Abstract
Lychnophora salicifolia plants collected from four different places in Brazil (three states: Goias, Minas Gerais and Bahia) revealed a conserved accumulation of vicenin-2, a di-C-glycosyl flavonoid. Quantitative studies by UPLC-MS/MS showed high concentration of vicenin-2 in leaves from sixty specimens of six Lychnophora species. So the tissue distributions of vicenin-2 were evaluated in wild Lychnophora leaves (Asteraceae) by laser based imaging mass spectrometry (IMS) to propose its distributions and possible functions for the species analyzed. Mass spectrometric imaging revealed that vicenin-2, unlike other flavonoids, was produced at the top of the leaves. The combination of localization and UV absorption properties of vicenin-2 suggests that it could act as a UV light barrier to protect the plants, since plants are sessile organisms that have to protect themselves from harsh external conditions such as intense sunlight.
Similar content being viewed by others
Introduction
In 2000, Myers and co-workers published an important paper calling attention for the global biodiversity hotspots and its priorities in conservation programs. Biodiversity hotspots are defined as a habitat with high concentrations of endemic species that are losing rapidly the original area1. Almost 44% of all vascular plants are located in one of the 25 hotspots reported. These habitats occupy only 1.4% of the total land surface of the Earth1. Recently, BIOTA/FAPESP started a successful Brazilian program in combining advance in scientific knowledge with improvement of public policies on biodiversity conservation2. Florist compilation done by the BIOTA program showed Asteraceae as the most diverse plant family of Cerrado, one of the important hotspots of Brazil3. In the campos rupestris (high altitute places) of Cerrado, there are only few herbaceous and arboreous species (see Supplementary Information) and species of the subtribe Lychnophorinae are one of the most abundant3, which showed sesquiterpene and flavonoids as the most representative secondary metabolites4.
In this context, the genus Lychnophora is the most abundant within the subtribe Lychnophorinae, which occurs at high altitudes in rocky fields (campos rupestris), a biome composed of grasses and herbaceous vegetation, where the exposure to UV radiation is intense5,6. In addition, these species show strong endemism and normally they occur in groups of 10 to 20 specimens6. So these small populations of Lychnophora are subjected to specific harsh environmental characteristics, such as high exposure to UV light, alternation between long periods of drought/rain and periodic burnings5,6. The survival characteristic of these plants under these evolutionary pressures can be related to these strong environmental characteristics. Recently, an ecological analysis of Lychnophorasalicifolia showed a correlation between the geographical localization of a sample and its polar metabolites (quantitative effects), such as vicenin-2, but no qualitative differences7. Vicenin-2 (Fig. 1), a di-C-glycosyl flavonoid isolated from L. ericoides, showed significant antioxidant and anti-inflammatory properties8. In a similar ecological study with Lychnophoraericoides from eight different provenances showed an increase in defensive metabolites (by sesquiterpene lactones biosynthesis). This increase was found to be correlated with maximal cytotoxic activities of plants growing at the interface between two types of forest, the campos rupestris and a semi-deciduous forest9. Vicenin-2 was also found to be present in every sample analyzed of L. ericoides, suggesting the conserved accumulation of this secondary metabolite and that there may be a conserved role for this substance9. We therefore set out to identify a physiological role for vicenin-2 mapping its location in the leaves from Brazilian wild Lychnophora plants by subjecting sectioned leaves for Imaging Mass Spectrometry (IMS) and to correlate its tissue distribution with possible physiological functions.
Laser based-IMS (Imaging Mass Spectrometry) was introduced in 1997 by Caprioli and it combines molecular mass analysis and spatial information in tissues10. Initially, this procedure was applied for protein and peptide analyses from animal tissues11. More recently the technique was adapted to capture low molecular weight metabolites (≤1200 Da) from plant tissues12,13,14 applying usually MS mode to analyze intact surfaces, such as the distribution of glucosinolates from foliar intact surfaces of Arabidopsis thaliana13, as well as flavonoids and hypericins from intact leaves and flowers of Hypericum perforatum and H. reflexum without matrix using LDI (Laser Desorption Ionization)-IMS14. However, unreliable data can be produced due to nonspecific method (MS analyses), since plant tissues are complex matrixes that can exhibit metabolites with the same molecular weight15, such as the flavonoids vicenin-2 and tiliroside (3-O-6″-O-(E)-p-coumaroyl-β-glucopyranosyl-kaempferol) present in leaves of L. ericoides, which can only be distinguished based on differences on the fragmentation pathway16. Thus, images created from MS data (using only the ions m/z 595 [M + H]+ and 593 [M − H]− from negative and positive modes) do not reveal the tissue distribution of vicenin-2 only.
The goals of our study are 1) the confirmation of conserved accumulation and high incidence of vicenin-2 in Lychnophora species and 2) to determine the location and thereby to develop a hypothesis for the physiological function of vicenin-2 and other flavonoids by LDI-IMS studies of sections from Lychnophora leaves.
Results
Analyses of extracts from L. salicifolia by LDI-MS
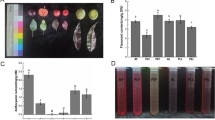
Specimens of L. salicifolia, collected from different regions (Brazilian states), were analyzed by LDI-MS metabolic fingerprinting. Despite the presence of various ions, every plant contained the ion m/z 593 [M − H]− (Fig. 2), which was confirmed to be vicenin-2 by MS/MS data and proved its conserved accumulation in specimens of L. salicifolia. These experiments also demonstrated the ionization of flavonoid vicenin-2 and other metabolites without the need for the addition of a matrix to assist in the ionization of these flavonoids, demonstrating the efficiency of method.
Quantitative analyses by UPLC-MS/MS
Sixty specimens from six species of Lychnophora (L. ericoides, L. pinaster, L. pseudovilosissima, L. salicifolia, L. stavioides and L. vilosissima) were analyzed by UPLC-MS/MS for quantification studies of vicenin-2 in leaves. The concentration of vicenin-2 concentration was approximately 1.4 μg/mg in dried leaves of all species studied (Fig. 3).
Analyses of fresh samples of Lychnophora species and vicenin-2 by LDI-MS and LDI-MS/MS
The fresh leaves from species L. salicifolia, L. ericoides and L. pinaster were extracted with methanol:water (9:1) and analyzed by LDI-MS. In all spectra, the ion m/z 593 [M − H]− was observed, indicating the presence of vicenin-2 in the species analyzed.
In the MS/MS spectrum of vicenin-2, the main fragment ions observed were the following: 575 (2%), 503 (46%), 473 (44%), 455 (3%), 413 (18%), 397 (10%), 383 (100%) and 353 (73%) (Fig. 2). The typical neutral losses from 90 and 120 u yielded by a charge remote mechanism (moiety 0,3X and 0,2X, respectively) were observed. In addition, for vicenin-2 the 0,3X and 0,2X pathway reactions were observed for both rings giving more confidence to the data (Fig. 4).
LDI-MS and MS/MS imaging
The transverse leaf sections of L. ericoides, L. salicifolia and L. pinaster were analyzed by LDI Imaging using MS and MS/MS. In addition, these sections were also investigated by a classical histological approach with Sudan, in order to aid the interpretation of LDI Imaging data and understand the anatomical features of the Lychnophora species studied. L. ericoides, L. salicifolia and L. pinaster showed peculiar leaf anatomy with xeromorphic features, such as the thick cuticles and cell walls, as well as abaxial stomatic crypts containing large amount of stomata and simple trichomes (Fig. 5).
Transverse sections of Lychnophora salicifolia leaf.
LDI-MS/MS(−) image reconstructed from the fragment ions m/z 503, 473 and 383 of vicenin-2 (A) Overview of the leaf - histological analyses by Sudan (B), showing the middle of leaf and its various anatomical features. Optical image (obtained by scanner), used to create the LDI-MS/MS image illustrated in A and details of anatomy (C); the marked regions are corresponding to same regions of A and tissue thickness are illustrated in all images. [Cu: cuticle, lEp: lower epiderm, MD: midrib, uEp: upper epiderm, PP: photosynthetic parenchyma, SC: stomatic crypt, TT: tector trichome).
The LDI-MS and MS/MS IMS confirmed the tissue distribution of vicenin-2, which produced a layer on the top of the leaf (Fig. 5,6,7). Vicenin-2 accumulated in some mesophyll cells and in the epidermis of upper leaf side of L. salicifolia (Fig. 5) and L. pinaster (Fig. 7). In addition, it accumulated from upper up to lower epidermal cells in L. ericoides and in palisade parenchyma cells. Besides it was also observed in the central rib close to the vessel element (xylem) and fiber cell walls for the three species analyzed. The flavonoid aglycones as pinobanksin, chrysin, pinocembrin and pinostrobin displayed different tissue distribution compared to vicenin-2, being identified in the abaxial stomatic crypts of L. ericoides (Fig. 8) inside the head of glandular trichomes, which confirms previous observations17.
Transverse sections of Lychnophora ericoides leaf.
LDI-MS/MS(−) image reconstructed from the fragment ions m/z 503, 473 and 383 of vicenin-2 (A). Overview of the leaf - histological analyses by Sudan (B), showing the middle of leaf and its various anatomical features. Optical image (obtained by scanner), used to create the LDI-MS/MS image illustrated in A and details of anatomy (C); the marked regions are corresponding to same regions of A and tissue thickness are illustrated in all images. [Cu: cuticle, GT: glandular trichome, lEp: lower epiderm, MD: midrib, uEp: upper epiderm, PP: photosynthetic parenchyma, SC: stomatic crypt, TT: tector trichome).
Transverse sections of Lychnophora pinaster leaf.
LDI-MS/MS(−) image reconstructed from the fragment ions m/z 503, 473 and 383 of vicenin-2 (A) Overview of the leaf - histological analyses by Sudan (B), showing the middle of leaf and its various anatomical features. Optical image (obtained by scanner), used to create the LDI-MS/MS image illustrated in A and details of anatomy (C); the marked regions are corresponding to same regions of A and tissue thickness are illustrated in all images. [Cu: cuticle, GT: glandular trichome, lEp: lower epiderm, MD: midrib, uEp: upper epiderm, PP: photosynthetic parenchyma, SC: stomatic crypt, TT: tector trichome).
LDI-MS (+) images of L. ericoides leaves (transversal sections), under the photo tissue, from the pinobanksin (A). chrysin (B), pinocembrin (C) and pinostrobin (D). LDI-MS images of L. ericoides leaves obtained from all identified flavonoid aglycones in positive (E) and negative (F) modes. The marked regions are represented by the histological section (Fig. 6B).
Discussion
The preliminary vicenin-2 occurrence analysis presented in this paper covers an area of more than 750000 km2 from different Brazilian States (São Paulo, Minas Gerais, Goias and Bahia). Initially, a systematic investigation concerning the effects of energy and frequency laser, number of shots and PIE was performed to develop the metabolic fingerprint method by LDI-MS, since this approach had not been explored previously. So a qualitative screening of L. salicifolia specimens was performed and it was consistent with previous investigations7,9,18, confirming the ability of the LDI-MS proposed method to analyze L. salicifolia specimens. Moreover, the flavonoid vicenin-2 was found in all specimens regardless of the origin of the plants.
The quantification of vicenin-2 of sixty specimens from six of Lychnophora species showed a high concentration of vicenin-2 in the leaves (Fig. 3). Because of the common occurrence and abundant quantities of vicenin-2 in all the plants studied, we set out to evaluate the tissue distribution by LDI mass spectrometric imaging to understand their locations and to propose the physiological role of this compound, which has not been previously described. In this context, there is ongoing interest in understanding the main physiological and ecological functions of flavonoids that includes ultraviolet (UV) protection, detoxifying agents, allelopathy, control of auxin transport, defense and pigmentation of flowers to attract pollinators, somatic embryogenesis and pollen tube germination19,20.
To provide insight of the distribution of the flavonoids in the plants, the transverse sections of leaves from the three different species were selected and analyzed by LDI imaging. As previously discussed, generation of images applying MS with absence of fragmentation data is an unspecific method to analyze mixtures of natural products due to its high complexity and the possibility of isobaric ions occurrence. Another important point is the use of time of flight (TOF) analyzer due to its good accuracy and resolution, improving the quality of the results, but no other structural information when the MS mode is used. So systematic MS/MS studies furnish mechanistically and structurally fragmentation information can highly improve the data confidence and quality of MALDI imaging mapping.
In this way, the fragmentation of vicenin-2 revealed distinct daughter ions, the 0,3X and 0,2X pathway reactions observed for both sugar rings21,22 and we subjected the leaves to LDI-MS and MS/MS IMS based on these data. This sequential MS/MS analysis made it possible to measure the spatial distribution by constructing ion intensity maps simultaneously with the structural information based on gas phase decomposition reactions, confirming the site proposed for vicenin-2.
Vicenin-2 produced a layer on the top of the leaves of L. ericoides, L. salicifolia and L. pinaster, which was present in epidermal and parenchyma cells of the upper leaf side, suggesting that it protects against UV radiation. For L. ericoides, the layers of cells between the epidermis (upper and lower) are thinner than the observed in L. salicifolia and L. pinaster and produced by palisade parenchyma only. So, it explains the distribution of vicenin-2 producing a layer on the top of the leaf, which set off from epidermal cells in upper up to lower leaf sides; different distribution in the tissue can be related to the position of the leaves in the plants. Based on the distribution and its absorption coefficient7, vicenin-2 probably acts as a sunlight barrier, mainly against UV-B radiation. The radiation is divided into lower energy UV-A (320–400 nm), higher energy UV-B (280–320 nm) and UV-C (254–280 nm), but UV-B causes the most severe damages23,24. It can induce damage in DNA, RNA and proteins that can affect certain physiological processes, such as photosynthesis25,26. The plant responses to UV-B radiation are mediated by UV-B specific and nonspecific signalling pathways that can include, for example, the production of reactive oxygen species and the increase of secondary metabolites, as flavonoids, which can also act reducing the level of insect herbivory and raising the defense against pathogens27,28,29. The phenolic compounds and flavonoids, located in vacuoles and/or bounded by cell walls, can absorb the radiation and thus reduce the extent of damage, acting as UV-filters25. So the high concentrations of vicenin-2 together with the thick cuticles reported in this study seem to be related to the high solar radiation incidence and high temperature described for the region where these plants have grown.
In addition, vicenin-2 was also observed in central rib close to the vessel element (xylem) and fiber cell walls, probably functioning as a defense against pathogens, since the xylematic cells can be an entry of microorganisms. Beckman (2000) reported that phenolic compounds, such as flavonoids, are stored strategically in specialized cells where they play a direct role in the defense or signaling, which can be infused into tissue, as xylem vessels30.
Other flavonoids showed a different tissue distribution than vicenin-2, suggesting different functions. The flavonoid aglycones (pinobanksin, chrysin, pinocembrin and pinostrobin) have been identified in the abaxial stomatic crypts of L. ericoides inside the head of glandular trichomes, confirming previous observations17. A number of flavonoid aglycones with UV protection functions have been detected in the cuticle19; however, these compounds were not identified in the cuticles of the species studied here. Besides, flavonoids can also act in auxin transport regulation31,32, including glycosides as kaempferol 3-O-rhamnoside-7-O-rhamnoside, an endogenous polar auxin transport inhibitor31. In contrast to this study, the tissue distribution analysis of metabolites is usually done based on nonspecific imaging data obtained, for example, by microscopy and they do not show the location of specific substances in the tissues33. The histological data are obtained with specific reagents (dyes) for certain metabolite classes, but there are drawbacks due to the false results.
To the best of our knowledge, this is the first evidence of a chemical barrier performed by a specific flavonoid for sunlight absorption in plants reported by the simultaneous quantification and structural elucidation of a di-C-glycosyl flavonoid, vicenin-2. It produced a layer on top of the epidermis and together with its UV absorption coefficient suggested that vicenin-2 acts as a UV light barrier to protect the plant from damage. Besides, our study contributes vastly for physiological knowledge about a single flavonoid and its spatial tissue distribution. We have also identified and located specific flavonoids with the support of quantitative studies and structural information based on the fragmentation patterns. The spatial distributions of each metabolite can only be proposed with the rather unusual analysis of transversal leaf sections that can help to understand ecological and physiological functions of secondary metabolites in plants. Previously published approaches to propose flavonoid sites have been based on nonspecific imaging (looking for a group of flavonoids) where data were obtained using microscopy, but our study demonstrated the powerful application of MALDI-IMS to analyze the tissue distribution of metabolites in plant transverse section, stimulating its use for this new approach.
Methods
Analyses of extracts from L. salicifolia by LDI-MS
32 specimens of L. salicifolia were obtained from voucher samples (collected from four different places in Brazil that were located in three states: Goias, Minas Gerais and Bahia) through the herbarium of Campinas State University (São Paulo, Brazil). The collected leaves from voucher specimens were powdered in liquid nitrogen. For screening analysis of extracts by LDI-MS, 5 mg of powdered leaves were extracted with 0.5 mL of MeOH:H2O (9:1, v:v) using a ultrasonic bath (10 minutes). Then the samples were centrifuged at 13,000 rpm (max. speed). Of each voucher specimen three extracts were prepared and 6 mass spectra of each plant extract (1 μL per spot in MTP BigAnchor Chip 384 TF plate of Bruker) were acquired in negative ion mode. A MALDI-TOF/TOF UltrafleXtreme (Bruker Daltonics, Bremen, Germany) instrument was used. The reflector mode was adjusted to 500 Hz in negative ion mode. A PIE (Pulsed Ion Extraction) of 250 ns was used in negative ion mode and 1000 laser shots per spectrum were acquired by the acquisition of 100 shots per position.
Quantitative analyses by UPLC-MS/MS
Sixty specimens of L. ericoides, L. pinaster, L. pseudovilosissima, L. salicifolia, L. stavioides and L. vilosissima were obtained from voucher samples (collected from different states in Brazil: São Paulo, Goias, Minas Gerais and Bahia) of the herbarium of Campinas State University (São Paulo, Brazil). The samples were powdered and extracted with methanol and water (9:1) and used for quantification studies. An ACQUITY UPLC-MS System (Waters Assoc., Milford, MA, USA) was used with an ACQUITY 1.7 μm C18 BEH column (2.1 mm × 50 mm, Waters Assoc., Milford, USA). The mobile phase was acetonitrile (B) and deionized water (A) containing 0.1% acetic acid. The column temperature was maintained at 30°C and the samples were conditioned at 10°C in the automatic injector. The mobile phase flow rate was 0.3 mL/min and injection volume was 5 μL. The extracts were filtered through a 13 mm GH Polypro membrane (0.22 μm pore size). The elution profile was as follows: 0 to 3 min, 3 to 15% B; 1 to 3.5 min, 15 to 100% B; 3.5 to 4.5 min, 100% B; 4.5 to 5 min, 100 to 3% B; and 5 to 6 min (column equilibration), 3% B. The multiple reaction monitoring (MRM) method for vicenin-2 quantification was performed in negative ionization mode with the following parameters: transition monitored at m/z 593 > 353; cone energy, 30 kV; collision energy, 35 eV; and capillary energy, 2.5 kV. Nitrogen was used as nebulizing and drying gas (600 L/h, 350°C) and argon was used as the collision gas. The analytical method was validated and the reliability and credibility of the results were ensured for the quantification of vicenin-2. The appropriate values for the limits of detection (0.5 ng/mL) and quantitation (10.0 ng/mL) were established and the concentration range required for the analysis of the content variability within the analyzed groups of specimens was adequately based on these limits established. The precision and recovery values satisfied the FDA acceptance value criteria.
Analyses of fresh samples of Lchnophora species and vicenin-2 by LDI-MS and LDI-MS/MS
The species L. salicifolia, L. ericoides and L. pinaster were collected in campos rupestris (Cerrado biome - Minas Gerais, Brazil). The analyses by LDI-MS and LDI-IMS were performed using these fresh samples. Firstly, their fresh leaves were powdered in liquid nitrogen and extracted with MeOH:H2O (9:1, v:v) using an ultrasonic bath, subsequently centrifuged and 1 μL was added on a spot of plate (MTP BigAnchor Chip 384 TF plate of Bruker). The method used in the analyses was the same for L.salicifolia, which was previously described, to confirm the presence of vicenin-2 in these fresh samples. Besides, the fragmentation pathway by MALDI-LIFT of vicenin-2 was studied using the same parameters described to LDI-MS/MS imaging.
LDI-MS and MS/MS imaging
The species L. salicifolia, L. ericoides and L. pinaster were collected as previously described. Transverse sections from fresh leaves of L. salicifolia, L. ericoides and L. pinaster were cut using a Leica RM2245 microtome at a thickness of 50 μm. Double-sided tape (3 M Co., USA) was used to adhere these sections to indium tin oxide-coated conductive slides for MALDI analysis (Bruker Daltonics). The sections were also stained with Sudan for histological analyses.
All of the analyses were performed in duplicate in both negative/positive ion modes, including the MS/MS imaging of vicenin-2 in negative ion mode. A MALDI-TOF/TOF UltrafleXtreme (Bruker Daltonics, Bremen, Germany) instrument was used. The following MALDI parameters were used: 110 ns PIE, 1000 Hz laser frequency, reflector mode, 250 shots per position and 500 shots per spectra acquisition. In addition, the ion of vicenin-2 was accelerated to 19 kV in the LIFT cell for MS/MS analyses.
References
Myers, N., Mittermeier, R. A., Mittermeier, C. G., Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Joly, C. A. et al. Biodiversity conservation research, training and policy in São Paulo. Science 328, 1358–1359 (2010).
Forzza, R. C. et al. [As Angiospermas do Brasil]. Catálogo de Fungos e Plantas do Brasil [Forzza, R. C. et al. (ed.)] [78–89] (Instituto de pesquisas Jardim Botânico do Rio de Janeiro, Rio de Janeiro, 2010).
Keles, L. C. et al. Lychnophorinae (Asteraceae): A Survey of its chemical constituents and biological activities. Quim. Nova 33, 2245–2260 (2010).
Simon, M. F. et al. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. PNAS 106, 20359–20364 (2009).
Semir, J., Monge, M., Rezende, A. R. & Lopes, N. P. [Química de Lychnophora] As Arnicas Endêmicas das Serras do Brasil – Uma Visão Sobre a Biologia e Química das Espécies de Lychnophora (Asteraceae) [Souza, G. H. B. & Lopes, N. P. (ed.)] [165–189] (UFOP, Ouro Preto, 2012).
Gouvea, D. R., Meloni, F., Ribeiro, A. B. B., Lopes, J. L. & Lopes, N. P. A new HPLC-DAD-MS/MS method for the simultaneous determination of major compounds in the crude extract of Lychnophora salicifolia Mart. (Brazilian arnicão) leaves: application to chemical variability evaluation. Anal. Chim. Acta 748, 28–36 (2012).
Gobbo-Neto, L. et al. Evaluation of the Anti-inflammatory and antioxidant activities of di-C-glucosylflavones from Lychnophora ericoides (Asteraceae). Planta Med. 71, 3–6 (2005).
Gobbo-Neto, L. et al. Differential metabolic and biological profiles of Lychnophora ericoides Mart. (Asteraceae) from different localities in the Brazilian “campos rupestres”. J. Braz. Chem. Soc. 21, 750–759 (2010).
Caprioli, R. M., Farme, T. B. & Gile, J. Molecular imaging of biological samples: Localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 69, 4751–4760 (1997).
Schwamborn, K. & Caprioli, R. M. Molecular imaging by mass spectrometry - looking beyond classical histology. Nat. Rev. Cancer 10, 639–646 (2010).
Lee, Y. J., Perdian, D. C., Song, Z., Yeung, E. S. & Nikolau, B. J. Use of mass spectrometry for imaging metabolites in plants. Plant J. 70, 81–95 (2012).
Shroff, R., Vergara, F., Muck, A., Svatoš, A. & Gershenzon, J. Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense. PNAS 105, 6196–6201 (2008).
Hölscher, D. et al. Matrix-free UV-laser desorption/ionization (LDI) mass spectrometric imaging at the single-cell level: distribution of secondary metabolites of Arabidopsis thaliana and Hypericum species. Plant J. 60, 907–918 (2009).
Lopes, A. A. et al. A biosynthetic pathway of sesquiterpene lactones in Smallanthus sonchifolius and their localization in leaf tissues by MALDI imaging. Chem. Commun. 49, 9989–9991 (2013).
Gobbo-Neto, L. & Lopes, N. P. Online identification of chlorogenic acids, sesquiterpene lactones and flavonoids in the Brazilian arnica Lychnophora ericoides Mart. (Asteraceae) leaves by HPLC-DAD-MS and HPLC-DAD-MS/MS and a validated HPLC-DAD method for their simultaneous analysis. J. Agric. Food Chem. 56, 1193–1204 (2008).
Gobbo-Neto, L., Gates, P. J. & Lopes, N. P. Negative ion ‘chip-based’ nanospray tandem mass spectrometry for the analysis of flavonoids in glandular trichomes of Lychnophora ericoides Mart. (Asteraceae). Rapid Commun. Mass Spectrom. 22, 3802–3808 (2008).
Moraes, S. L., Gregório, L. E., Tomaz, J. C. & Lopes, N. P. Rapid screening and identification of polar constituents from Brazilian Arnica Lychnophora sp. by LC-UV/DAD-ESI-MS and LC-UV/DAD-ESI-MS/MS analysis. Chromatographia 69, S157–S165 (2009).
Veitch, N. C. & Grayer, R. J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 28, 1626–1695 (2011).
Taylor, L. P. & Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 8, 317–323 (2005).
Domon, B. & Costello, C. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 5, 397–409 (1988).
Waridel, P. et al. Evaluation of quadrupole time-of-flight tandem mass spectrometry and ion-trap multiple-stage mass spectrometry for the differentiation of C-glycosidic flavonoid isomers. J. Chromatog. A 926, 29–41 (2001).
Tevini, M. & Teramura, A. H. UV-B effects on terrestrial plants. J. Photochem. Photobiol. 50, 479–487 (1989).
Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 7, 581–591 (2005).
Schnitzler, P. P. et al. Tissue localization of UV-B screening pigments and of chalcone synthase mRNA in needles of Scots pine seedlings. New Phytol. 132, 247–258 (1996).
Strid, A., Chow, W. S. & Anderson, J. M. UV-B damage and protection at the molecular level in plants. Photosynth. Res. 39, 475–489 (1994).
Jenkins, G. I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 60, 407–431 (2009).
Stratmann, J. Ultraviolet-B radiation co-opts defense signaling pathways. Trends Plant Sci. 8, 526–533 (2003).
Treutter, D. Significance of flavonoids in plant resistance: a review. Environ. Chem. Lett. 4, 147–157 (2006).
Beckman, C. B. Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defense responses in plants? Physiol. Mol. Plant Pathol. 57, 101–110 (2000).
Peer, W. A. et al. Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiol. 126, 536–548 (2001).
Yin, R. et al. Kaempferol 3-O-rhamnoside-7-O-rhamnoside is an endogenous flavonol inhibitor of polar auxin transport in Arabidopsis shoots. New Phytol. 201, 466–475 (2014).
Hutzler, P. et al. Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J. Exp. Bot. 49, 953–965 (1998).
Acknowledgements
We acknowledge FAPESP (Process: 2012/18031-7, 2009/54098-6 and 2010/50811-7 and CNPq (INCT-if) and CAPES for financial support. We thank CGEN/CNPq for a license for biological acess and Prof. Dr. João Semir for the plant identifications. The authors dedicate this paper in memoriam of Profa. Dra. Suely Galdino.
Author information
Authors and Affiliations
Contributions
D.B.S. and N.P.L. designed the research; D.B.S. and I.C.C.T. conducted the imaging experiments; D.B.S. and S.P.T. conducted the histological experiments; D.R.G. collected the quantitative data; M.E. and D.B.S. collected the LDI analyses of extracts; D.B.S. and N.P.L. wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Support Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Silva, D., Turatti, I., Gouveia, D. et al. Mass Spectrometry of Flavonoid Vicenin-2, Based Sunlight Barriers in Lychnophora species. Sci Rep 4, 4309 (2014). https://doi.org/10.1038/srep04309
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04309
This article is cited by
-
Brazilian arnicas: bioactive compounds, pharmacological properties, potential use and clinical applications
Phytochemistry Reviews (2024)
-
A thorough evaluation of matrix-free laser desorption ionization on structurally diverse alkaloids and their direct detection in plant extracts
Analytical and Bioanalytical Chemistry (2020)
-
Development and Characterization of Phytocosmetic Formulations with Saccharum officinarum
Revista Brasileira de Farmacognosia (2020)
-
Metabolomic and gene expression approaches reveal the developmental and environmental regulation of the secondary metabolism of yacón (Smallanthus sonchifolius, Asteraceae)
Scientific Reports (2019)
-
Untargeted LC–MS metabolomic studies of Asteraceae species to discover inhibitors of Leishmania major dihydroorotate dehydrogenase
Metabolomics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.