Abstract
Hierarchical Li4Ti5O12/TiO2 tubes composed of ultrathin nanoflakes have been successfully fabricated via the calcination of the hydrothermal product of a porous amorphous TiO2 precursor and lithium hydroxide monohydrate. The hierarchical tubes are characterized by powder X-ray diffraction, nitrogen adsorption/desorption, scanning electron microscopy and transmission electron microscopy techniques. These nanoflakes exhibit a quite complex submicroscopic structure with regular structural imperfection, including a huge number of grain boundaries and dislocations. The lithium ion storage property of these tubes is evaluated by galvanostatic discharge/charge experiment. The product shows initial discharge capacities of 420, 225 and 160 mAh g−1 at 0.01, 0.1 and 1.0 A g−1, respectively. After 100 cycles, the discharge capacity is 139 mAh g−1 at 1.0 A g−1 with a capacity retention of 87%, demonstrating good high-rate performance and good cycleability. The high electrochemical performance is attributed to unique structure and morphology of the tubes. The regular structural imperfection existed in the nanoflakes also benefit to lithium ion storage property of these tubes. The hierarchical Li4Ti5O12/TiO2 tubes are a promising anode material for lithium-ion batteries with high power and energy densities.
Similar content being viewed by others
Introduction
Rechargeable lithium ion batteries have been successfully adopted as power sources for portable electronic devices because of their high energy density and long cycle life1, whereas the practical application of lithium ion batteries to (hybrid) electric vehicles and stationary electric energy storage largely depends on the electrochemical performance of the electrode materials, such as large specific capacities, high rate capability and good cycling stability. It is envisaged that the introduction of a nanostructure and the increase of crystallographic defect concentration and grain boundary (GB) density are feasible strategies to improve the electrochemical performance of electrode materials. Nanostructured materials with small particle size and large specific surface area can provide relatively shortened mass and charge transport pathways and charming surface activities, resulting in enhanced rate capabilities2.
The research on structural imperfection derived from crystallographic defects is a very active and rapidly developing area in solid-state chemistry3. Crystallographic defects can bring forth rich structural chemistry and unexpected properties to the crystalline materials. For example, the smooth mass transfer in a solid-state reaction can be achieved only when the reactants have certain defect concentration. Oxygen-deficient ferrites with a spinel structure can efficiently decompose CO2 into carbon through the transfer of oxygen to the oxygen vacancies4. The presence of defects in the crystal structures may enhance the lithium ion transport properties and electronic conductivity of the electrode materials5,6,7,8,9,10,11,12,13. Therefore, it is promising to enhance the rate performance of lithium ion batteries to a great extent by introducing defects to the electrode materials.
A large number of lithium ions can be stored on the interfacial area of the nanostructured materials with a high grain boundary density, increasing the specific capacities of these materials14,15. It is of interest that the capacity introduced by the interfacial lithium storage is almost independent on the charge-discharge rate due to its thermodynamic characteristics16,17. Dual- and multi-phase materials are expected to provide increased grain boundary density. Recently, the interface in dual-phase Li4Ti5O12/TiO2 materials is demonstrated to be superior for storing the extra lithium18,19,20,21.
Herein, the synergistic effect of nanostructure, crystallographic defect and grain boundary on the lithium ion storage properties of electrode materials is observed for dual-phase hierarchical Li4Ti5O12/TiO2 tubes prepared via the calcination of a lithium titanium oxide hydrate, which is the hydrothermal product of a porous amorphous TiO2 precursor and lithium hydroxide monohydrate. The hierarchical tubes are composed of ultrathin nanoflakes with an ordered defect structure. Plenty of grain boundaries are present within the Li4Ti5O12 crystallites and among Li4Ti5O12 and TiO2 crystallites in the nanoflakes. These hierarchical tubes exhibit large reversible capacities and good cycling performance.
Results
The X-ray diffraction (XRD) pattern of the produced tubes is shown in Figure 1a The broadened peaks located at 18.44°, 35.59°, 43.40° and 62.92° can be readily indexed to the (111), (311), (400) and (440) diffractions, respectively, of Li4Ti5O12 with a cubic spinel structure (JCPDS No. 49-0207). The additional peaks at 25.34°, 38.03°, 47.99°, 54.25° and 55.04° are ascribed to the (101), (004), (200), (105) and (211) diffractions, respectively, of anatase TiO2 (JCPDS No. 21-1272). The XRD analysis indicates that both spinel Li4Ti5O12 and anatase TiO2 exist in the sample obtained, generating a dual-phase Li4Ti5O12/TiO2 composite material. The anatase TiO2 may arise from the crystallization of residual amorphous titania surviving the hydrothermal treatment. The content of spinel Li4Ti5O12 in the composite is approximately 64 wt% based on the XRD analysis. The morphology of the Li4Ti5O12/TiO2 composite is revealed by SEM and TEM observation (Figure 1b, c). The Li4Ti5O12/TiO2 exhibits a tube-like morphology assembled by interleaving ultrathin nanoflakes. The diameter of the tubes ranges from 0.5 to 2.0 μm with a length of 5–20 μm. The nanoflakes with a smooth surface are approximately 1 μm in length and 200–400 nm in width. As observed in the TEM image, plenty of void space exists among the nanoflakes, forming a three-dimensional (3D) channel system which facilitates the transportation of the electrolyte and lithium ions. The inner side of the nanoflakes is fused together to form the wall of the tubes, ensuring the good electronic contact of these flakes.
X-ray diffraction pattern and microscopy images of the hierarchical Li4Ti5O12/TiO2 tubes.
(a) X-ray diffraction pattern of the hierarchical tubes, indicating that both spinel Li4Ti5O12 and anatase TiO2 exist in the sample obtained. (b) SEM image shows that a tube-like morphology assembled by interleaving ultrathin nanoflakes. (c) TEM image of the hierarchical Li4Ti5O12/TiO2 tubes with an individual nanoflake indicated in the white circle.
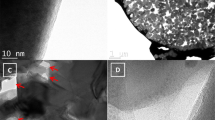
Figure 2 shows further structural feature of the hierarchical Li4Ti5O12/TiO2 tubes. A schematic illustration of the individual nanoflake is drawn to show the regions revealed by the HRTEM observation (Figure 2a). The HRTEM image of the nanoflake taken in region I clearly exhibits a periodic arrangement of fringes with an average distance of approximately 1.7 nm, obviously larger than the lattice fringe spacings of Li4Ti5O12 and TiO2 (Figure 2b). The lattice-fringe-like periodic pattern is believed to be derived from defects, demonstrating the regular structural imperfection of these nanoflakes. However, the corresponding selected area electron diffraction (SAED) (inset in Figure 2b) along the [011] zone axis (perpendicular to the nanoflake surface) exhibits a series of spot pattern, characteristic for a single-crystal-like structure. All of the diffraction spots can be well indexed to the spinel Li4Ti5O12 with a cubic structure in which the direction [hkl] is perpendicular to the (hkl) plane. Therefore, it can be concluded that the Li4Ti5O12 nanoflakes are bound by (011) facets on both the top and bottom surfaces. The HRTEM images (Figure 2c, d) of the nanoflake in region I and II are taken along the direction that almost parallels with the nanoflake plane. As shown in Figure 2c, the lattice fringes with a spacing of 0.50 nm attributing to the (111) plane of spinel Li4Ti5O12 with a lattice fluctuation are clearly observed. A careful examination of the HRTEM image indicates that structural imperfection is derived from the interlacing of two or three closely packed parallel (111) planes. This layer-by-layer-like stacking can be clearly observed in the TEM images viewing along or perpendicular to the surface of the nanoflakes (Figure 2d, e). The interlayer spacing of the lattice-fringe-like structural imperfection is approximately 1.7 nm, consistent with that observed in Figure 2b. For a cubic structure, the dihedral angle between the (111) planes is 70.5°. Distinct lattice fringes with a distance of approximately 0.48 nm are observed, corresponding to the (111) planes of spinel Li4Ti5O12 (Figure 2e). The angle between these lattice fringes is approximately 70.5°, consistent with the cubic structure of Li4Ti5O12. It is interesting that plenty of grain boundaries exist among the crystalline domains in the nanoflakes (Figure 2f). These attractive grain boundaries are expected to provide extra diffusion path and storage sites for lithium ions. Figure 2g shows the HRTEM image taken in region III, the root section of the nanoflake. The lattice fringe spacings of 0.49 and 0.36 nm observed are ascribed to the (111) plane of spinel Li4Ti5O12 and (101) plane of anatase TiO2, respectively, confirming the co-existence of the spinel Li4Ti5O12 and anatase TiO2. In addition, a great deal of grain boundaries among the spinel Li4Ti5O12 and anatase TiO2 are also observed in the region. The HRTEM observation is in agreement with that of the XRD analysis. The presence Li4Ti5O12 and TiO2 dual phase significantly increases the grain boundary density.
Schematic drawing and electron microscopy images of the nanoflake.
(a) Schematic representation of a structural model of the nanoflake, showing a regular lamellar structure with defects. (b, c) High resolution TEM (HRTEM) images of the nanoflake in region I. Regular structural imperfection with an average distance of approximately 1.7 nm is clearly observed in the TEM image (b). In the SAED pattern inset in b, the electron diffraction along the [011] zone axis (perpendicular to the nanoflake surface) is indexed to the cubic structure of spinel Li4Ti5O12, each stacked layer are offset and stepping upwards layer by layer like a staircase (c). (d, e, f) HRTEM images of the nanoflake in region II. The layers stacked in the nanoflakes form a dihedral angel {111} vs. {111} of approximately 70.5°, consistent with the cubic structure of Li4Ti5O12. Grain boundaries and dislocations present in the nanoflakes (f). (g) HRTEM image of the nanoflake in region III, confirming the co-existence of the spinel Li4Ti5O12 and anatase TiO2.
The textural feature of the hierarchical Li4Ti5O12/TiO2 tubes is characterized by nitrogen adsorption/desorption measurements (Supplementary Figure 1). The isotherms are identified as of type IV with an H3 hysteresis loop, indicating the presence of slit-like pores. This is consistent with the SEM and TEM observation. A hysteresis loop with a sharp capillary condensation is presented at a relative pressure of 0.80 to 0.97, which is due to the existence of macroporous structures. The pore size distribution shows feature characteristic for hierarchical materials. The specific Brunauer-Emmett-Teller (BET) surface area and the pore volume are determined to be 107 m2 g−1 and 0.52 cm3 g−1, respectively.
Cyclic voltammetry (CV) was used to investigate the electrochemical behavior of the hierarchical Li4Ti5O12/TiO2 tubes with regular structural imperfection. Figure 3 shows the CV curves of the hierarchical Li4Ti5O12/TiO2 tubes at a scan rate of 0.2 mV s−1 in the potential range from 2.5 to 1.0 V. In the first cycle, three pairs of peaks were observed. Two pairs of cathodic/anodic peaks located at 1.43/1.65 V and 1.64/2.06 V versus Li+/Li are associated with lithium ion insertion/extraction within the spinel Li4Ti5O12 and anatase TiO2 lattices, respectively. An extraordinary pair of peaks located at 1.27/1.78 V versus Li+/Li are distinctly observed, denoted as S peaks. It is generally accepted that the S peaks are related to the surface-confined charge-transfer processes with the presence of amorphous phase in the organized skeleton22,23. The S peaks have been well documented in the literature to explain the electrochemical behavior of Ti-based compounds, such as TiO2 (B)24, anatase22, spinel Li4Ti5O1225, dual-phase Li4Ti5O12–TiO218 and layered hydrogen titanate26. For the hierarchical Li4Ti5O12/TiO2 tubes, the appearance of S peaks is attributed to the presence of the regular structural imperfection in the nanoflakes. In the following cycle, the peak potentials of insertion and extraction in/from spinel Li4Ti5O12 lattices process slightly shift to 1.46 and 1.66 V, respectively and the pair of peaks corresponding to the TiO2 − LixTiO2 redox couples almost disappears. It means that the electrode reaction of the hierarchical Li4Ti5O12/TiO2 tubes is controlled by a mixed process of the faradaic pseudocapacitive and diffusion-limited reaction, benefiting performance enhancement of electrode active materials18,25,27.
The electrochemical performance of the hierarchical Li4Ti5O12/TiO2 tubes was evaluated by galvanostatic charge-discharge technique. Figure 4a displays the initial three discharge/charge curves of the hierarchical Li4Ti5O12/TiO2 tubes at a current density of 0.01 A g−1 in the potential window of 2.5–1.0 V (versus Li+/Li). In the discharge process, two potential plateaus at potentials of approximately 1.75 and 1.55 V are clearly observed. The first plateau at 1.75 V is ascribed to the phase transition from anatase TiO2 to orthorhombic LixTiO2 upon lithium ion insertion. The plateau at 1.55 V is attributed to the generation of a rocksalt-structured Li7Ti5O12, resulting from the lithium ion insertion into the spinel Li4Ti5O12. A long sloped region observed from 1.55 to 1.0 V can be assigned to the pseudocapacitive lithium storage behavior of the Li4Ti5O12/TiO2 tubes, characteristic of the electrochemical reactions involving surface-confined charge-transfer or interfacial storage processes14,15,16,25. At 0.01 A/g, the discharge capacity of the first cycle can reach a high lithiation capacity of 420 mAh g−1 and a de-lithiation capacity of 315 mAh g−1. The discharge capacities of the second and third cycles are approximately 286 and 271 mAh g−1, respectively, with the coulombic efficiency significantly improved. The initial discharge capacity of the hierarchical Li4Ti5O12/TiO2 tubes at 0.01 A g−1 (approximately C/16) is much higher than that of the nanostructured Li4Ti5O12 at even lower current density (approximately 228 mAh g−1 at C/24)28. As revealed by the TEM observed, the grain boundary density of the Li4Ti5O12/TiO2 tubes can be significantly increased by introducing TiO2 crystallites into the nanoflakes. Thus, the high specific capacity of the Li4Ti5O12/TiO2 tubes is mainly attributed to the extra amount of lithium ions stored on the interfacial area of the crystalline Li4Ti5O12 and TiO2 grains. In addition, the electrochemical properties of lithium ion insertion/extraction largely depend on the crystal surface plane orientation of the electrode materials. The lowest energy (011) surface of Li4Ti5O12 is predicted to be energetically favorable for lithium ion insertion into the vacant 16c sites29. But for Li4Ti5O12 nanoflakes with nearly 100% exposed (011) facets, the intercalation of lithium ions into empty tetrahedral 8a sites in the near surface region is not feasible. Thus, the extra charge capacity must result from the insertion of lithium ions into the ordered defect layers in spinel Li4Ti5O12.
Electrochemical characterizations of the hierarchical Li4Ti5O12/TiO2 tubes.
(a) The initial three discharge/charge curves of the hierarchical Li4Ti5O12/TiO2 tubes at 0.01 A g−1. (b) Cycling performance of the hierarchical Li4Ti5O12/TiO2 tubes at 0.1 and 1.0 A g−1. The electrochemical evaluation suggests the large specific capacity, high rate capability and excellent cycling stability of these hierarchical tubes.
Cycling performance of the hierarchical Li4Ti5O12/TiO2 tubes at the discharge/charge current density of 0.1 and 1.0 A g−1 is shown in Figure 4b. Cycled at 0.1 A g−1, a reversible capacity of 146 mAh g−1 is observed for the 100th cycle, giving a capacity retention rate of approximately 65%. Cycled at a current density of 1.0 A g−1, the hierarchical Li4Ti5O12/TiO2 tubes show a discharge capacity of 160 mAh g−1 in the first cycle. Good capacity retention is realized over extended cycling. A charge capacity of 139 mAh g−1, approximately 87% of its initial charge capacity is retained after 100 cycles. After 800 cycles, the discharge capacity of the hierarchical Li4Ti5O12/TiO2 tubes is still of 134 mAh g−1 (Supplementary Figure 2b). The cycling stability for the hierarchical Li4Ti5O12/TiO2 tubes at a large current density is distinctly superior to those for the related composite materials described in the literature18,19,20,21. Thus, the high cycling stability of the Li4Ti5O12/TiO2 tubes is mainly ascribed to their unique hierarchical tubular structure built up by thin nanoflakes.
The high specific capacity, good cycleability of the hierarchical Li4Ti5O12/TiO2 tubes constructed by nanoflakes are attributed to the synergistic effect of the hierarchical nanostructure, the ordered fringe-like crystallographic defect and the large number of grain boundaries among the spinel Li4Ti5O12 and anatase TiO2 nanocrystals. First, the 3D porous channel system of the composite material facilitates the transport of electrolyte and lithium ions within the electrode and the small thickness of the nanoflakes shortens the diffusion distance for both electron and lithium ions. Second, the fringe-like structural imperfection, abundant grain boundaries and phase interfaces in the hierarchical Li4Ti5O12/TiO2 composite favor the storage of extra lithium ions, increasing the specific capacity of the composite material. In addition, the abundant phase interfaces are also favorable for the fast electrochemical lithium-ion insertion and extraction processes, improving the kinetics of the electrode. Third, the tubular structure and thin nanoflakes effectively buffer the stress induced during the discharge/charge process, contributing to the cycling performance improvement of the composite. Because of these structural features, the hierarchical Li4Ti5O12/TiO2 composite exhibits large specific capacities and good cycling stability.
In summary, hierarchical Li4Ti5O12/TiO2 tubes composed of nanoflakes have been successfully fabricated through the conversion of a lithium titanium oxide hydrate upon calcination at 400°C. The structural imperfection formed in the nanoflakes during the phase transformation from lithium titanium oxide hydrate to spinel Li4Ti5O12 has proved to be advantageous for lithium ion storage of these tubes. The obtained hierarchical Li4Ti5O12/TiO2 tubes exhibit a high initial discharge capacity of 420 mAh g−1 at 0.01 A g−1. Cycling at 1.0 A g−1, a capacity of 139 mAh g−1 is retained even after 100 cycles, indicating good cycling stability. The high electrochemical performance of the tubes is attributed to their unique nanostructure, including the 3D porous channel system, the structural imperfection in Li4Ti5O12 nanoflakes and the high grain boundary density among the nanoparticles. The 3D porous channel system facilitates the mass transport of the electrolyte and lithium ions and provides sufficient void space to accommodate the volume change during the discharge/charge process, ensuring the good cycling performance. Such elaborate combination of crystal structure and architectural mode could be helpful for designing new high rate composite electrodes.
Methods
Preparation of hierarchical Li4Ti5O12/TiO2 tubes
The hierarchical Li4Ti5O12/TiO2 tubes were prepared from a porous amorphous TiO2 as precursor by a hydrothermal process and subsequent calcination. Typically, titanium glycolate was first synthesized via heating the mixture of ethylene glycol and tetrabutyl titanate (1:100 by volume) at 160°C for 2 h under vigorous stirring. The porous amorphous TiO2 were prepared through the irradiation of titanium glycolate under ultraviolet light (Supplementary Figure 3a–b). A mixture of the amorphous titania (240 mg, 3.00 mmol) and lithium hydroxide monohydrate (111 mg, 2.65 mmol) was added to 24 mL of ditilled water with continuous stirring. The obtained suspension was transferred into a 30 mL Teflon-lined stainless-steel autoclave, which was then heated at 130°C for 7 d. The white precipitate of layered hydrous lithium titanate was collected by filtration, washed with ethanol thoroughly and dried naturally at room temperature (Supplementary Figure 3c–d). The final product of hierarchical Li4Ti5O12/TiO2 tubes was generated by the calcination of the layered hydrous lithium titanate at 400°C for 1.5 h.
Characterization
Powder X-ray diffraction (XRD) patterns were recorded on a Rigaku Dmax-2200 diffractometer (Rigaku, Japan) with Cu Kα radiation (λ = 1.5418 Å). The morphology of the sample was observed with a scanning electron microscope (SEM, JSM-7401F, JEOL, Japan). The microstructure of the sample was characterized by using transmission electron microscope (TEM, JEM-2100F, JEOL, Japan) and high resolution transmission electron microscope (HRTEM, JEM-2100F, JEOL, Japan), operating at 200 kV. The specific surface areas of the samples were measured on a Micromeritics ASAP 2010 M + C nitrogen adsorption instrument (Micrometritices Inc., USA) at 77 K.
Electrochemical properties of the hierarchical Li4Ti5O12/TiO2 tubes were evaluated with CR2016 coin cells. The working electrode was prepared on an aluminum foil by using a doctor-blade method with a slurry composed of 75 wt.% active materials (TiO2), 15 wt.% conductivity agent (acetylene black) and 10 wt.% binder (polyvinylidene fluoride, PVDF). Lithium metal foil was used as both the counter and reference electrodes. A microporous polypropylene membrane (Celgard 2500) was used as the separator. Coin cells were assembled in an argon filled glove box with both moisture and oxygen contents below 1.0 ppm. The electrolyte was 1.0 M of LiClO4 in ethylene carbonate/dimethyl carbonate (EC/DEC, 1:1 by volume). The galvanostatic charge and discharge experiment was performed with a battery tester LAND-CT2001A in the voltage range of 1.0–2.5 V at room temperature. Cyclic voltammograms (CV) measurement was conducted by using an electrochemical cell with a three-electrode configuration. Li metal foil was selected as the reference and counter electrode. The electrolyte was 1 M LiClO4 in ethylene carbonate/dimethyl carbonate (EC/DEC, 1:1 by volume). The cyclic voltammograms (CV) were conducted on a CHI 660B electrochemical workstation at a scanning rate of 0.2 mV s−1 in a potential range of 2.5–1.0 V (vs Li+/Li).
References
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Bruce, P. G., Scrosati, B. & Tarascon, J. M. Nanomaterials for Rechargeable Lithium Batteries. Angew. Chem. Int. Ed. 47, 2930–2946 (2008).
West, A. R. Solid State Chemistry and its Applications (J. Wiley & Sons, Chichester, 1984).
Nordhei, C., Mathisen, K., Safonova, O., van Beek, W. & Nicholson, D. G. Decomposition of Carbon Dioxide at 500°C over Reduced Iron, Cobalt, Nickel and Zinc Ferrites: A Combined XANES–XRD Study. J. Phys. Chem. C 113, 19568–19577 (2009).
Badi, S. P. et al. Direct synthesis of nanocrystalline Li0.90FePO4: observation of phase segregation of anti-site defects on delithiation. J. Mater. Chem. 21, 10085–10093 (2011).
Chung, S. Y., Choi, S. Y., Yamamoto, T. & Ikuhara, Y. Atomic-Scale Visualization of Antisite Defects in LiFePO4 . Phys. Rev. Lett. 100, 125502 (2008).
Gibot, P. et al. Room-temperature single-phase Li insertion/extraction in nanoscale LixFePO4 . Nat. Mater. 7, 741–747 (2008).
Hamelet, S. et al. Existence of Superstructures Due to Large Amounts of Fe Vacancies in the LiFePO4-Type Framework. Chem. Mater. 23, 32–38 (2010).
Janssen, Y., Middlemiss, D. S., Bo, S. H., Grey, C. P. & Khalifah, P. G. Structural Modulation in the High Capacity Battery Cathode Material LiFeBO3 . J. Am. Chem. Soc. 134, 12516–12527 (2012).
Lee, J., Zhou, W., Idrobo, J. C., Pennycook, S. J. & Pantelides, S. T. Vacancy-Driven Anisotropic Defect Distribution in the Battery-Cathode Material LiFePO4 . Phys. Rev. Lett. 107, 085507 (2011).
Luo, J. Y., Chen, L. J., Zhao, Y. J., He, P. & Xia, Y. Y. The effect of oxygen vacancies on the structure and electrochemistry of LiTi2(PO4)3 for lithium-ion batteries: A combined experimental and theoretical study. J. Power Sources 194, 1075–1080 (2009).
Wang, Y. et al. Defect effects on the physical and electrochemical properties of nanoscale LiFe0.92PO4 and LiFe0.92PO4/C/graphene composites. Nanoscale 5, 3704–3712 (2013).
Zheng, J. et al. Enhanced Li+ ion transport in LiNi0.5Mn1.5O4 through control of site disorder. Phys. Chem. Chem. Phys. 14, 13515–13521 (2012).
Guo, X. et al. Capacitive Energy Storage on Fe/Li3PO4 Grain Boundaries. J. Phys. Chem. C 115, 3803–3808 (2011).
Yu, X. Q. et al. Reversible lithium storage in LiF/Ti nanocomposites. Phys. Chem. Chem. Phys. 11, 9497–9503 (2009).
Jamnik, J. & Maier, J. Nanocrystallinity effects in lithium battery materials - Aspects of nano-ionics. Part IV. Phys. Chem. Chem. Phys. 5, 5215–5220 (2003).
Maier, J. Nanoionics: ion transport and electrochemical storage in confined systems. Nat. Mater. 4, 805–815 (2005).
Li, X., Lai, C., Xiao, C. W. & Gao, X. P. Enhanced high rate capability of dual-phase Li4Ti5O12-TiO2 induced by pseudocapacitive effect. Electrochim. Acta 56, 9152–9158 (2011).
Rahman, M. M. et al. Basic molten salt process A new route for synthesis of nanocrystalline Li4Ti5O12-TiO2 anode material for Li-ion batteries using eutectic mixture of LiNO3-LiOH-Li2O2 . J. Power Sources 195, 4297–4303 (2010).
Rahman, M. M., Wang, J. Z., Hassan, M. F., Wexler, D. & Liu, H. K. Amorphous Carbon Coated High Grain Boundary Density Dual Phase Li4Ti5O12-TiO2: A Nanocomposite Anode Material for Li-Ion Batteries. Adv. Energy Mater. 1, 212–220 (2011).
Wang, J. et al. Li4Ti5O12–TiO2 composite anode material for lithium-ion batteries. J. Power Sources 222, 196–201 (2013).
Kavan, L., Rathousky, J., Gratzel, M., Shklover, V. & Zukal, A. Surfactant-templated TiO2 (anatase): Characteristic features of lithium insertion electrochemistry in organized nanostructures. J. Phys. Chem. B 104, 12012–12020 (2000).
Kavan, L., Rathousky, J., Gratzel, M., Shklover, V. & Zukal, A. Mesoporous thin film TiO2 electrodes. Microporous and Mesoporous Mater. 44, 653–659 (2001).
Zukalova, M., Kalbac, M., Kavan, L., Exnar, I. & Graetzel, M. Pseudocapacitive lithium storage in TiO2(B). Chem. Mater. 17, 1248–1255 (2005).
Lai, C., Dou, Y. Y., Li, X. & Gao, X. P. Improvement of the high rate capability of hierarchical structured Li4Ti5O12 induced by the pseudocapacitive effect. J. Power Sources 195, 3676–3679 (2010).
Li, J. R., Tang, Z. L. & Zhang, Z. T. Layered hydrogen titanate nanowires with novel lithium intercalation properties. Chem. Mater. 17, 5848–5855 (2005).
Kavan, L. et al. Lithium storage in nanostructured TiO2 made by hydrothermal growth. Chem. Mater. 16, 477–485 (2004).
Chiu, H. c. et al. Aqueous Synthesized Nanostructured Li4Ti5O12 for High-Performance Lithium Ion Battery Anodes. J. Electrochem. Soc. 160, A3041–A3047 (2013).
Ganapathy, S. & Wagemaker, M. Nanosize Storage Properties in Spinel Li4Ti5O12 Explained by Anisotropic Surface Lithium Insertion. ACS Nano 6, 8702–8712 (2012).
Acknowledgements
This work was financially supported by the National Basic Research Program (2013CB934102, 2011CB808703) and the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Contributions
Y.J. and K.W. developed the idea and designed the experiments. Y.J. performed the sample fabrication, measurements and data analysis. Y.J. and H.Z. performed the electrochemical experiments. Y.J., K.W., H.Z., J.W. and J.C. analyzed the data and discussed the results. Y.J., K.W. and J.C. co-wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Jiang, YM., Wang, KX., Zhang, HJ. et al. Hierarchical Li4Ti5O12/TiO2 composite tubes with regular structural imperfection for lithium ion storage. Sci Rep 3, 3490 (2013). https://doi.org/10.1038/srep03490
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03490
This article is cited by
-
Polygonal multi-polymorphed Li4Ti5O12@rutile TiO2 as anodes in lithium-ion batteries
Nano Research (2019)
-
Lithium Titanate/Carbon Nanotubes Composites Processed by Ultrasound Irradiation as Anodes for Lithium Ion Batteries
Scientific Reports (2017)
-
Fabrication of Li4Ti5O12-TiO2 Nanosheets with Structural Defects as High-Rate and Long-Life Anodes for Lithium-Ion Batteries
Scientific Reports (2017)
-
Si doped T6 carbon structure as an anode material for Li-ion batteries: An ab initio study
Scientific Reports (2016)
-
Fabrication of Nb2O5 Nanosheets for High-rate Lithium Ion Storage Applications
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.