic solvent (n-hexane, ethyl acetate) as the oil phase were mixed individually into a measuring cylinder with plug and vibrated violently for 5 min at 40°C. The tubes were kept for 30 min at 40°C. The volume of the emulsified layer was measured in triplicate and the average value was calculated34.
References
Holmberg, K. [Novel surfactants, Preparation, applications and biodegradability, Second Edition, Revised and Expanded]. Surfactant Science Series [Holmberg, K. (ed.)] (Marcel Dekker, New York, 1998).
Pere, C., Infante, I. & Maria, R. Amino acid-based surfactants: enzymatic synthesis, properties and potential applications. Biocat. Biotrans. 20, 215–233 (2002).
Furutani, T., Ooshima, H. & Kato, J. Preparation of n-, o-diacyl ethanolamine from n-acyl ethanolamine using lipase preparations. Enzy. Microb. Technol. 20, 214–220 (1997).
Gallot, B. & Hassan, H. H. Lyotropic Lipo-Amino-Acids: Synthesis and Structural Study. Mol Cryst. Liq. Cryst. 170, 195–214 (1989).
Godtfredsen, S. E. & Bjoerkling, F. inventors; An enzyme-catalyzed process for preparing N-acyl amino acids and N-acylamino acid amides. European Patent EP 0, 473, 700 (A1) 1992 March 11.
Nagao, A. & Kito, M. Synthesis of o-acyllhomoserine by lipase. J. Am. Oil Chem. Soc. 66, 710–713 (1989).
Nnanna, I. A. & Xia, J. Protein-based Surfactants; Surfactant Science series (Marcel Dekker, New York, 2001).
Valivety, R., Jauregui, P. & Vulfson, E. N. Chem-enzymatic synthesis of amino acid-based surfactants. J. Am. Oil Chem. Soc. 74, 879–886 (1997).
Presenz, P. Lipoamino acids and lipopeptides as amphiphilic compounds. Govi-Verlag, Pharmazie Verlag GmbH Pharmazie 51, 755–758 (1996).
Infante, M. R., Pinazo, A. & Seguer, J. Non-conventional surfactants from amino acids and glycolipids: structure, preparation and properties. Colloid Surface A 123, 49–69 (1997).
Takehara, M. Properties and applications of amino acid based surfactants. Colloids Surface A 38, 149–167 (1989).
Yang, J., Kimura, M., Kuwano, E. & Suzuki, K. Isolation of L(+)-methionine sulfoxide related to diapause induction in the silkworm, Bombyx mori. Insec Biochem. Mol. Biol. 25, 975–980 (1995).
Wu, C., Yamashita, T., Taira, H. & Suzuki, K. Free nucleotides related to the induction of diapause in the silkworm, Bombyx mori. J. Insect. Physiol. 42, 441–447 (1996).
Tamura, Y., Nakajima, K., Nagayasu, K. & Takabayashi, C. Flavonoid 5-glucosides from the cocoon shell of the silkworm, Bombyx mori. Phytochem. 59, 275–278 (2002).
Itoh, M., Hattori, A., Nomura, T., Sumi, Y. & Suzuki, T. Melatonin and arylalkylamine N-acetyltransferase activity in the silkworm, Bombyx mori. Mol. Cell. Endocrinol. 115, 59–64 (1995).
Cha, J., Kim, Y., Moon, H. & Cho, Y. Hepatoprotective effects on alcoholic liver disease of fermented silkworms with Bacillus subtilis and Aspergillus kawachii. Int. J. Food Sci. Nutr, Epub ahead of print 25 August (2011).
Yokota, H., Sagawa, K., Eguchi, C. & Takehara, M. New amphoteric surfactants derived from lysine. I. Preparation and properties of Nε-acyllysine derivatives. J. Am. Oil Chem. Soc. 62, 1716–1719 (1985).
Baschang, G. & Hartmann, A. Aminosulphonic acid derivatives and process for their preparation. United States patent patent US5, 342, 977. 1994 Aug 30.
Braun, D. B. Developments with lipoamino acids and their salts. Cosmet. Toiletries. 104, 87–96 (1989).
Gibson, H. & Holah, J. T. in Preservation of Surfactant Formulations [Morpeth, F. F.(ed.)] (Blackie Academic and Professional, Glasgow, 1995).
Montet, D. et al. Enzymatic of N-ε-acyllysines. J. Am. Oil Chem. Soc. 66, 710–713 (1989).
Nagao, A. & Kito, M. Synthesis of o-acyl-L-homoserine by lipase. J. Am. Oil Chem. Soc. 66, 710–713 (1989).
Soo, E., Salleh, A. B., Basri, M. & Kamaruddin, K. Optimization of the enzyme-catalyzed synthesis of amino acid-based surfactants from palm oil Fractions. J. Biosci. Bioenginer. 95, 361–367 (2003).
Hu, J. W. & Fan, Q. S. Review on Refined Techniques of Protein frOm Silkworm Chrysalis. China Food Addit. 5 (2004).
Pan, W. J., Fang, T. T., Liao, A. M., Zhang, H. Y. & Wei, Z. J. Character and fatty acid composition of silkworm pupa oil. Food Sci. 32, 148–151 (2011).
Rosenfeld, J. M. Application of analytical derivatizations to the quantitative and qualitative determination of fatty acids. Anal. Chim. Acta. 465, 93–100 (2002).
Li, H., Liu, L. & Li, J. S. Synthesis of N-lauroyl amino acid surfactants from the pupa protein. Guang-Xi J. Light Ind. 24, 11–12 (2008).
Gamazo-Vazquez, J., Garcia-Falcon, M. S. & Simal-Gandara, J. Control of contamination of olive oil by sunflower seed oil in bottling plants by GC-MS of fatty acid methyl esters. Food Control. 14, 463–467 (2003).
Ren, Q. Q. & Zhao, T. Synthesis and application of modified vegetable oils in water-repellent finishing of cotton fabrics. Carbohyd. Polym. 80, 381–386 (2010).
Wu, M. H. & Zhang, Y. Q. Nanofiltration recovery of sericin from silk production waste: synthesis and characteristics of lauroyl sericin-based surfactant. RSC Adv. 4, 4140–4145 (2014).
Shiomi, H., Yamada, H. & Nomura, M. Surfactant. 1992 March 11.Japan Patent JP 1, 127, 687 (6A). 1999 October 12.
Biermann, M., Lange, F., Piorr, R., Ploog, U., Rutzen, H. et al. in Surfactants in Consumer Products [Falbe, J. (ed.)] (Springer-Verlag, Heidelberg, 1987).
Rosen, M. J., Cohen, A. W., Dahanayake, M. & Hua, X. Y. Relationship of structure to properties in surfactants. 10. Surface and thermodynamic properties of 2-dodecyloxypoly (ethenoxyethanol)s, C12H25(OC2H4)x OH, in aqueous solution. J. Phys. Chem. 86, 541–545 (1982).
Xia, J. d., Qian, J. h. & Nnanna, I. A. Synthesis and surface properties of amino acid surfactants from industrial waste proteins. J. Agric. Food Chem. 44, 975–979 (1996).
Acknowledgements
The authors gratefully acknowledge the earmarked fund (CARS-22-ZJ0504) for China Agriculture Research System (CARS) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, P. R. China.
Author information
Authors and Affiliations
Contributions
M.H.W. and Y.Q.Z. designed and performed research. M.H.W. performed the experiments in Figures 1–7 and Table 1. L.Z.W. performed the experiments in Tables 2–3. The main manuscript text, L.Z.W. prepared Figures 1–2. Y.Q.Z. revised this manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Wu, MH., Wan, LZ. & Zhang, YQ. A novel sodium N-fatty acyl amino acid surfactant using silkworm pupae as stock material. Sci Rep 4, 4428 (2014). https://doi.org/10.1038/srep04428
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04428
This article is cited by
-
Preparation and surface active properties of coconut and sunflower protein-based diethanolamides
Biomass Conversion and Biorefinery (2016)
-
The Rebirth of Waste Cooking Oil to Novel Bio-based Surfactants
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.