Abstract
The end-Permian mass extinction was followed by the formation of an enigmatic rock layer with a distinctive macroscopic spotted or dendroid fabric. This deposit has been interpreted as microbial reef rock, digitate dendrolite, digital thrombolite, dendritic thrombolite, or bacterial deposits. Agreement has been reached in considering them as microbialites, but not in their formation. This study has revealed that the spotted and dendroid microbialites were composed of numerous fossil casts formed by the planktic cyanobacterium, Microcystis, a coccoid genus that at the present-day commonly forms blooms in modern lakes, rivers and reservoirs. The abundance of the fossils and the diagenesis they experienced has determined the macroscopic fabric: where they abundant, the rock appears as dendroid, otherwise, it appears as spotted. The ancient Microcystis bloom might produce toxin to kill other metazoans and be responsible for the oceanic anoxia that has puzzled so many researchers for so many years.
Similar content being viewed by others
Introduction
The most severe crisis in Earth history was the sudden disappearance of 90% of marine and most land species1, just before 252.3 Ma ago2, probably due to extreme oceanic and climate changes3,4 caused by massive release of thermogenic carbon dioxide and methane2. The main extinction episode5 was followed by the formation of a distinctive rock layer with a macroscopic spotted6,7 or dendroid8 fabric. This deposit has been interpreted as microbial reef rock built by Renalcis or Renalcis-like microbes9,10,11, digitate dendrolite composed of lobate objects8,12, or digital thrombolite and dendrolite13 built by Renalcis-group calcimicrobes14,15. Other researchers have described them as spotted and dendritic thrombolite consisting of mesoclots with spheroids16,17,18, or as deposits rich in bacterial colonies and altered by diagenesis19,20. Here we show that all these microbialites were formed by the planktic cyanobacterium, Microcystis, a coccoid genus that at the present-day commonly forms blooms in modern lakes, rivers and reservoirs. Microcystis fossilization together with diagenetic alteration has determined the distinctive macroscopic appearance of these microbialites. Microcystis blooms could have contributed to the marine dysoxia or anoxia following this extinction event. Freshwater algal bloom in Permian-Triassic transition probably caused by green alga Spirogyra has been reported21. Here is the first report of a massive marine algal bloom preserved in fossil record.
Results
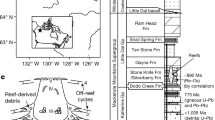
The microbialite interval in southern China is generally 2 to 10 m thick and consists of two parts7,17,18,19,20. The lower part, dominantly gray in color with brown spots (Fig. 1, A), has been described as spotted microbialite. The upper part is brown in color, with vertically-elongate gray patches (Fig. 1, B), has commonly been described as dendroid microbialite. Microscopic examination of the thin sections showed that both the brown “spots” of the spotted rock and the brown patches of the dendroid rock consist of abundant fossils in a micritic (fine-grained carbonate) matrix. The fossils are casts, composed of minerals filling the spaces left by decay of the original organisms. These preserves only the shape of the precursor organisms, but not their soft tissues. The cast fossils are all composed of calcite crystals (occasionally dolomite) that is coarser than the micritic matrix, with crystals generally 0.01 ∼ 0.6 mm wide. The micritic matrix between the fossils consist of tiny calcite crystals (<0.005 mm).
Features of the spotted rock (A) and dendroid rock (B). (C): Fossils without orientation. (D): Thick dendroid rock bed and intercalated thin marl layer. Br-brown spot or patch; Gr-gray areas or patched; F-the problematic fossils, here interpreted as Microcystis colonies; Mic-rock of micrites; Mar-marl; PR-the dendroid rock.
The fossil casts are of different sizes, ranging up to more than 3.5 mm and various shapes (Fig. 2). The smaller fossils (<0.5 mm) are generally subspherical to subelliptic. The medium sizes are 0.5 ∼ 1.0 mm wide, in peanut-shape, reniform, trilobate, embryo-like and other irregular shapes. The large fossils (>1.0 mm) consist of partially connected small and medium fossils, in irregular or dendritic arrangements.
Shapes and sizes of the fossils in the spotted and dendroid rocks.
(A, B): large treelike; (C): medium treelike; (D): medium irregular; (E): medium embryo-like; (F): medium treelike; (G): medium circular; (H): medium trilobate; (I, J): medium kidney-like; (K): medium 8-like; (L): small subellipitical. The boundaries of some fossils are marked by yellow dotted lines. All bars 0.5 mm long.
The internal composition and organization of the fossil casts includes at least three types: (1) needle-like calcites in which all the needles are perpendicular to the edges of the fossils and about 0.05 mm long and 0.01 mm wide; (2) needlelike calcite at the edge and large (e.g., 0.24 mm wide) blocky calcite in the interior (e.g., Fig. 2: D, F); (3) yellowish granular calcite (0.04 ∼ 0.06 mm wide) or dolomite at the edge and colorless granular calcites in the interior (Fig. 2: H, K). We interpret the needle-like calcite as syndepositional cement that formed during organic matter decay.
The preservation of the shapes of the precursor organisms requires that the sediments were lithified prior to decay of the organisms. In present-day shallow marine environments, sediments can lithify within several months22. Thus, in order for their shapes to be preserved, the precursor organisms, or at least their external coverings, would needed to survive within the sediments for this period. Colonies of the cyanobacterium, Microcystis, can retain their shape in sediments for up to 15 years, due to their protective mucilaginous envelopes, which are difficult to decompose. Since there is no evidence of a hard skeleton in the fossils, we infer that the precursor organisms might have possessed such a mucilaginous envelope.
The gray areas between the brown spots of the spotted rock and the brown patches of the dendroid rock are composed of micrite (i.e., calcites crystals < 0.005 mm; Fig. 1: C). Micrites is generally considered to have formed in calm or protected water23. The fossils in the spotted rock are randomly distributed, showing no preferred orientation (Fig. 1: C). If the organisms adhered to the sea floor during life, the fossils should remain in their upward growth orientation. However, this is not the case. Thus, we infer that the organisms were planktic.
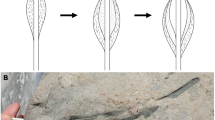
The precursor organisms of the fossils are very similar in shape to the extant cyanobacterium Microcystis. As plankton, mainly in freshwater, Microcystis generally occur as greenish colonies (Fig. 3) consisting of masses of cells in a transparent shared mucilaginous envelope24,25,26,27. The colonies range 0.1 to more than 3.3 mm in size and small ones are generally subspherical or subelliptic in form. The medium-sized colonies consist of two to several small colonies, in peanut-shape, reniform, trilobate, embryo-like, treelike and other irregular shapes. The large colonies consist of numerous small and medium sized colonies and can be treelike, netlike, or irregular in shape.
Shapes and sizes of colonies of present-day Microcystis.
(A, B): large tree-like; (C, D): medium irregular; (E): medium treelike; (F): medium circular; (G), medium embryo-like; (H): small subspherical, medium 8-shaped; (I): medium trilobate, embryo-like; (J): large irregular. Bars 0.1 mm in (A–I) and 1 mm in (J). (A) and (B) are cited from Fig. 7.2 of reference29; (C, D, E, F, H) cited from reference26; (I) cited from reference27; (G) from reference25; (J): taken in this study.
Three other cyanobacteria, Woronichinia, Gloeocapsa and Aphanocapsa and one chlorophyte, Botryococcus, have some of the colonial shapes of modern Microcystis. However, they also show differences. Woronichinia colonies are generally subspherical or embryo-like, but are small (<0.1 mm) and do not exhibit other shapes. The colonies of Gloeocapsa are generally subspherical or multiple-spherical, but also are small (<0.17 mm) and not varied in shape. The colonies of Aphanocapsa are generally subspherical and <0.06 mm in size, even though their sheet-like colonies can reach 1.5 cm. The colonies of Botryococcus are generally multiple-subspherical and do not show other forms and are all small.
Both the precursor organisms of the fossils and modern Microcystis have mucilaginous envelopes and are inferred to be able to stay alive in sediments for more than 14 years28, sufficiently long for lithification of the sediments to occur. Modern Microcystis29 is planktic and so were the precursor organisms as inferred.
The precursor organisms are very similar in appearance to colonies of modern Microcystis, suggesting that they might be Microcystis and that the cast fossils originated from them. However, it is also possible that some of the fossils also originated from ancient Woronichinia, Gloeocapsa, Aphanocapsa and Botryococcus.
The formation of these fossils from Microcystis colonies requires four steps: (1) burial of Microcystis colonies by micritic sediments, (2) lithification that retained the shapes of the original colonies, (3) decay of the Microcystis colonies, leaving cavities, (4) growth of needle-like cements on the inner wall of the cavities with their basal ends perpendicular to the cavity walls, (5) further diagenesis. Lithification of the micritic sediments before the decay of the mucilaginous envelope was responsible for the preservation of the shape of the cyanobacterium. The decay left cavities, which were filled in by early needle-like aragonite and later blocky calcite cements. The spotted and dendroid structures have the same carbon and oxygen isotope composition as the micritic matrix6, which implies that the diagenesis has homogenized them.
Recognization of these Microcystis fossils clarifies several problems that have puzzled researchers for a long time. The first is the origin of these microbialites. Some researchers14,15 thought the microbialites (or microbial framestones) were built by Renalcis-like microbes. Others17,18 considered that they were constructed by unidentified spherical or globular objects, or by irregular bacterial colonies19. Our study identifies these Renalcis-like spheroidal objects and irregular colonial fossils as casts of Microcystis colonies. The appearance of the microbialites depends on the abundance of the cast fossils. Where the fossils are sparse, the microbialites appear look spotted. If they are very abundant, ∼>70% of the rock volume, then the microbialites have a dendroid or reticulate macrofabric.
Optimal temperatures for Microcystis growth are 15 ∼ 29°C29. Growth ceases below 14°C. Microcystis is tolerant of high temperatures, unless they exceed 45°C. These microbialites formed in low latitudes15, with seawater surface temperatures in the range 27 ∼ 35°C4, which partially overlaps with the optimal temperature of present-day Microcystis.
Fossil casts account for 50 ∼ 75% by volume of the dendroid microbialites in our samples and for 30 ∼ 50% of the spotted microbialites. The dendroid microbialites always overlie the spotted microbialites, suggesting progression from low abundance to blooming.
Blooms of modern Microcystis are harmful to other organisms for two reasons. Firstly, some species of modern Microcystis produce toxins lethal to most metazoans including humans30. Secondly, Microcystis blooms can create anoxia29 which will kill most other organisms. This effect is consistent with the scarcity of other organisms associated with the microbialites. Our study shows 417 species of marine invertebrates in the crinoid limestone underlying the microbialites compared with 25 species in the microbialites, including ostracods, small gastropods, small worms and tiny foraminifers.
Conflicting estimates have been made of the oxygen levels during formation of the microbialites in the Laolongdong section15. Some researchers found that the ostracods occurring in the microbialites were those tolerant of low oxygen31. We found pyrite grains (0.5 ∼ 2 mm wide) in the microbialites, but not in the immediately underlying strata. We infer that the pyrite was transformed from framboid, which are considered to form in dysoxic water. The mean size of the framboid in the microbialites and the overlying strata is about 8.4 microns and is considered to reflect dysoxic conditions32 and upwelling of low-oxygen water has been proposed as the cause of the oceanic anoxia15. Since modern Microcystis bloom can cause dysoxia or anoxia of the water column29, we suggest that dysoxia or anoxia of the sea water in which the microbialites formed was caused by Microcystis blooms.
Methods
Five outcropped profiles of the spotted and dendroid microbialites in southern Chin, three at Laolongdong, about 30 km north of Chongqing, one in Xiushui, Jiangxi Province and the last in Xuanhan, Sichuan Province, were examined inch by inch. Details of the spotted and dendroid fabric were observed and pictured. More than 150 oriented samples were collected, slabbed and thin-sectioned (0.035 mm) using standard techniques. All thin sections were observed under 2×, 5× and 10× objective lens of Olympus BX41P Microscope. All fossils were pictured and compared with similar modern colonial cyanobacteria. Their similarities were evaluated carefully.
References
Erwin, D. H. The Great Paleozoic Crisis: Life and Death in the Permian. (Columbia Univ. Press, New York, 1993).
Shen, S. Z. et al. Calibrating the End-Permian Mass Extinction. Science 334, 1367–1372 (2011).
Wu, Y. S. Conodont, Reef Evolution and Mass Extinction across Permian-Triassic Boundary. (Geological Publishing House, Beijing, 2005).
Sun, Y. D. et al. Lethally hot temperatures during the Early Triassic greenhouse. Science 338, 366–370 (2012).
Yin, H. F., Feng, Q. L., Lai, X. L., Baud, A. & Tong, J. N. The protracted Permo-Triassic crisis and multi-episode extinction around the Permian–Triassic boundary. Global and Planet. Change 55, 1–20 (2007).
Wang, Y. B., Tong, J. N., Wang, J. S. & Zhou, X. G. Calcimicrobialite after end-Permian mass extinction in South China and its palaeoenvironmental significance. Chin. Sci. Bul. 50, 665–671 (2005).
Jiang, H. X. & Wu, Y. S. Origin of Microbialite-like Dendroid Rocks in the Permian-Triassic Boundary Section in Xiushui, Jiangxi Province. Geol. Review. 53, 323–329 (2007a).
Kershaw, S., Zhang, T. & Lan, G. A. Microbialite carbonate crust at the Permian-Triassic boundary in South China and its palaeoenvironmental significance. Palaeogeogr., Palaeoclimat., Palaeoecol. 146, 1–18 (1999).
Lehrmann, D. J. Early Triassic calcimicrobialmounds and biostromes of theNanpanjiang Basin, South China. Geology 27, 359–362 (1999).
Lehrmann, D. J. et al. Permian–Triassic boundary sections from shallow marine carbonate platforms of the Nanpanjiang Basin, South China: implications for oceanic conditions associated with the end-Permian extinction and its aftermath. Palaios 18, 138–152 (2003).
Payne, J. L., Lehrmann, D. J., Wei, J. Y. & Knoll, A. H. The pattern and timing of biotic recovery from the end-Permian extinction on the Great Bank of Guizhou, Guizhou Province, China. Palaios 21, 63–85 (2006).
Kershaw, S., Li, Y., Crasquin-Soleau, S., Feng, Q. L. & Mu, X. N. Earliest Triassic microbialites in the South China block and other areas: controls on their growth and distribution. Facies 53, 409–425 (2007).
Riding, R. Microbial carbonates: the geological record of calcified bacterial-algal mats and biofilms. Sedimentology 47,179–214 (2000).
Yang, H. et al. Composition and structure of microbialite ecosystems following the end-Permian mass extinction in South China. Palaeogeogr., Palaeoclimat., Palaeoecol. 308, 111–128 (2011).
Kershaw, S. et al. Microbialites and global environmental change across the Permian–Triassic boundary: a synthesis. Geobiology 10, 25–47 (2012).
Ezaki, Y., Liu, J. B. & Adachi, N. Earliest Triassic microbialite micro- to megastructures in the Huaying area of Sichuan Province, South China: Implications for the nature of oceanic conditions after the end-Permian extinction. Palaios 18, 388–402 (2003).
Ezaki, Y., Liu, J. B., Nagano, T. & Adachi, N. Geobiological aspects of the earliest Triassic microbialites along the southern periphery of the tropical Yangtze Platform: Initiation and cessation of a microbial regime. Palaios 23, 356–369 (2008).
Wang, H. F., Liu, J. B. & Ezaki, Y. Sea-Level Changes at the Dawen Permian-Triassic Boundary Section of Luodian, Guizhou Province, South China: A Global Correlation. Acta Scient. Natur. Uni. Pekin. 48, 589–602 (2012).
Jiang, H. X. & Wu, Y. S. Restudy of the microbialite from the Permian-Triassic boundary section, Chongqing. Acta Petrol. Sin. 23, 1189–1196 (2007b).
Jiang, H. X. & Wu, Y. S. Diagenesis of the microbialites in the Permian-Triassic boundary section at Laolongdong, Chongqing, South China. J. Palaeogeogr. 2, 183–191 (2013).
Afonin, S. A., Barinova, S. S. & Krassilov, V. A. A bloom of Tympanicysta Balme (green algae of zygnematalean affinities) at the Permian-Triassic boundary. Geodiversitas 23, 481–487 (2001).
Shinn, E. A. Submarine lithification of Holocene carbonate sediments in the Persian Gulf. Sedimentology 12, 109–144 (1969).
Folk, R. L. Pratical petrographic classification of limesonte. Bull. Am. Ass. Petrol. Geol. 43, 1–38 (1959).
Komárek, J. & Anagnostidis, K. Cyanoprokaryota 1. Teil: Chroococcales. Süβwasserflora von Mitteleuropa 19/1. Ettl, H., Gartner, G., Heynig, H. & Mollenhauer, D. (eds.). 164–190. (Gustav Fischer, Ulm, 1999).
Komarek, J. & Komarkova, J. Review of the European Microcystis-morphospecies (Cyanoproryptes) from nature. Czech Phycol. 2, 1–24 (2002).
Yu, G. L., Song, L. R. & Li, R. H. Taxonomic notes on water bloom forming Microcystis species (Cyanophyta) from China–An example from samples of the Dianchi Lake. Acta Phytot. Sin. 45, 727–741 (2007).
Xu, Y. et al. Non-microcystin producing Microcystis wesenbergii (Komarek) Komarek (Cyanobacteria) representing a main waterbloom-forming species in Chinese waters. Environment. Poll. 156, 162–167 (2008).
Latour, D., Salencon, M. J., Reyss, J. L. & Giraudet, H. Sedimentary imprint of Microcystis aeruginosa (cyanobacterium) blooms in Grangent reservoir (Loire, France). J. Phycol. 43, 417–425 (2007).
Šejnohová, L. & Maršálek, B. Microcystis. Their Diversity in Space and Time. Whitton, B. A. (ed.). 195–228. (Springer Science, 2012).
Carmichael, W. W. Health effects of toxin-producing cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk. Assess. 7, 1393–1407 (2001).
Crasquin-Soleau, S. & Kershaw, S. Ostracod fauna from the Permian–Triassic boundary interval of South China (Huaying Mountains, eastern Sichuan Province): palaeoenvironmental significance. Palaeogeogr., Palaeoclimat., Palaeoecol. 217, 131–141 (2005).
Liao, W., Wang, Y., Kershaw, S., Weng, Z. & Yang, H. Shallow marine dysoxia across the Permian-Triassic boundary; evidence from pyrite framboid in the microbialite in South China. Sed. Geol. 232, 77–83 (2010).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 40472015, 40802001 and 41372121). Special thanks also to Jiří Komárek for his helpful comments on identification of the fossils.
Author information
Authors and Affiliations
Contributions
Y.S.W. wrote the manuscript. G.L.Y. and R.H.L. helped in identifying the fossils. L.R.S. provided modern cyanobacterial samples. D.Y.L. gave helpful advice. R.R. revised the manuscript. H.X.J., L.J.L. and R.Z. joined the field work.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Wu, Y., Yu, G., Li, R. et al. Cyanobacterial fossils from 252 Ma old microbialites and their environmental significance. Sci Rep 4, 3820 (2014). https://doi.org/10.1038/srep03820
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03820
This article is cited by
-
Microfabric features of microbial carbonates: experimental and natural evidence of mold holes and crusts
Journal of Palaeogeography (2021)
-
Calcilobes wangshenghaii n. gen., n. sp., microbial constructor of Permian–Triassic boundary microbialites of South China, and its place in microbialite classification
Facies (2021)
-
Permian–Triassic boundary microbialites (PTBMs) in southwest China: implications for paleoenvironment reconstruction
Facies (2017)
-
Microconchids from microbialites near the Permian-Triassic boundary in the Zuodeng Section, Baise area, Guangxi Zhuang Autonomous Region, South China and their paleoenvironmental implications
Journal of Earth Science (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.