Abstract
We demonstrate a simple technique to transfer chemical vapour deposited (CVD) graphene from copper and platinum substrates using a soak-and-peel delamination technique utilizing only hot deionized water. The lack of chemical etchants results in cleaner CVD graphene films minimizing unintentional doping, as confirmed by Raman and electrical measurements. The process allows the reuse of substrates and hence can enable the use of oriented substrates for growth of higher quality graphene and is an inherently inexpensive and scalable process for large-area production.
Similar content being viewed by others
Introduction
Graphene, a monolayer honeycomb lattice structure of sp2-bonded carbon atoms, has become a subject of great interest due to its extraordinary optical, mechanical and electronic properties1,2,3. Successful isolation of graphene by the mechanical exfoliation of highly oriented pyrolytic graphite (HOPG) has opened doors for new innovations in the field of nanoelectronics4,5,6. Since then many new methods have emerged to synthesize and isolate single to few-layer graphene3 especially on large area substrates. These methods include reduction of graphite oxide7, ultrasonication of graphite8, synthesis on SiC substrate9,10 and chemical vapour deposition (CVD) on metal substrates such as Ni11, Cu12,13, Ru14 and Pt15,16.
Of all these methods, the low-pressure growth of graphene on Cu foils, in particular, is known to be advantageous in terms of controlled graphene size, number of layers and quality12. It has also been shown that better quality graphene can be grown on Cu (111) oriented grains17. Thus CVD graphene growth on Cu produces large areas of mostly monolayer graphene and is a promising way of producing large area graphene for practical nanoelectronics applications3,18. To fully realize the advantages of the CVD graphene growth there must be a reliable method for transferring the graphene from metallic Cu substrates to more useful substrates like insulating substrates19, flexible/stretchable substrates20 and transparent electrodes21,22. Pt (111), as a substrate for CVD graphene growth, is also interesting because it has minimum effect on the physical properties of graphene due to its very weak graphene-substrate interaction. Further, it has been shown that the electronic structure of the graphene grown on Pt is nearly the same as that of the free standing graphene23. In addition, Pt does not get oxidized easily like other metal substrates such as Cu.
Currently the processes used to transfer large-area and high-quality graphene synthesized on metal substrates require wet etching of the metal substrates12,24. These processes trap ionic species between graphene and substrate interface which act as scattering centers and lead to degradation of the electrical properties of the devices fabricated on the graphene. Further, the etching process also results in loss of metal ultimately increasing the cost of the transfer process; this is especially true for precious metals and oriented single crystal substrates that are expensive. Electrochemical methods to transfer graphene without metal loss have been demonstrated but they involve chemicals like NaOH and are complex25,26. An intercalation method to transfer graphene from Pt to other substrates has also been shown, but for small size graphene flakes only27. To overcome these problems, we demonstrate a novel facile method to transfer graphene from metal substrates (Cu and Pt) with hot deionized (DI) water without using any chemical etchants. This results in transferred graphene layers that are clean and show improved properties compared to graphene layers transferred using the conventional etching route.
DI water has potential use in transfer processes due to its capability to penetrate nanoscale hydrophobic-hydrophilic interfaces and separate them. Such methods have been used to selectively position21 and transfer28 graphene flakes and other nanostructures using difference in affinity to water. In our method, we first coat the graphene layer with poly(methyl methacrylate) (PMMA) as a support material. PMMA has been used to support and transfer mechanically exfoliated graphene flakes29,30 and CVD-grown graphene to target substrates19. We then exploit the differential interaction of water with the hydrophobic graphene31,32 and the hydrophilic metal like Cu33 or Pt34 to delaminate the graphene from the substrate used for CVD growth.
The most important difference in our DI water Soak-and-Peel Delamination (SPeeD) method and presently established methods to transfer graphene from Cu12,20,22,24,29,30,35,36,37 and Pt substrates26 is that, our SPeeD method is very simple since it does not involve use of any chemical etchants and hence provides cleaner graphene. Additionally the metal substrate (Cu and Pt) can be reused – an aspect desirable for industrial production. As our technique utilizes the difference in the interaction of the graphene and substrates with water, this method can be extended to a larger class of CVD substrates for a variety of applications. The SPeeD technique uses only DI water, hence contamination due to ionic species can be significantly reduced ensuring that the electrical properties are not degraded as typically seen for graphene transferred via processes using chemical etchants to remove the Cu substrate.
Results
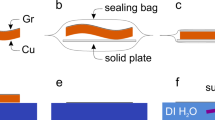
CVD graphene was grown both in continuous12 and island growth38 modes on Cu and Pt substrates (details about the growth provided in Supplementary Section I). The SPeeD transfer process is schematically depicted in Figure 1. After the CVD growth of graphene on Cu foil (Figure 1(a)), we spin-coated the graphene with PMMA (310 nm thick resist (Microchem EL-9) at a speed of 3200 rpm for 45 s) followed by baking for 7 minutes at 175°C (Figure 1(b)). To avoid crumpling of the resist after delamination and for easier handling, Kapton tape (3M 5413) is stuck on the resist and uniform pressure is applied using a teflon roller (Figure 1(c)).
Schematic showing the steps of the DI water SPeeD graphene transfer method.
(a) CVD graphene is grown on Cu substrate. (b) PMMA is spin-coated on the graphene grown on Cu/Pt substrate. (c) Kapton tape is pressed on PMMA with a teflon roller. (d) The stack is immersed in DI water at 90°C. Water penetrates between graphene and Cu substrate and separates them. (e) Kapton tape with PMMA and graphene on it is pressed against the target substrate and heated for 40 minutes at 140°C. (f) The PMMA on the target substrate is removed with acetone and RTA.
The Cu foil, with the tape attached, is then immersed in a beaker of DI water maintained at 90°C for 2 hours (Figure 1(d)). During this period DI water penetrates the graphene-Cu interface. Subsequently, the Kapton tape (to which the PMMA/graphene stack is attached) is slowly peeled away with tweezers leaving behind the Cu foil (see Supplementary Movie S1 which shows the key steps of our SPeeD process). The target substrate, a 300 nm thick SiO2-coated p-type silicon wafer, was cleaned using oxygen plasma reactive ion etching to ensure better adhesion between graphene and the substrate. The Kapton tape, clamped to a glass slide and the target substrate are brought into contact and heated for 40 minutes at 140°C (Figure 1(e)). After allowing the sample to cool for 20 minutes, the Kapton tape is detached from the glass slide and the stack is put in acetone to remove the EL-9 layer. Subsequently, rapid thermal annealing (RTA) (300°C for 10 minutes and 350°C for 5 minutes in 100 sccm Ar) is done to remove any residual PMMA (Figure 1(f)), thus completing the transfer process. The success rate of our transfer process is about 90 percent (for statistics of the samples transferred by SPeeD method, see Supplementary Section II).
We used the DI water based SPeeD method to transfer graphene grown on Pt foils as well. With our technique, this can be easily done without curling of graphene and without using any chemical like NaOH that can lead to unintentional doping of graphene26. The only difference from the process for releasing used for graphene on Cu is that a thicker resist layer was used for Pt compared to Cu and the resist was not baked. The remaining procedure for transfer of graphene from Pt is same as that for graphene on Cu.
To benchmark the quality of graphene transferred using our SPeeD process, we transferred two graphene samples, grown on Cu foils from the same batch and in the same graphene growth run, by two different transfer methods. Though the quality of our CVD graphene is not as good as the best reported in the literature12,19,39, the comparison of graphene samples grown in the same run under identical conditions but transferred by different processes should show the influence of the transfer process on the defect level, doping level and quality of graphene. The first sample was transferred onto a SiO2-coated Si substrate using conventional etching of Cu with ammonium persulphate solution (details in Supplementary Section III). The second sample was transferred to an identical substrate using our SPeeD method with DI water without using any etchant. We compare the two samples transferred using Raman spectroscopy measurements40 and electrical transport measurements. The results of the comparison are discussed in the following sections.
Discussion
Confocal Raman spectroscopy measurements were performed on both the samples using a WITec Alpha 300R confocal Raman microscope. Figures 2(a) and (b) compare the Raman maps of the full width at half maximum (FWHM) of the G peak for the two graphene samples transferred by the two different methods. Figures 2(c) and (d) compare colourscale maps of 2D/G peak intensity ratio for same area of graphene transferred by two different methods. The spatially-averaged Raman spectra of graphene over a 9 μm × 9 μm area for both samples are shown in Figure 2(e). To evaluate shift in peak positions, the spectra are aligned with reference to the Si substrate peak (520 cm−1) and to compare relative intensity of the various features, the spectra are intensity normalized to the graphene G peak value. The ratio of the intensity of the 2D peak to G peak in both the samples is comparable and has the value of ~2, which indicates that the graphene is monolayer40.
Comparative Raman spectroscopy of graphene transferred by etching Cu to release graphene and by using the SPeeD process.
Integrated Raman mapping over an area of 9 μm × 9 μm for the FWHM of the G peak of graphene transferred by (a) our SPeeD method using DI water and (b) conventional etching of Cu by ammonium persulphate solution. Colourscale maps of 2D/G peak intensity ratio for same area of graphene transferred by (c) our SPeeD method and (d) conventional Cu etching method. (e) Comparison of the spatially averaged Raman scattering spectra of graphene transferred by the two different methods. Inset of (e) shows the blue shift of 8 cm−1 in the G peak for graphene transferred by Cu etching which indicates that it is p-type doped.
The interesting observation is that the integrated Raman spectrum of the graphene transferred by the SPeeD method has a lower D peak intensity than that of the sample transferred by conventional Cu etching (Figure 2(e)). This suggests that the graphene transferred by the SPeeD process has less defects compared to the one transferred by conventional Cu etching41. Further, the inset (Figure 2(e)) shows that the FWHM of the G peak of the graphene transferred by the SPeeD process is narrower than that of the graphene transferred by Cu etching. This points to a lower disorder in the SPeeD transferred sample42. Additionally, the G peak is blue shifted43 in the graphene transferred by Cu etching. This indicates that the graphene transferred by Cu etching is p-type doped compared to the other sample40,43. This p-type doping can be attributed to charge impurities present in the Cu etchant or the presence of defects in the graphene. Thus, transferring graphene using our SPeeD method without any use of Cu etchant reduces the probability of graphene getting doped by ionic impurities. Transport measurements on two graphene samples (discussed later) grown using the same recipe but different transfer methods corroborate this observation about reduced doping in the SPeeD transferred samples.
The SPeeD method has been successfully applied to transfer CVD graphene grown on Pt foils as well. Raman measurements on the CVD graphene transferred via the SPeeD method from Pt foils to 300 nm SiO2-coated p-doped Si are shown in Figure 3.
Furthermore, the reusability of Cu and Pt substrates using our SPeeD method was investigated. A Raman spectroscopy comparison of CVD graphene grown using a recipe for continuous growth of few-layer graphene on fresh and reused Cu foils, showed that the quality of graphene does not degrade on reusing the metal substrate (for details, see Supplementary Section IV).
The Dirac point for graphene transferred by Cu etching is shifted to the high positive gate voltage region (>50 V) (Figure 4(b)) indicating that it is highly p-type doped which also agrees well with the Raman measurements. The Dirac point is observable in DI water transferred graphene at ~15 V (Figure 4(c)) indicating that the sample is much cleaner in comparison. The electrical transport measurements for the devices fabricated using graphene samples transferred by two different methods were done in air at room temperature. Prior to measurement the samples were annealed at 350°C in 100 sccm forming gas for 20 min.
Electrical transport measurements for devices fabricated using graphene samples transferred by two different methods.
(a) Optical image of CVD graphene grown on Cu transferred by SPeeD method. (b) Gating curve for the device fabricated with graphene transferred by conventional Cu etching method. (c) Gating curve for device fabricated with graphene transferred by SPeeD method. The source-drain spacing of the devices used for measurements was ~5 μm.
An optical image of the graphene transferred from Pt substrate by SPeeD method on 300 nm SiO2-coated p-type doped silicon target substrate is shown in Figure 5(a). The gating curve for the device fabricated using our transfer method shows the presence of the Dirac peak at ~15 V (Figure 5(b)) once again indicating a relatively clean sample.
Electrical transport measurement for device fabricated using graphene sample transferred from Pt substrate.
(a) Optical image of the graphene transferred by SPeeD from Pt substrate to 300 nm SiO2-coated p-type doped Si substrate. (b) Transport measurement for the device fabricated on the graphene transferred from Pt. The source-drain spacing of the device used for measurement was ~7 μm.
The Raman and electrical data unequivocally demonstrate that the SPeeD method using hot DI water is an effective method to transfer CVD graphene. We observe that the delamination of freshly grown CVD graphene layers is more difficult than the delamination of graphene that has been exposed to air for a few days. This allows us to propose a possible mechanism for the efficacy of the SPeeD method. Our observations suggest that diffusion of oxygen at the interface of graphene and copper plays an important role44. The intercalation of oxygen modifies the nature of interaction between graphene and substrate leading to a wicking effect where a thin layer of water separates graphene and substrate. This process is expedited on heating in DI water. In addition, the presence of nanometer scale holes in the CVD graphene greatly accelerates the delamination process45 by providing several pathways for delamination. This process is analogous to the previous reports46,47 where the buffered HF – SiO2 etch rate along the interface of SiO2 and graphene is significantly enhanced due to a wicking effect.
In summary, we have successfully demonstrated a novel and simple Soak-and-Peel Delamination method using DI water to transfer CVD-grown graphene from metal substrates like copper and platinum to other substrates of interest. This method does not expose graphene to any harsh chemicals and hence ensures that electrical properties of graphene are not affected. This method is cost effective because no etchant is used and since the metal is not etched it can also be recycled many times reducing large scale production costs. It will also allow the use of single crystals of Cu (111) for improved growth without consuming the copper single crystals. This simple technique demonstrates low cost, clean transfer of graphene and opens doors for its widespread use. The SPeeD process may also provide a generic route to exploit differential hydrophilic/hydrophobic interactions to delaminate other 2D layered materials from grown substrates.
References
Geim, A. K. & Novoselov, K. S. The rise of graphene. Nature Mater. 6, 183–191 (2007).
Neto, A. H. C., Guinea, F., Peres, N. M. R., Novoselov, K. S. & Geim, A. K. The electronic properties of graphene. Rev. Mod. Phys. 81, 109–162 (2009).
Novoselov, K. S. et al. A roadmap for graphene. Nature 490, 192–200 (2012).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Zhang, Y., Small, J. P., Pontius, W. V. & Kim, P. Fabrication and electric-field-dependent transport measurements of mesoscopic graphite devices. Appl. Phys. Lett. 86, 073104 (2005).
Wang, L. et al. One-dimensional electrical contact to a two-dimensional material. Science 342, 614–617 (2013).
Stankovich, S. et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45, 1558–1565 (2007).
Hernandez, Y. et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nature Nanotech. 3, 563–568 (2008).
Berger, C. et al. Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J. Phys. Chem. B 108, 19912–19916 (2004).
Kim, J. et al. Layer-resolved graphene transfer via engineered strain layers. Science 342, 833–836 (2013).
Yu, Q., Lian, J., Siriponglert, S., Li, H., Chen, Y. P. & Pei, S.-S. Graphene segregated on Ni surfaces and transferred to insulators. Appl. Phys. Lett. 93, 113103 (2008).
Li, X. et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324, 1312–1314 (2009).
Hao, Y. et al. The role of surface oxygen in the growth of large single-crystal graphene on copper. Science 342, 720–723 (2013).
Sutter, P. W., Flege, J.-I. & Sutter, E. A. Epitaxial graphene on ruthenium. Nature Mater. 7, 406–411 (2008).
Kang, B. J., Mun, J. H., Hwang, C. Y. & Cho, B. J. Monolayer graphene growth on sputtered thin film platinum. J. Appl. Phys. 106, 104309 (2009).
Sutter, P., Sadowski, J. T. & Sutter, E. Graphene on Pt (111): Growth and substrate interaction. Phys. Rev. B 80, 245411 (2009).
Wood, J. D., Schmucker, S. W., Lyons, A. S., Pop, E. & Lyding, J. W. Effects of polycrystalline Cu substrate on graphene growth by chemical vapor deposition. Nano Lett. 11, 4547–4554 (2011).
Geim, A. K. Graphene: status and prospects. Science 324, 1530–1534 (2009).
Reina, A. et al. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 9, 30–35 (2009).
Kim, K. S. et al. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457, 706–710 (2009).
Bonaccorso, F., Sun, Z., Hasan, T. & Ferrari, A. C. Graphene photonics and optoelectronics. Nature Photon. 4, 611–622 (2010).
Bae, S. et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nature Nanotech. 5, 574–578 (2010).
Gao, M. et al. Epitaxial growth and structural property of graphene on Pt (111). Appl. Phys. Lett. 98, 033101 (2011).
Li, X. et al. Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 9, 4359–4363 (2009).
Wang, Y. et al. Electrochemical delamination of CVD-grown graphene film: toward the recyclable use of copper catalyst. ACS Nano 5, 9927–9933 (2011).
Gao, L. et al. Repeated growth and bubbling transfer of graphene with millimetre-size single-crystal grains using platinum. Nat. Commun. 3, 699 (2012).
Ma, D. et al. Clean transfer of graphene on Pt foils mediated by a carbon monoxide intercalation process. Nano Res. 6, 671–678 (2013).
Schneider, G. F., Calado, V. E., Zandbergen, H., Vandersypen, L. M. K. & Dekker, C. Wedging transfer of nanostructures. Nano Lett. 10, 1912–1916 (2010).
Bonaccorso, F. et al. Production and processing of graphene and 2d crystals. Mater. Today 15, 564–589 (2012).
Reina, A. et al. Transferring and identification of single- and few-layer graphene on arbitrary substrates. J. Phys. Chem. C 112, 17741–17744 (2008).
Bianco, V., Iskrov, S. & Franzese, G. Understanding the role of hydrogen bonds in water dynamics and protein stability. J. Biol. Phys. 38, 27–48 (2012).
Wang, S., Zhang, Y., Abidi, N. & Cabrales, L. Wettability and surface free energy of graphene films. Langmuir 25, 11078–11081 (2009).
Valatte, G. Hydrophilicity of metal surfaces: silver, gold and copper electrodes. J. Electroanal. Chem. Interfacial Electrochem. 139, 285–301 (1982).
Gardner, J. R. & Woods, R. The hydrophilic nature of gold and platinum. J. Electroanal. Chem. Interfacial Electrochem. 81, 285–290 (1977).
Levendorf, M. P., Ruiz-Vargas, C. S., Garg, S. & Park, J. Transfer-free batch fabrication of single layer graphene transistors. Nano Lett. 9, 4479–4483 (2009).
Lee, Y. et al. Wafer-scale synthesis and transfer of graphene films. Nano Lett. 10, 490–493 (2010).
Regan, W. et al. A direct transfer of layer-area graphene. Appl. Phys. Lett. 96, 113102 (2010).
Han, Z. et al. Suppression of multilayer graphene patches during CVD graphene growth on copper. arXiv: 1205.1337 (2012).
Li, X. et al. Large-area graphene single crystals grown by low-pressure chemical vapor deposition of methane on copper. J. Am. Chem. Soc. 133, 2816–2819 (2011).
Ferrari, A. C. & Basko, D. M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nature Nanotech. 8, 235–246 (2013).
Ferrari, A. C. Raman spectroscopy of graphene and graphite: disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 143, 47–57 (2007).
Cançado, L. G. et al. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 11, 3190–3196 (2011).
Das, A. et al. Monitoring dopants by Raman scattering in an electrochemically top-gated graphene transistor. Nature Nanotech. 3, 210–215 (2008).
Kidambi, P. R. et al. Observing graphene grow: catalyst–graphene interactions during scalable graphene growth on polycrystalline copper. Nano Lett. 13, 4769–4778 (2013).
O'Hern, S. C. et al. Selective molecular transport through intrinsic defects in a single layer of cvd graphene. ACS Nano 6, 10130–10138 (2012).
Bolotin, K. I. et al. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 146, 351–355 (2008).
Du, X., Skachko, I., Barker, A. & Andrei, E. Y. Approaching ballistic transport in suspended graphene. Nature Nanotech. 3, 491–495 (2008).
Acknowledgements
The work at TIFR was supported by the Government of India. M.M.D. acknowledges the support of the Department of Science and Technology, India, through the Swarnajayanti fellowship.
Author information
Authors and Affiliations
Contributions
P.G. and P.D.D. contributed equally to this work. They developed the process, performed the measurements, analyzed the data and wrote the manuscript. S.G. and S.D. contributed to the device fabrication and measurements. H.M. helped with Raman measurements. A.B. and M.M.D. planned and supervised the study. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
A facile process for soak-and-peel delamination of CVD graphene from substrates using water (Supplementary Material)
Supplementary Information
Supplementary Movie S1
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Gupta, P., Dongare, P., Grover, S. et al. A facile process for soak-and-peel delamination of CVD graphene from substrates using water. Sci Rep 4, 3882 (2014). https://doi.org/10.1038/srep03882
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03882
This article is cited by
-
Influence of plasma treatment on SiO2/Si and Si3N4/Si substrates for large-scale transfer of graphene
Scientific Reports (2021)
-
A Universal Stamping Method of Graphene Transfer for Conducting Flexible and Transparent Polymers
Scientific Reports (2019)
-
Determination of quantitative structure-property and structure-process relationships for graphene production in water
Nano Research (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.