Abstract
Cerebral asymmetries result from hemispheric specialization and interhemispheric communication pattern that develop in close gene-environment interactions. To gain a deeper understanding of developmental and functional interrelations, we investigated interhemispheric information exchange in pigeons, which possess a lateralized visual system that develops in response to asymmetrical ontogenetic light stimulation. We monocularly trained pigeons with or without embryonic light experience in color discriminations whereby they learned another pair of colors with each eye. Thereby, information from the ipsilateral eye had to be transferred. Monocular tests confronting the animals with trained and transferred color pairs demonstrated that embryonic light stimulation modulates the balance of asymmetrical handling of transfer information. Stronger embryonic stimulation of the left hemisphere significantly enhanced access to interhemispheric visual information, thereby reversing the right-hemispheric advantage that develops in the absence of embryonic light experience. These data support the critical role of environmental factors in molding a functionally lateralized brain.
Similar content being viewed by others
Introduction
Brains of humans and many other animals are characterized by a functional dominance of one hemisphere for different perceptual, motor or cognitive processese.g.1,2. But hemispheric lateralization can only be functional when efficient interhemispheric connections control and mediate the degree and direction of information exchange. The interplay between hemispheric specialization and communication is shaped during development and requires differentiation of specialized intrahemispheric circuits as well as interhemispheric communication systems. While some aspects of asymmetry have a genetic foundation, the mature lateralized pattern only emerges in interaction with epigenetic factors like hormones, environmental stimulation, or social learning2,3,4. These factors develop their effects partly by interhemispheric systems, which thereby acquire own asymmetrical features. The development of specialized intrahemispheric circuits cannot be separated from that of lateralized interhemispheric components but is still poorly understood5,6,7,8,9. Deeper insight requires model systems allowing modulations of their asymmetrical functional organization.
The visual system of birds constitutes an excellent animal model to investigate how epigenetic factors influence visual lateralization10,3,11. Birds like chicks or pigeons demonstrate a pronounced left-hemispheric advantage in discriminating and memorizing visual objects, categorizing, or visuomotor control. In contrast, the right hemisphere dominates visuospatial attention and controls aspects of social behavior like attack, copulation or escape from predators3,11,12.
These behavioral asymmetries can be easily demonstrated just by occluding one eye since the optic nerves of these birds cross virtually completely at the optic chiasm. Functional asymmetries are accompanied by structural left-right differences in the visual pathways3,13. While chicks display transient projection asymmetries within the thalamofugal pathway13, pigeons develop lifelong structural left-right differences within the tectofugal system that mediates foveal vision and controls visuomotor control3. Within this system, retinal input is transferred to the contralateral mesencephalic optic tectum. Bilateral projections ascend to the diencephalic nucleus rotundus, which in turn projects to the ipsilateral telencephalic entopallium. Apart from soma size asymmetries at the tectal and rotundal level20,21,22, there are more fibers crossing from the right tectum to the left rotundus than vice versa14. In contrast, there is no evidence for left-right differences in the number of retinotectal projections. Accordingly, asymmetrical recrossing of tectorotundal fibers results in a more bilateral representation of visual information within the left hemisphere14, allowing it enhanced access to information learnt with the ipsilateral eye11. The better information transfer from the right to the left hemisphere might be related to a left hemispheric dominance in learning and visuomotor control3,15 and enables the integration of hemispheric-specific knowledge16.
Critical (albeit not all) aspects of visual asymmetries develop in response to asymmetrical photic stimulation during development. Due to an asymmetrical position of avian embryos within the egg, light shining through the egg shell stimulates the right eye, while the left eye is visually deprived. The resulting differences in retinal activity induce asymmetrical differentiation processes in left and right hemispheric visual circuits, which ultimately establish the adult functional lateralization pattern17,3,18. As a result, depriving the embryos from light during development prevents the formation of visuomotor as well as anatomical asymmetries in chickens and pigeons13,19,20,21,22. In the altricial pigeon, manipulations of the visual experience after hatching still modify the typical pattern20,23,24,25. These studies show that the final lateralization pattern is not simply the result of activity-dependent differentiation processes within the stronger light stimulated hemisphere; it arises from subtle changes in the balance of left-right development. This suggests that visually driven asymmetry formation affects commissural systems, which in turn mediate interhemispheric communication and information exchange3,18. Accordingly, modulating the embryonic light experiences of pigeon embryos allows investigating in how far the asymmetrical development of intra- and interhemispheric communication systems is interrelated16. In the present study, we investigated the question whether asymmetrical information transfer is controlled by the ontogenetic light conditions.
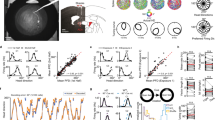
To this end, we trained pigeons with (‘normal’) or without embryonic light experience (‘dark-incubated animals’) on a monocular color discrimination test. Thereby, each hemisphere learnt to discriminate a different pair of colors11. By testing the hemispheric-specific knowledge, animals of both experimental groups have to answer to the color pair learned by the other hemisphere (Figure 1). Therefore, we can estimate the access to transfer information with each hemisphere depending on the embryonic light conditions.
During training, the pigeons learnt to discriminate a different color pair with each eye/hemisphere.
Because of the almost complete crossing of the optic nerves, occlusion of one eye restricted visual input primarily to the contralateral hemisphere. Accordingly, there was a pair of trained (I) and transfer colors (II) for each eye/hemisphere after training. Photograph was taken by M.M.
Results
Monocular testing of a first group of normal pigeons (n = 9) demonstrated a left-hemispheric dominance of transfer performance that confirmed previous data qualitatively11 but not quantitatively (t-test for dependent samples: t = 1.631, p = 0. 142).
In order to verify the observed behavioural asymmetries in normal birds (see below), we decided to test a second group of birds (n = 8). In a first statistical step, we analyzed whether the two normal groups differed in any parameter of performance. Since we found no statistical differences between the groups (neither with parametric t- nor with non-parametric Mann-Whitney-U tests), we pooled the data of normal birds and compared the performances of this group (n = 17) with that of the dark-incubated birds (n = 9).
Monocular training
First, the animals were monocularly trained in color discriminations so that each hemisphere learned another pair of colors. On average, normal animals needed 7 ± 0.5 and dark-incubated pigeons required 9 ± 0.5 sessions to achieve the learning criterion of more than 90% correct responses during three consecutive sessions under each seeing condition. There was no difference in learning speed between groups or hemispheres. After training, both groups demonstrated comparable discrimination accuracies (controls: 96% ± 0.034%; dark-incubated: 95% ± 0.04%) without any difference between the monocular seeing conditions (Figure 2).
Monocular testing
During monocular tests that directly followed training, the animals were confronted with a mixture of trained and transfer color pairs, which were learnt while seeing with the same or the other eye, respectively. Reward contingencies of transfer color pairs had to be transferred at some point in time during or after training. Therefore, performance during the discrimination of transfer colors could detect hemispheric-specific efficiency of accessing transfer information.
Surprisingly, normal animals increased left-hemispheric discrimination of trained color pairs during the testing phase (t-test for dependent samples: t = 2.232, p = 0.040; Cohen's d = 0.644; Figure 2), whereas right-hemispheric performances did not show any differences between training and testing (t-test for dependent samples: t = −0.418, p = 0.681, Cohen's d = 0.109). Dark-incubated animals did not show comparable changes in discrimination accuracy between training and test sessions (t-test for dependent samples: ns; Figure 2).
Comparing discrimination of trained and transfer pairs during the testing phase, normal and dark-incubated birds displayed hemispheric-specific variances (Figures 2, 3) that were analyzed in a 2 × 2 × 2 mixed ANOVA with “group” (normal – dark) as independent factor and “pair-type” (trained - transfer) and “hemisphere” (left-right) as dependent variables. Mean discrimination performance did not differ between the experimental groups (F1, 24 = 0.01, p = 0.942) but discrimination of the trained color pairs was significantly better than that of the transfer pairs (F1, 24 = 11.05, p = 0.0003; partial eta-squared ηp2 = 0.315). Nevertheless, transfer was successful since accuracies of transfer color pairs did not undershoot the learning criterion of 90% even with the minor hemisphere (one sample t-tests: ns). Discrimination accuracy of the respectively better hemisphere even outperformed the learning criterion significantly (one sample t-tests: p < 0.05; Figure 3).
Discrimination accuracy of transfer color pairs during testing expressed as the percentage of trials responded correctly for the left (dark gray) and right (light gray) hemispheres of normal (left columns) and dark-incubated (right columns) pigeons; dashed line indicates the learning criterion of 90% correct choices (asterisks indicate performances significantly higher than 90% criterion according to one sample t-tests).
Bars represent standard errors; * = p < 0.05, ** p < 0.01, ***p < 0.001, (*) = p = 0.05 + Cohen's d > 0.7 according to posthoc comparison.
In general, normal pigeons displayed better discrimination accuracies with the left hemisphere and dark-incubated animals with the right one (interaction “hemisphere” × “group”: F1/24 = 7.80, p = 0.010; ηp2 = 0.245); however, discrimination success also depended on whether the stimuli were learned or transferred (interaction “pair-type” × “hemisphere” × “group”: F1/24 = 6.44, p = 0.018; ηp2 = 0.212). Only the hemispheric-specific discrimination accuracies of transfer color pairs differed between the groups. Left hemispheric discrimination was significantly higher in normal compared to dark-incubated birds (t = 2.886, p < 0.008, Cohen's d = 1.238; Figure 3). Right-hemispheric differences between the groups were on the other hand present but not significant (t = −1.651, p = 0.112, Cohen's d = 0.709; Figure 3). In normal pigeons, left-right differences in discrimination accuracy were substantial for transfer color pairs (t-test for dependent samples: t = 2.043, p = 0.056, Cohen's d = 0.727, Figure 3), but also present for trained color pairs (t-test for dependent samples: t = 2.100, p = 0.052, Cohen's d = 0.484). The left-hemispheric advantage in discriminating transfer color pairs (Figure 4a) was present in most of the individuals (Figure 4b) with a remarkable variation of right hemispheric performances ranging between 43%–100%. Left-hemispheric scores, in contrast, varied only between 86%–100%.
Degree of performance asymmetries for trained and transferred color pairs calculated as: (left-hemispheric performance – right-hemispheric performance)/mean (left- + right-hemispheric performance) *100; negative values indicate a right-hemispheric (RH) and positive values indicate a left-hemispheric (LH) advantage; (a) mean asymmetries of normal (white) and dark-incubated (black) pigeons; (b) individual asymmetries of transfer performance in normal (white) and dark-incubated (black) pigeons.
Bars represent standard errors; * = p < 0.05 according to posthoc comparison in (a).
In dark-incubated birds, discrimination of trained color pairs did not differ between the hemispheres (t-test for dependent samples: t = 0.354, p = 0.732, Cohen's d = 0.113), but transfer pairs were profoundly better discriminated with the right hemisphere (t-test for dependent samples: t = −2.2231, p = 0.057, Cohen's d = 1.110; Figure 3). This led to a degree of asymmetry that was significantly reversed compared to normal pigeons (t = 2.621, p = 0.015, Cohen's d = 1.125; Figures 4a). A right-hemispheric advantage was present in nearly all dark-incubated animals (Figure 4b) whereby the discrimination accuracy ranged from 89% to 100%. The left-hemispheric performance varied between 68% and 97%.
The observed differences in transfer performances were presumably not related to variations in response rates. Although both groups responded during a lower number of trials when confronted with transfer color pairs (normal: 98 ± 2.7% of trained/94 ± 8.5% of transfer trials; dark-incubated: 99 ± 0.4% of trained/92 ± 13.5% of transfer trials; Figure 5). These differences were not significant neither for left- nor right-hemispheric trials (Friedmans ANOVA: normal: ANOVA Chi2 (N = 15, FG = 3) = 6.052, p = 0.905; dark-incubated: ANOVA Chi2 (N = 9, FG = 3) = 0.561, p = 0.109).
Discussion
Pigeons with and without embryonic light experience, show an opposite hemispheric lateralization pattern in accessing transfer information. This demonstrates that the balance of sharing visual information between the hemispheres is shaped by the ontogenetic light experiences. Environmental factors can counteract endogenous developmental tendencies supporting their critical role for the development of both intrahemispheric specialization as well as interhemispheric communication.
Normal pigeons performed better in transfer color pair discriminations when tested with the right eye. These results support an enhanced left-hemispheric access to information that has been primarily experienced with the contralateral right hemisphere and confirms the asymmetry pattern observed by Valencia-Alfonso et al.11. In contrast, dark-incubated pigeons performed better with the right hemisphere and hence, showed a reversed asymmetry of sharing visual information. On the one hand, this is the first example for a functional lateralization that appears without visual experience during embryonic development in pigeons. In chickens, independence from embryonic light stimulation was previously shown for the right hemispheric dominance of novelty detection26,27, visual choice to approach a social partner28, to detour an obstacle29, or prevalently monocular sleep30. So, functional asymmetries are present in dark-incubated birds, but when the embryos have been exposed to light, the endogenous asymmetry pattern can change. Light levels an inherent asymmetry in detouring an obstacle to reach a target29, reverses the left-right direction of eye opening during post-hatching sleep in chicks30,31 and the preferential access to transfer information in pigeons.
A shift in the hemispheric lateralization pattern affects information exchange between the hemispheres6,3,8. Visual processing comprises ascending bottom-up as well as descending top-down pathways and both include commissural projections3. It is therefore critical to disentangle at which level light is effective in modulating lateralized interhemispheric communication. As outlined below, our data suggest that biased visual experience during development influences interhemispheric components of both systems.
It is likely that light input primarily impacts on the development of ascending visual pathways and hence, bottom-up processing17,3,18 since visual experience is a critical factor for the activity-dependent fine tuning of visual circuitse.g.32,33. Thus, embryonic light deprivation might impair basic visual processing within dark-incubated birds or within the less stimulated right hemisphere of normal pigeons. But visual acuity does not differ between the hemispheres34 and dark-incubated pigeons do not display signs of impaired visual perception or simple cognitive abilities22,16. Accordingly, normal and dark-incubated pigeons in our experiments demonstrated no differences in learning speed or discrimination accuracy after training. Moreover, on average both experimental groups displayed successful transfer of conditioned stimulus contingencies. It is rather the efficiency in accessing transfer information that is dependent on the ontogenetic light experiences.
As already mentioned in the introduction, functional dominances are accompanied by structural left-right differences within the tectofugal system in pigeons3,35. A subpopulation of fibers ascending from the mesencephalic tectum to the thalamic nucleus rotundus cross through the ventral part of the diencephalic supraoptic decussation. Thereby, the number of fibers projecting from the right tectum to the left rotundus is essentially higher than vice versa providing the left hemisphere with a more complete representation of information from both eyes14. Integrity of the supraoptic commissure is critical for interocular transfer of pattern, brightness or color discrimination36,37,38. Therefore, the left-hemispheric advantage in accessing transfer information of normal pigeons can be the direct consequence of a stronger bilateral tectorotundal input11.
Structural asymmetries in the visual pathways of pigeons and chickens are shaped by the ontogenetic light conditions39,13,20,21,22,23,19. This suggests that the proportion of crossing tectorotundal fibers could be regulated by asymmetrical photic input. Stronger stimulation of the left hemisphere during embryonic development may promote the growth and/or stabilization of fibers crossing to this brain side, causing the observed enhancement of left-hemispheric access to transfer information. But a direct relation between the degree of bilateral input and efficiency of accessing interhemispheric visual information would imply the presence of an asymmetrical tectorotundal projection in dark-incubated birds, too. Although not tested directly yet, this is questionable since asymmetries of tectal and rotundal soma sizes, which would indicate asymmetries of axodendritic complexity, are missing in light-deprived pigeons22,23,19. Therefore, it is unlikely that lateralized access to transfer information is only caused by asymmetries of ascending projections and hence, simply by the degree of input from the ipsilateral eye.
Emergence of functional asymmetries seems to depend on more complex mechanisms, specifically those affecting processing of information from the ipsilateral eye. Physiological and behavioral data show a differential lateralized transmission of ipsilateral input by each hemisphere40. Cells that respond to ipsilateral stimulation were nearly exclusively detected within the left rotundus, but their number is remarkably small even on the left side41. Moreover, interocular transfer of learnt color discrimination is asymmetrically delayed up to three hours42. These data provide evidence for the action of neuronal mechanisms that regulate access and/or handling of ipsilateral input. Therefore, it is conceivable that asymmetries of these mechanisms might be as important as bottom-up systems for the generation of asymmetrical functional asymmetries.
It is likely that top-down projections mediate these mechanisms. Tectofugal processing is asymmetrically controlled by telencephalic top-down systems that arise in the forebrain41,3,11 and descend towards the midbrain where inhibitory commissural systems regulate hemispheric dominance, communication and cooperation43,3,16. Blocking neuronal activity within the left hemisphere inhibits access to transfer information11 and abolishes dominance of the left hemisphere in making a decision between conflicting information44. If top-down systems are ultimately decisive for the balance of hemispheric-specific access to visual information, their lateralized action might be a secondary but critical consequence of asymmetrical visual stimulation during ontogeny18,17,3.
Ontogenetic plasticity studies demonstrate that monocular modulations of visual experience exert their effects within both brain halves17,3,18. Light stimulation increases visuoperceptual skills in the left hemisphere but reduces right-hemispheric visuomotor speed22. Major effects of unilateral ocular manipulations can even be manifested within the primarily unaffected brain side24,25. Transient silencing of retinal activity enhances the performance of the non-affected eye24. Thus, already during development, it is the balance of left- and right-hemispheric differentiation that reflects the action of asymmetrical visual stimulation. Although light effects must be primarily mediated by ascending visual pathways, permanent asymmetrical effects are only stabilized by linking the action of top-down and interhemispheric systems17,3. As a consequence, critical aspects of visuomotor functioning are shifted to the left hemisphere even counteracting endogenously present asymmetries. These mechanisms allow the left hemisphere a better access to interhemispheric visual information and provide it with an enhanced capacity to integrate information from both left and right visual hemi-fields during visual discrimination45,22 and pecking control46,47. To this regard, the observed left-hemispheric advantage in accessing information learnt by the ipsilateral eye might be related to a dominant role in visuomotor control. In sum, our data indicate that biased visual experience induces structural left-right differences within the ascending visual pathways, but major functional consequences are manifested only at higher cognitive level within systems that regulate the balance of interhemispheric communication and exert executive control onto visuomotor behavior.
Methods
17 adult homing pigeons (Columba livia) of undetermined sex from local breeders (originating from two experimental groups with n = 9 and n = 8) as well as 9 adult dark-incubated animals from lab-own breeding pairs were used for the study. For dark incubation, fertilized eggs from eight pairs of breeding homing pigeons (Columba livia) were incubated in two still-air incubator kept in darkness at constant temperature (38.3°C) and humidity (60–75%) throughout the entire period of incubation. Directly after hatching, the nestlings were banded and swapped with the artificial eggs the breeding birds were sitting on22.
The birds were housed individually and were placed on a 12/12 h light/dark cycle. Animals were maintained on 85%–90% of their free feeding weight throughout the experiments. Food was provided during the experiment and after experimental sessions.
All experiments were performed according to the principles regarding the care and use of animals adopted by the German Animal Welfare Law for the prevention of cruelty to animals as suggested by the European Communities Council Directive of November 24, 1986 (86/609/EEC) and were approved by the animal ethics committee of the Landesamt für Natur, Umwelt und Verbraucherschutz NRW, Germany. All efforts were made to minimize the number of animals used and to minimize their suffering.
Behavioural training and testing
Experiments were performed within a conventional skinner box (32 (w) × 34 (d) × 32 (h) cm) equipped with a house light on the ceiling and two transparent square pecking keys (5 cm × 5 cm) on the front panel located horizontally with a distance of 11.5 cm to side panels and floor of the box. Behind the wall, a flat screen (HP with a resolution of 1024 × 768 Pixel) projected the particular color stimuli onto the transparent pecking keys. Centrically below the pecking keys, a magnetic food hopper delivered food reward (mixed grains) in case of correct responses. Conditioning was enhanced by an additional feeder light that was lit simultaneously with feeder activation located 10 cm above the floor. All programs used during training and testing were programmed with the Matlab-toolbox48.
Monocular color discrimination training
After a hand- and autoshaping procedure where the animals learnt to associate key pecking with food, birds were subjected to a monocular training on a forced choice paradigm. The animals had to discriminate between a rewarded (S+) and a non-rewarded (S−) color displayed simultaneously on the two pecking keys. The four colors (red, green, blue, yellow) used were isoluminant and balanced across subjects and presentation side. Color pairs were presented to one eye while the other one was temporally covered with an eye cap. Owing to the almost total crossing in the visual pathway of the pigeon49, occlusion of an eye restricts visual input primarily to the hemisphere contralateral to the seeing eye. Thus, at the end of the training sessions, each hemisphere had direct experience with only one pair of colors. As a consequence, there was a pair of “trained” (learned with the contralateral eye of a hemisphere) and “transfer” (learned with the ipsilateral eye) colors for each hemisphere11 (Figure 1).
During training sessions, pecking on the rewarded color (S+) led to food access for 3 sec. while pecking on the non-rewarded one (S−) resulted in 20 sec. of darkness. Training sessions with 60 trials per day were performed with daily alternating eyes until the animals reached 90% of correct responses in three successive sessions with each eye. Subsequently, the continuous reinforcement schedule was replaced by a variable ratio (VR 80) in which 80% of trials was rewarded. This adapts animals for the testing phase that included non-rewarded catch trials (see below). Animals were retrained until they reached learning criterion under VR 80 with each eye.
Monocular test sessions
After reaching the learning criterion, three monocular test sessions for each eye were conducted. In these sessions, animals were confronted with 48 “trained” color pairs randomly interspersed with 12 “transfer” color pairs. Responses to transfer stimuli were not rewarded. This allowed comparing monocular performance of trained and transfer color pairs in order to evaluate access to contralaterally learnt information and hence, efficiency in accessing transfer information.
Data analysis
Statistical analysis was carried out using the program Statistica 10 (StatSoft, Tulsa, USA). Normal distribution was evaluated by Kolmogorov–Smirnov and Shapiro-Wilk tests and homogeneity of variance by Levene as well as Brown-Forsythe-tests. Discrimination accuracies were analysed with mixed repeated measures ANOVA. Dependent and independent-t-tests were conducted in cases of significant factor effects. Since sample sizes of the two experimental groups differed substantially, we repeated all analyses with non-parametric statistics. The nonparameteric tests led to the same results demonstrating significant differences between normal and dark-incubated birds only for the left-hemispheric performance (Mann-Whitney U-test: Z = 2.178, p = 0.029) and for asymmetries in discrimination accuracy of transfer color pairs (Mann-Whitney U-test: Z = 2,483, p = 0.013). As the non-parametric results proved to be comparable to the parametric results, we decided to use parametric statistics since they allow analyzing factorial interactions. Pecking activity wasanalyzed by nonparametric Friedmans ANOVAs since data were not normally distributed.
References
Vallortigara, G. & Rogers, L. J. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–589 (2005).
Ocklenburg, S. & Güntürkün, O. Hemispheric asymmetries: the comparative view. Front Psychol. 3, 1–9 (2012).
Manns, M. & Güntürkün, O. Dual coding of visual asymmetries in the pigeon brain: the interaction of bottom-up and top-down systems. Exp. Brain Res. 199, 323–332 (2009).
Concha, M. L., Bianco, I. H. & Wilson, S. W. Encoding asymmetry within neural circuits. Nat. Rev. Neurosci. 13, 832–843 (2012).
Schaafsma, S. M., Riedstra, B. J., Pfannkuche, K. A., Bouma, A. & Groothuis, T. G. Epigenesis of behavioural lateralization in humans and other animals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 915–927 (2009).
Bloom, J. S. & Hynd, G. W. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol. Rev. 15, 59–71 (2005).
van der Knaap, L. J. & van der Ham, I. J. How does the corpus callosum mediate interhemispheric transfer?. Behav. Brain Res. 223, 211–221 (2011).
Hervé, P. Y., Zago, L., Petit, L., Mazoyer, B. & Tzourio-Mazoyer, N. Revisiting human hemispheric specialization with neuroimaging. Trends Cogn. Sci. 17, 69–80 (2013).
Güntürkün, O. & Hausmann, M. The dual coding hypothesis of human cerebral asymmetries. J. Neurol. Sci. (Turk.). 20, 140–150 (2003).
Rogers, L. J. Factors influencing development of lateralization. Cortex. 42, 107–109 (2006).
Valencia-Alfonso, C. E., Verhaal, J. & Güntürkün, O. Ascending and descending mechanisms of visual lateralization in pigeons. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 955–963 (2009).
Rogers, L. J., Vallortigara, G. & Andrew, R. J. Divided brains: the biology and behaviour of brain asymmetries (Cambridge University Press, Cambridge, 2013).
Deng, C. & Rogers, L. J. Prehatching visual experience and lateralization in the visual wulst of the chick. Behav. Brain Res. 134, 375–385 (2002).
Güntürkün, O., Hellmann, B., Melsbach, G. & Prior, H. Asymmetries of representation in the visual system of pigeons. NeuroReport. 9, 4127–4130 (1998).
Verhaal, J., Kirsch, J. A., Vlachos, I., Manns, M. & Güntürkün, O. Lateralized reward-related visual discrimination in the avian entopallium. Eur. J. Neurosci. 35, 1337–1343 (2012).
Manns, M. & Römling, J. The impact of asymmetrical light input onto hemispheric specialization and cooperation. Nat. Commun. 3, 696 (2012).
Manns, M. The epigenetic control of asymmetry formation - lessons from the avian visual system. In: Malashichev, Y. & Deckel, W. (eds.) Behavioral and Morphological Asymmetries in Vertebrates. Landes Bioscience, Georgetown. 13–23 (2006).
Güntürkün, O. & Manns, M. The embryonic development of visual asymmetry in the pigeon. In: Hugdahl, K. & Westerhausen, R. (eds.) The two Halves of the Brain - Information Processing in the Cerebral Hemispheres. MIT Press, Cambridge. 121–142 (2010).
Freund, N., Güntürkün, O. & Manns, M. A morphological study of the nucleus subpretectalis of the pigeon. Brain Res. Bull. 75, 491–493 (2008).
Manns, M. & Güntürkün, O. Monocular deprivation alters the direction of functional and morphological asymmetries in the pigeon's visual system. Behav. Neurosi. 113, 1257–1266 (1999a).
Manns, M. & Güntürkün, O. Light experience induces differential asymmetry pattern of GABA- and parvabumin-positive cells in the pigeon's visual midbrain. J. Chem.Neuroanat. 25, 249–259 (2003).
Skiba, M., Diekamp, B. & Güntürkün, O. Embryonic light stimulation induces different asymmetries in visuoperceptual and visuomotor pathways of pigeons. Behav.Brain Res. 134, 149–156 (2002).
Manns, M. & Güntürkün, O. ‘Natural’ and artificial monocular deprivation effects on thalamic soma sizes in pigeons. NeuroReport. 10, 3223–3228 (1999b).
Prior, H., Diekamp, B., Güntürkün, O. & Manns, M. Posthatch activity-dependent modulation of visual asymmetry formation in pigeons. NeuroReport. 15, 1311–1314 (2004).
Manns, M., Freund, N., Leske, O. & Güntürkün, O. Breaking the balance: ocular BDNF-injections induce visual asymmetry in pigeons. Develop. Neurobiol. 68, 1123–1134 (2008).
Rogers, L. J. Development and function of lateralization in the avian brain. Brain Res. Bull. 76, 235–244 (2008).
Rogers, L. J., Andrew, R. J. & Johnston, A. N. Light experience and the development of behavioural lateralization in chicks III. Learning to distinguish pebbles from grains. Behav. Brain Res. 177, 61–69 (2007).
Andrew, R. J., Johnston, A. N., Robins, A. & Rogers, L. J. Light experience and the development of behavioural lateralisation in chicks. II. Choice of familiar versus unfamiliar model social partner. Behav. Brain Res. 155, 67–76 (2004).
Chiandetti, C., Galliussi, J., Andrew, R. J. & Vallortigara, G. Early-light embryonic stimulation suggests a second route, via gene activation, to cerebral lateralization in vertebrates. Sci. Rep. 3, 2701 (2013).
Mascetti, G. G. & Vallortigara, G. Why do birds sleep with one eye open? Light exposure of the chick embryo as a determinant of monocular sleep. Curr. Biol. 11, 971–974 (2001).
Bobbo, D., Galvani, F., Mascetti, G. G. & Vallortigara, G. Light exposure of the chick embryo influences monocular sleep. Behav. Brain Res. 134, 447–466 (2002).
Berardi, N., Pizzorusso, T., Ratto, G. M. & Maffei, L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 26, 369–378 (2003).
Espinosa, J. S. & Stryker, M. P. Development and plasticity of the primary visual cortex. Neuron. 75, 230–249 (2012).
Güntürkün, O. & Hahmann, U. Visual acuity and hemispheric asymmetries in pigeons. Behav. Brain Res. 60, 171–175 (1994).
Ströckens, F., Freund, N., Manns, M., Ocklenburg, S. & Güntürkün, O. Visual asymmetries and the ascending thalamofugal pathway in pigeons. Brain Struct. Funct. 218, 1197–1209 (2013).
Burkhalter, A. & Cuénod, M. Changes in pattern discrimination learning induced by visual deprivation in normal and commissurotomized pigeons. Exp. Brain Res. 31, 369–385 (1978).
Francesconi, W., Fogassi, L. & Musumeci, D. Interocular transfer of visual discriminations in wulst-ablated pigeons. Behav. Brain Res. 5, 399–406 (1982).
Watanabe, S. Interhemispheric transfer of visual discrimination in pigeons with supraoptic decussation (DSO) lesions before and after monocular learning. Behav. Brain Res. 17, 163–170 (1985).
Rogers, L. J. & Deng, C. Light experience and lateralization of the two visual pathways in the chick. Behav. Brain Res. 98, 277–287 (1999).
Mpodozis, J., Cox, K., Shimizu, T., Bischof, H. J., Woodson, W. & Karten, H. J. GABAergic inputs to the nucleus rotundus (pulvinar inferior) of the pigeon (columba livia). J. Comp. Neurol. 374, 204–222 (1996).
Folta, K., Diekamp, B. & Güntürkün, O. Asymmetrical modes of visual bottom-up and top-down integration in the thalamic nucleus rotundus of pigeons. J. Neurosci. 24, 9475–9485 (2004).
Skiba, M., Diekamp, B., Prior, H. & Güntürkün, O. Lateralized interhemispheric transfer of color cues: evidence for dynamic coding principles of visual lateralization in pigeons. Brain Lang. 73, 254–273 (2000).
Güntürkün, O. & Böhringer, P. G. Lateralization reversal after intertectal commissurotomy in the pigeon. Brain Res. 408, 1–5 (1987).
Freund, N., Brodmann, K., Manns, M. & Güntürkün, O. Pigeons being spoilt for choice: a study on hemispheric dominance. refereed abstract T16-11B, 8th Meeting of the German Neuroscience Society Göttingen, Germany (2009).
Güntürkün, O. & Kesch, S. Visual lateralization during feeding in pigeons. Behav. Neurosci. 101, 433–435 (1987).
Güntürkün, O. Lateralization of visually controlled behavior in pigeons. Physiol. Behav. 34, 575–577 (1985).
Güntürkün, O. & Hoferichter, H. H. Neglect after section of a left telencephalotectal tract in pigeons. Behav. Brain Res. 18, 1–9 (1985).
Rose, J., Otto, T. & Dittrich, L. The biopsychology-toolbox: a free, open-source matlab-toolbox for the control of behavioral experiments. J. Neurosci. Methods. 175, 104–107 (2008).
Weidner, C., Reperant, J., Miceli, D., Haby, M. & Rio, J. P. An anatomical study of ipsilateral retinal projections in the quail using radioautographic, horseradish peroxidase, fluorescence and degeneration techniques. Brain Res. 340, 99–108 (1985).
Acknowledgements
This research was supported by a DFG grant from the German Science Foundation DFG to M.M. and by a Marie Curie Early Stage Research Training Fellowship of the European Community's Sixth Framework Program under contract number MESTCT-2005-020385 to J.V.
Author information
Authors and Affiliations
Contributions
S.L. conducted the experiments, S.L. & M.M. analyzed the data, J.V., N.P. & M.M. planned and supervised the experiments, M.M. wrote the manuscript and all authors reviewed it.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Letzner, S., Patzke, N., Verhaal, J. et al. Shaping a lateralized brain: Asymmetrical light experience modulates access to visual interhemispheric information in pigeons. Sci Rep 4, 4253 (2014). https://doi.org/10.1038/srep04253
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep04253
This article is cited by
-
Pigeons show how meta-control enables decision-making in an ambiguous world
Scientific Reports (2021)
-
Visuospatial attention in the lateralised brain of pigeons – a matter of ontogenetic light experiences
Scientific Reports (2017)
-
A new method to manipulate broiler chicken growth and metabolism: Response to mixed LED light system
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.