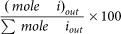Abstract
Design of catalytic materials has been highlighted to build ultraclean use of heavy oil including liquid-to-gas technology to directly convert heavy hydrocarbons into H2–rich gas fuels. If the H2 is produced from such heavy oil through high-active and durable catalysts in reforming process that is being constructed in hydrogen infrastructure, it will be addressed into renewable energy systems. Herein, the three different hollow fiber catalysts networked with perovskite nanoparticles, LaCr0.8Ru0.2O3, LaCr0.8Ru0.1Ni0.1O3 and LaCr0.8Ni0.2O3 were prepared by using activated carbon fiber as a sacrificial template for H2 production from heavy gas oil reforming. The most important findings were arrived at: (i) catalysts had hollow fibrous architectures with well-crystallized structures, (ii) hollow fibers had a high specific surface area with a particle size of ≈50 nm and (iii) the Ru substituted ones showed high efficiency for H2 production with substantial durability under high concentrations of S, N and aromatic compounds.
Similar content being viewed by others
Introduction
An oil jackpot of up to 233 billion barrels has been discovered in Australia, the new Saudi Arabia, which is foreseeable to extend the practical use of petroleum to about 200 years from 40 years at today's oil consumption rate. With a rising concern for gradual fossil fuel exhaustion, an efficient use of such oil resources is faced to an imperative emerging issue towards hydrogen economy based on sustainable energy generation. Hydrogen-based fuel cells that can be used for both automotive and stationary applications require a stable source of H2 produced with a high efficiency reforming process1,2,3,4. The use of pure H2 as a fuel in automotive and residential applications faces the costly process of distillation and/or hydro-treatment of naphtha. One approach to overcoming such limitations is the use of direct reforming of gas oil (heavy hydrocarbons) over a heteroatom-resistant catalyst. This process would be profitable because of the infrastructural reasons and the economical price of starting feeds compared with naphtha hydrocarbon fuels5,6. Gas oils contain various hetero-atoms such as S and N, including those present in aromatic species, depending on the mining location. In particular, the high content of S molecules, especially refractory species such as alkyl substituted dibenzothiophene (x,y-DBT) in gas oil, compared to other liquid hydrocarbons like logistic fuel or commercial diesel, is a crucial factor to consider when trying to achieve value-added products7,8,9. This needs to be considered when designing the catalyst since such hetero-atoms can be deactivating10,11,12. Nevertheless, in order to be commercially viable, gas oil appears to be an attractive feed for reforming because of its similar molecular structure to conventional diesel and/or liquid hydrocarbons, apart from its higher S content. There are no reports on reforming using gas oil, which is likely due to the rapid deactivation of conventional catalysts by large amounts of S species during the reaction. Hence, the development of a highly active and stable reforming catalyst under high S and N conditions would be desirable in order to apply the reforming process to gas oil.
Perovskite materials with a formula of ABO3, where the A cation is 12-fold coordinated and the B cation is 6-fold coordinated with the oxygen, have been interested in various applications such as catalysis, energy, environment and bioscience applications13,14,15,16,17,18. Recently, noble methods for preparation of perovskite with unique architecture and morphology have emerged in the form of porous, scaffold and egg-shell skeleton due to their high geometric surface area with large cavities, light weight and flexibility for mass and heat transfer19,20,21,22,23. To achieve such perovskite materials, templates such as polymer and biomolecules have been explored to manipulate their structure, crystallinity, size and shape, which cannot be obtained by the conventional methods24,25,26,27. In such a way, crystal growth and configuration of the perovskites can be controlled since templates act as a dispersion agent and steric stabilizer for the deposition of metal precursors on them. Perovskite catalysts composed of highly reactive metal oxides have been highlighted as an alternative to conventional noble metal catalysts for the reforming of hydrocarbons. These catalyst materials have a number of advantages, including stability at high temperatures, in redox environments and in the presence of H2-rich gases. Moreover, perovskites are known to be significantly resistant to deactivation since their chemical binding energy for S is very low28,29. In particular, the perovskite of LaCrO3 is stable because it is well known to strongly prefer six-fold coordination and the Cr in the B-site can be partially substituted for Ru, Ni, or Fe etc, providing catalytic activity that is extremely promising for the reforming of heavy hydrocarbons like gas oils28,29,30,31.
In this work, a hollow fibrous perovskite structure composed of nanoparticles network incorporated with Ru and Ni was prepared through a novel process by employing activated carbon fiber (ACF) as a sacrificial template. The catalytic activity of the prepared hollow perovskite fibers in the production of H2 from gas oils with different S and N contents through autothermal reforming (ATR) was investigated for the first time.
Results
Hollow fiber architechtures with a multi mixed perovskite nanoparticles are designed for an efficient mass tranfer including short diffusion and high contact time with the reactants in the autothermal reforming system32. The Synthesis procedure of the perovskite hollow fiber was shown in the Fig. 1A. The acid-treated ACF template was immersed in a solution of stoichiometric LaIII, CrIII, NiII and RuIII ions, resulting in ionic binding of the metal cations to the negatively charged surface of the ACF. The hollow fiber composed of a perovskite nanoparticle network could then be successfully formed by mild heat treatment of the each LaIIICrIII0.8RuIII0.2/ACF, LaIIICrIII0.8RuIII0.1NiII0.1/ACF and LaIIICrIII0.8NiII0.2/ACF composite at 1027 K under air flow to remove the C content via combustion. For the formation of crystalline networks, ACF promotes the nucleation of nanoparticles at mild temperatures by an in-situ CO generation from the oxidative exothermic decomposition of ACF at the interface with metal cations25,26,27.
According to the powder X-ray diffraction (XRD) patterns shown in Fig. 1B, all perovskites exibit major diffraction peaks in similar angle regions, corresponding to the (hkl) indices (112), (022), (220), (222), (312) and (040). These are exactly the same as those from the orthorhombic structure of LaCrO3 perovskite with space group Pbnm, which matches the set of peaks reported in the powder diffraction file (PDF) no. 00-024-101631,33. The stoichiometric chemical composition for all the perovskites was found to be by ICP-AES analysis (Table S1). From the magnified image showing the (112) reflection, substitution of RuIII and NiII into LaCrO3 can be seen to have caused a peak displacement to a lower angle when the substitution level is fixed with the ratio of 0.2 at the B-site. This implies an increase in the d-value (basal spacing) from 2.739 Å (LaCrO3) to 2.764 Å (LaCr0.8Ru0.2O3), 2.761 Å (LaCr0.8Ru0.1Ni0.1O3) and 2.756 Å (LaCr0.8Ni0.2O3), in addition to the cell parameters and cell volume. As can be seen in Table S2, there were slight increases for the LaCr0.8Ru0.2O3 in b-axis and c-axis cell parameters from 5.478 Å to 5.526 Å (Δb = 0.048 Å) and from 7.736 Å to 7.835 Å (Δc = 0.099 Å), respectively. These differences can be attributed to the different crystal ionic radii by partial substitution of CrIII ion with RuIII ion in the B site34. When the RuIII metal ion is more substituted in perovskite lattices, their d-values were further shifted rather than the only NiIII-substituted one.
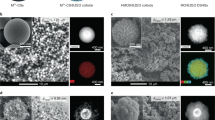
The SEM images in Fig. 2A show the perovskite with a relatively 1D hollow fibrous structures for different chemical compositions with outer and inner fiber diameters in the ranges of 4.5–6.4 μm and 4.1–6.0 μm, respectively and a length/diameter ratio of around 50, which corresponded well to the ACF template structure. Furthermore, in the photograph, different colors of the perovskite hollow fibers were clearly obtained from the spesific incorporation metals.
As illustrated in HR-TEM images in Fig. 2B, it was clearly observed that all perovskite fibers were made up of spherical nanoparticles with a average size of approximately 30, 50 and 80 nm in diameter for LaCr0.8Ru0.2O3, LaCr0.8Ru0.1Ni0.1O3 and LaCr0.8Ni0.2O3, respectively, which are networked each other to form the hollow fibrous structure. Furthermore, the HR-TEM analysis provides strong evidence for the formation of the micro-tubular feature from the wall with a thickness of over 50 nm at the end of highly interconnected nanoparticles as the dark contrast. Both features provided a large specific surface area of 14.26, 13.65 and 12.33 m2/g for LaCr0.8Ru0.2O3, LaCr0.8Ru0.1Ni0.1O3 and LaCr0.8Ni0.2O3, respectively, which are ~3 times larger than that of grain nanoparticles prepared in the absence of the ACF template (4.39 m2/g with a size of ≈50 nm in Fig. S1 and Table S3). It is likely that these hollow fibrous morphology comprising of nanosized particles and their large surface area would provide a short distance of mass transfer for the hydrocarbons in and desired H2 out easily, resulting in an excellent catalytic performance in the process of oil reforming. As presented in right panel of HR-TEM images, the selected area electron diffraction (SAED) spot patterns for all perovskite hollow fibers were assigned to the [010] and [001] planes along the [100] direction. As shown in the left panel of magnified HR-TEM images, the interplanar distances of LaCr0.8Ru0.2O3, LaCr0.8Ru0.1Ni0.1O3 and LaCr0.8Ni0.2O3 were observed as 0.277, 0.275 and 0.273 nm, respectively, from the regular lattice fringes along the [112] direction. These results are well consistent with the values estimated from the XRD patterns.
To investigate the chemical bonding nature of the Ru/Ni substituted perovskites, the x-ray photoelectron spectroscopy (XPS) peaks corresponding to La 3d, Cr 2p, O 1s, Ru 3p3/2,and Ni 3p3/2 of the hollow fibers are displayed in Fig. 3. Binding energies and atomic ratios are determined from the peak fittings are summarized in Table S4 and S5. The two typical peaks of La 3d3/2 and La 3d5/2 are observed in Fig. 3A, close to those expected for a La3+ ion in an oxide environment. No significant differences were observed in the La 3d5/2 doublets (La2O3 at 838.4 eV and La(OH)3 at 833.9 eV) for the perovskite hollow fibers28. For the Cr peaks shown in Fig. 3B, the Cr 2p3/2 zone was deconvoluted into two valence states: low-valence, Cr3+, at ca. 576.1 eV and high-valence, Cr5+ or Cr6+, at ca. 577.6 or 579.2 eV, respectively30. Referring to the Ru and Ni substituted perovskite hollow fibers, the Cr 2p3/2 peaks was more shifted toward higher valence state along with relatively higher proportion of Cr5+ or Cr6+ than LaCrO3 hollow fiber indicating a sharing of electron density between the Ru/Ni and less electronegative Cr when no significant changes in peak shape was involved. The O 1s spectra shown in Fig. 3C were also split into two peaks: lattice oxygen (O2−) at the lower binding energy (529.4 eV) and surface adsorbed oxygen (O−) at the higher binding energy (531.2 eV)30,31. Considering the higher proportion of adsorbed oxygen on surface for the metal substituted LaCrO3, the spectra were used to estimate the ratio of Osurface/Olattice for each hollow fiber, with values of 1.24, 1.57, 1.00 and 0.56 for the LaCr0.8Ru0.2O3, LaCr0.8Ru0.1Ni0.1O3, LaCr0.8Ni0.2O3, LaCrO3, respectively. The higher proportion of surface oxygen of the Ru/Ni substituted hollow fibers compared to the LaCrO3 was likely due to the deformation of the perovskite structure by the metal substitution, which is expected to enhance the catalytic activity at the reaction. In Fig. 3D, the Ru 3p3/2 peaks (463.9–464.2 eV and 466.8–467.3 eV) of the LaCr0.8Ru0.2O3 and LaCr0.8Ru0.1Ni0.1O3 hollow fibers showed a higher binding energy of RuHigh-Valence, formally in the Ru4+/5+ mixed-valence states, in the BO3 inner lattice rather than the outer crystal surface28,35. In Fig. 3E, the Ni 3p3/2 peaks (66.9–68.5 eV and 71.8–74.5 eV) are also shifted to the higher binding energy of NiHigh-Valence29,36. From these results, the prepared perovskites with metal substitution of Cr3+, Ru3+, or Ni3+ are enriched with high-valence of metal cations along with O− species. These are related to the oxygen surface storage capacity which demonstrates the prevalent role of the Ru, Ni and Cr redox cycles on the catalytic activity.
The temperature-programmed reductions of H2 (H2-TPR) of the perovskite structures are displayed in Fig. 4A. As has been previously reported, the position of the reduction peaks can be affected by crystallite size, oxygen defects, bonding strength, transition metal state/location and doping level of the perovskites28,30. The profile from the LaCr0.8Ru0.2O3 hollow fiber showed a two-step consecutive reduction with H2 consumption peaks at around 325°C and 625°C while two different H2 consumption peaks was revealed at around 434°C and 516°C for the LaCr0.8Ni0.2O3 hollow fiber. The low-temperature peak is likely to include the reduction of Ru or Ni and Cr ions on the surface, changing them from an oxidized to a lower valance state, while the reduction peak at the higher temperature can be attributed to the further reduction of Ru or Ni and Cr ions in the perovskites framework. The first reduction peak of LaCr0.8Ru0.2O3 was observed to shift to a lower temperature on partial substitution of Cr by Ru in the B site of the LaCrO3 structure. In case of the LaCr0.8Ru0.1Ni0.1O3 hollow fiber showed small peak shifted to the lower temperature of 222 °C at first reduction than LaCr0.8Ru0.2O3 hollow fiber; this may be due to the outer-lattice Ru/Ni metals rather than inner-lattice Ru/Ni oxides. Compared to the other perovskites, the highest reduction peak for the LaCr0.8Ru0.2O3 hollow fiber appeared with a greater peak area at the relatively higher temperature which can be inferred to the presence of a higher quantity of Ru incorporation into the lattice, along with the CrHigh-Valence species that are strongly bonded to O30. The profile of LaCr0.8Ru0.2O3 hollow fiber indicated then an improved reducibility with higher amount of H2 consumption and material structural stability from TPR profiles, which is accessible to ATR reaction.
A. H2–TPR profiles of the hollow fibers: (a) LaCr0.8Ru0.2O3, (b) LaCr0.8Ru0.1Ni0.1O3, (c) LaCr0.8Ni0.2O3 and (d) LaCrO3. B. H2 production (mol%) of as-prepared perovkskite catalysts for ATR reaction using hexadecane (C16H34), with an addition of sulphur, Dibenzothiophene, at content of 100 ppm: (a) LaCr0.8Ru0.1Ni0.1O3 hollow fiber, (b) LaCr0.8Ru0.2O3 hollow fiber, (c) LaCr0.8Ru0.1Ni0.1O3 grain, (d) Pt-GDC, (e) LaCr0.8Ru0.2O3 grain, (f) LaCr0.8Ni0.2O3 hollow fiber and (g) LaCrO3 hollow fiber. (C16H34 = 0.012 ml·min−1, H2O/O2/C = 1.25/0.4/1, 1027 K, GHSV = 4000 h−1).
It should be noted that the ACF template used in the preparation of the perovskite hollow fibers can act as a combustible agent when hot spots due to burning of the carbon could be responsible for higher actual temperatures to induce mixed valance states that would enhance the mobility of oxygen in the lattice25,26,27. As is well known, when hydrocarbon species adsorb along with the O atoms, electrons transfer from the active metals neighbouring the vacancy at the B site to the reactants, facilitating cleavage of the C–H bond to release H2. It is, therefore, expected that the hollow fiber with a high percentage of RuHigh-Valence, NiHigh-Valence, CrHigh-Valence and O− species correlated to partial incorporation of Ru/Ni is superior in the redox catalysis in the reforming of hydrocarbon fuels to H2 gas.
Discussion
In a preliminary study as plotted in Fig. 4B and Fig. S2, the effects of the fibrous structure and Ru/Ni substitution in the catalyst activity were investigated: H2 production from heavy hydrocarbon, hexadecane (C16H34), with an addition of sulphur, Dibenzothiophene, at content of 100 ppm. All tested catalysts had conversions of the liquid hexadecane over 99% at the conditions of 1027 K, molar ratio of H2O/O2/C = 1.25/0.4/1 and GHSV of 4000 h−1. For a comparison with the hollow fiber catalyst, the grain powder catalyst was prepared by using the similar method without ACF template as a reference. Notably, the hollow fibers show not only a superior reforming activity but also a better durability on the H2 production than the grain powder without any drop of performance during 50 hr operation. As displayed in Fig. 4B(a)–4B(g), the LaCr0.8Ru0.1Ni0.1O3 and LaCr0.8Ru0.2O3 hollow fibers exhibited about 1.5 fold improvements in the H2 production activity than those corresponding grain powders. This result is due to the better mass/heat transfer of the tubular architecture through ACF template during the autothermal reforming process as well as more structural oxidation by combustion effect of the ACF.
The LaCr0.8Ru0.2O3 hollow fiber achieved a high initial H2 production of 66 mol% (H2 mol%) including no degradation in the performance for 50 hr with COx selectivity values of nearly 90% due to the active phase and stable structure in the redox catalysis reaction proved from the characterization data. Furthermore, the LaCr0.8Ni0.2O3 and LaCr0.8Ru0.1Ni0.1O3 fibers demonstrated a very high level of the production of H2, 71 mol% and 73 mol%, respectively. For 50 hr reaction, sustainable H2 production activities are observed in both Ru-substituted hollow fibers (Fig. 4B(a) and 4B(b)). Only Ni-substituted hollow fiber suffered in regards to durability for the long-term reaction because nickel-contained catalysts have generally shown catalyst deactivation by heavy carbon deposition (i.e. coke) on the catalyst surface37. This can be confirmed by large deposited carbon amount on the catalyst surfaces based on the TGA data (Fig. S3). As for comparison with the conventional 5% Pt-gadolinium-doped CeO2 (GDC) which is considered as a noble catalyst prepared by incipient wetness impregnation, there was also a remarkable drop of the H2 production efficiency under sulphur (100 ppm) contained hydrocarbon in spite of the similar initial performance. It can be understood as sulphur poisoning on the Pt metal surface being the active site for the large coke deposition (Fig. S3) whereas great sulphur endurance was revealed at the LaCr0.8Ru0.2O3 catalyst. As a result, it was found that the LaCr0.8Ru0.2O3 hollow fiber in particular, was seen to be highly effective in converting the surrogate fuel to H2-rich reformates and much more stable than the other perovskites.
For the first try of the gas oil reforming, gas oils obtained from Arabian light oil (LGO), medium oil (MGO), heavy oil (HGO) and diesel were subjected to detailed analysis to assess levels of reactive and refractory S, N and C species, using GC coupled with an atomic emission detector (GC–AED), as illustrated in Figs. 5A–5C and summarized the amount of each species in Table 1. Refractory S and alkyl-carbazole (Cx-Cz) contents were significantly (3–4 times) higher for the HGO than the LGO. Although the boiling point ranges of the gas oils were same (220–340°C), the distribution of C species varied between the samples, as is evident in the GC–AED chromatographs7,8,9. HGO contained almost no species less than C12 and had high amounts of C18–C20. The diesel exhibited a similar C profile to LGO, with much smaller molecules evident. The distribution of S species in the gas oil was significantly different. The major S species were alkyl-benzothiophenes (Alkyl-BTs) comprising C2–C5 alkyl chains, dibenzothiophene (DBT) and considerable amounts of alkyl-DBTs. HGO contained the largest amount of alkyl-DBTs with two or more alkyl C atoms, which are classed as the refractory S species8. The GC–AED chromatographs of N species, presented in Fig. 4C, clearly indicate a significantly higher N content in HGO, which is mainly due to Cx–Cz9. The concentrations of S and N for commercial diesel were found to be negligible compared to the gas oils.
GC–AED profiles for A. carbon, B. sulfur and C. nitrogen species of the (a) HGO, (b), MGO (c) LGO and (d) diesel. D. H2 production for diesel of the (a) LaCr0.8Ru0.2O3 hollow fiber and (b) Pt–GDC. E. H2 production of the (a) LGO (LaCr0.8Ru0.2O3 hollow fiber), (b) MGO (LaCr0.8Ru0.2O3 hollow fiber), (c) HGO (LaCr0.8Ru0.2O3 hollow fiber), (d) HGO (LaCr0.8Ru0.2O3 grain), (e) LGO (Pt–GDC), (f) MGO (Pt–GDC) and (g) HGO (Pt–GDC). F. Recycling test of the LaCr0.8Ru0.2O3 hollow fiber using HGO. (all fuels = 0.012 ml·min−1, H2O/O2/C = 1.25:0.4:1, 1027 K, GHSV = 4000 h−1). G. SEM image and XRD patterns (inset) of the LaCr0.8Ru0.2O3 hollow fiber refreshed after HGO reforming.
The time dependance of ATR experiments involving the three different gas oils, as well as the commercial diesel, were subsequently conducted over the Pt–GDC and the as-prepared LaCr0.8Ru0.2O3 catalysts, as shown in Figs. 5D, 5E, S4 and S5. The product distributions from S- and N-free diesel reforming are shown in Figs. 5D and S4. H2 production by the hollow fiber was continuously stable at 53 mol% over the time period tested and there was no evidence of catalyst deactivation. The loss in catalytic activity of the Pt–GDC (from 50 to 37 mol%) while reforming the diesel may be partially attributed to the presence of coke from unreacted polyaromatics in the fuel.
Fig. 5E show the H2 produced when the gas oils were homogeneously fed in the presence of high quantities of S and N (up to 17,000 ppm and 312 ppm, respectively). It was found that excellent hydrogen production, together with the tolerance of S and N compounds on the feeds could be achieved over perovskite hollow fiber comparing to conventional Pt–GDC. For the long periods of time-on-stream (~50 h), the H2 production by the hollow fiber, averaged from the steady state, achieved almost 47.4 mol% for LGO, 39.3 mol% for MGO and 34.6 mol% for HGO (Fig. 5E). H2 production from HGO varied significantly depending on the catalyst, with average values of 34.6 mol%, 23.3 mol% and 7.7 mol% for the LaCr0.8Ru0.2O3 hollow fiber, LaCr0.8Ru0.2O3 grains and Pt–GDC, respectively, with a decrease in the order: LaCr0.8Ru0.2O3 hollow fiber > LaCr0.8Ru0.2O3 grain > Pt–GDC. In addition, the higher reforming capacity of the LaCr0.8Ru0.2O3 hollow fiber was reflected in the lower proportion of non-reformed hydrocarbons (CH4, C2H4, C2H6 and C3H6) in the products, as displayed in Fig. S5. The increase in H2 was also accompanied by a decrease in COx for all of the tested catalysts. As expected, it was observed that the LaCr0.8Ru0.2O3 hollow fiber material exhibited higher activity and increased stability with a lower degree of deactivation, whereas the perovskite grain and the Pt–GDC were substantially deactivated over the reaction time period. This loss in ATR activity can be attributed to S poisoning and coke formation on the catalytic surface. As can be seen in Table 2, the quantity of C and S residues deposited on each used catalyst correlated with the concentrations of S, N and aromatic species contained in the gas oil fuels. After long-term reaction, even in HGO, the decrease in the deposited quantities of S and C were close to 80%, 61% and 52% for the hollow fiber, grain and Pt–GDC, respectively. This better tolerance under severe gas oil is in accordance with the higher reforming efficiency and stability observed for the LaCr0.8Ru0.2O3 hollow fiber, which is strongly associated with the improved mass/heat transfer rates by the microtubular structure with higher effective surface area and reactivity owing to the well incorporation of Ru in the perovskite lattice.
The durable stability of perovskite catalysts is important issue for their practical industry applications. The reforming process using HGO was repeated four times, as shown in Fig. 5F. When reforming process using HGO was repeated for each cycle, the used LaCr0.8Ru0.2O3 hollow fiber was routinely refreshed by combusting under air atmosphere at 1027 K to remove carbonaceous deposits (coke) and supplied to the reactor. The same quantities of products were constantly achieved for each 24 h cycle, which may be due to the sustainable hollow fibrous architecture composed of LaCr0.8Ru0.2O3 nanoparticles networks along with slight sintering and stable structure without any reduction or segregation of the metals as from the SEM and XRD analysis in Fig. 5G.
In conclusion, we demonstrated the synthesis of the hollow fiber networked with perovskite nanoparticles of LaCr0.8Ru0.2O3, LaCr0.8Ru0.1Ni0.1O3 and LaCr0.8Ni0.2O3 using ACF as a sacrificial template for heavy oil reforming. The hollow fiber had a well-crystallized structure and a high specific surface area of ≈13 m2/g was revealed with an average particle size of ≈50 nm, which is ~3 times larger than that of grains. The LaCr0.8Ru0.2O3 hollow fibrous architecture had a favorable effect on the capacity to yield H2 directly from heavy gas oil reforming, at 34.6 mol% compared to grain (23.3 mol%) and Pt–GDC (7.7 mol%), with substantial durability even for long-term reactions with high concentrations of S, N and aromatic compounds.
Methods
Synthesis of perovskite hollow fibers of LaCrAO3 (A = Ru and/or Ni)
Perovskite hollow fibers were prepared by using aqueous (solution) impregnation synthesis which is a rapid and convenient method to provide high surface area oxides. An ACF template (1 g) was serially treated with H2SO4 (6 M) and HNO3 (6 M) and then soaked in a mixed aqueous solution of La(NO3)3·9H2O (0.15 M), Cr(NO3)3·9H2O (0.1 M), Ni(NO3)2·9H2O (0.01 M or 0.02 M),and HN4O10Ru (0.01 M or 0.02 M) for 24 hours. After washing with deionized water, the ACF impregnated with LaIII/CrIII0.8/(RuIII and/or NiII) ions was then calcined at 1027 K for 6 h in sufficient air flow to form perovskite hollow fibers. For comparison, LaCr0.8Ru0.2O3 grain was prepared in the absence of the ACF under the same conditions. The 5% Pt–GDC (gadolinium-doped CeO2) was prepared by incipient wetness impregnation in a similar manner6.
Analyses of the commercial diesel and gas oils
Three different fuels were selected: hexadecane (Sigma–Aldrich) containing 100 ppm of sulphur (Dibenzothiophene, Sigma–Aldrich), commercial diesel (GS Caltex Co. Korea) and gas oils to investigate the reforming performances of the test catalysts. Gas oils were produced from the light (LGO), medium (MGO) and heavy (HGO) Saudi Arabian crude oils. Commercial diesel was collected from GS Caltex Co. Korea. Carbon, sulfur, nitrogen species of all fuels were monitored by gas chromatography (GC, HP6890) equipped with an atomic emission detector (AED, G2350A) at conditions summarized in Table S6. High performance liquid chromatography (HPLC) technique was applied for determination of aromatic components using Hitachi L-6200 unit equipped with D-700 DAD detector (λ = 254 nm). A 0.46 cm × 25 cm column, packed with 5 μm Zorbax-ODS, was used at 25°C. Mobile phase was a mixture of methanol and water (85:15 vol/vol) with the flow rate of 1.0 ml/min.
H2 Production test
The autothermal reforming of gas oil fuels was carried out at atmospheric pressure in a fixed-bed stainless steel flow reactor with 8 mm internal diameter. The temperature of the reactor was monitored and controlled by thermocouples that were coaxially inserted into the center of the catalyst bed. The fuels were injected by liquid pump and ultrasonic injector for homogeneous fuel supply. For ATR experiments of the hollow fiber catalysts, each feed, with a molar ratio of H2O/O2/C = 1.25:0.4:1, was introduced into the reactor by a mass flow controller (flow rate = 0.012 ml·min−1 and GHSV = 4000 h−1), where the reforming reaction of each hollow fiber catalyst (0.5 g) was carried out for 50–100 h at 1027 K at atmospheric pressure. All reformates (H2, CO2, CO, CH4, C2,3Hn) were periodically measured using on-line GC (Youglin-6000) equipped with a TC detector and an FI detector which were programmed to operate under high-sensitivity conditions and the product distributions were averaged from the steady state. The conversion and hydrogen mole percentage of products by hexadecane, diesel and gas oils are defined as follows:
-
Fuel conversions at the liquid base were over 99%.
-
Product distribution: (%) (i: H2, CO, CH4, CO2, C2,3Hn (C2H4 + C2H6 + C3H6)):
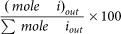
References
Yu, K. et al. Non-syngas direct steam reforming of methanol to hydrogen and carbon dioxide at low temperature. Nat. Commun. 3, 1230 (2012).
Choudhary, T. V. & Choudhary, V. R. Energy-Efficient Syngas Production through Catalytic Oxy-Methane Reforming Reactions. Angew. Chem. Int. Ed. 47, 1828–1847 (2008).
Deluga, G. A., Salge, J. R., Schmidt, L. D. & Verykios, X. E. Renewable hydrogen from ethanol by autothermal reforming. Science 303, 993–997 (2004).
Baudouin, D. et al. Nickel–Silicide Colloid Prepared under Mild Conditions as a Versatile Ni Precursor for More Efficient CO2 Reforming of CH4 Catalysts. J. Am. Chem. Soc. 134, 20624–20627 (2012).
Navarro, R. M. et al. Catalysts for Hydrogen Production from Heavy Hydrocarbons. ChemCatChem 3, 440–457 (2011).
Kang, I., Bae, J. M. & Bae, G. J. Performance comparison of autothermal reforming for liquid hydrocarbons, gasoline and diesel for fuel cell applications. J. Power Sources 163, 538–546 (2006).
Park, J. I. et al. Characteristics on HDS over amorphous silica-alumina in single and dual catalytic bed system for gas oil. Catal. Today 164, 100–106 (2011).
Kim, T. G. et al. Analysis and deep hydrodesulfurization reactivity of Saudi Arabian gas oils. J. Ind. Eng. Chem. in press (2013).
Murti, S. D. S., Yang, H., Choi, K. H., Korai, Y. & Mochida, I. Influences of nitrogen species on the hydrodesulfurization reactivity of a gas oil over sulfide catalysts of variable activity. Appl. Catal. A-Gen. 252, 331–346 (2003).
Chen, Y., Xie, C., Li, Y., Song, C. & Bolin, T. B. Sulfur poisoning mechanism of steam reforming catalysts: an X-ray absorption near edge structure (XANES) spectroscopic study. Phys. Chem. Chem. Phys. 12, 5707–5711 (2010).
Xie, C., Chen, Y., Engelhard, M. H. & Song, C. Comparative Study on the Sulfur Tolerance and Carbon Resistance of Supported Noble Metal Catalysts in Steam Reforming of Liquid Hydrocarbon Fuel. ACS Catal. 2, 1127–1137 (2012).
Nikolla, E., Holewinski, A., Schwank, J. & Linic, S. Controlling Carbon Surface Chemistry by Alloying: Carbon Tolerant Reforming Catalyst. J. Am. Chem. Soc. 128, 11354–11355 (2006).
Pena, M. A. & Fierro, J. L. G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 101, 1981–2017 (2001).
Suntivich, J. et al. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat. Chem. 3, 546–550 (2011).
Ogawa, M. & Kuroda, K. Photofunctions of Intercalation Compounds. Chem. Rev. 95, 399–438 (1995).
Ida, S. et al. Photoluminescence of Perovskite Nanosheets Prepared by Exfoliation of Layered Oxides, K2Ln2Ti3O10, KLnNb2O7 and RbLnTa2O7 (Ln: Lanthanide Ion). J. Am. Chem. Soc. 130, 7052–7059 (2008).
Jin, H., Rhim, S. H., Im, J. & Freeman, A. J. Topological Oxide Insulator in Cubic Perovskite Structure. Sci. Rep. 3, 1651 (2013).
Meyers, D. et al. Zhang-Rice physics and anomalous copper states in A-site ordered perovskites. Sci. Rep. 3, 1834 (2013).
Zhou, H. et al. Leaf-architectured 3D Hierarchical Artificial Photosynthetic System of Perovskite Titanates towards CO2 Photoreduction into Hydrocarbon Fuels. Sci. Rep. 3, 1667 (2013).
Ball, J. M., Lee, M., Heya, A. & Snaith, H. J. Low-temperature processed meso-superstructured to thin-film perovskite solar cells. Energy Environ. Sci. 6, 1739–1743 (2013).
Dong, D. et al. Eggshell membrane-templated synthesis of highly crystalline perovskite ceramics for solid oxide fuel cells. J. Mater. Chem. 21, 1028–1032 (2011).
Park, D. H., Kim, J. E., Oh, J. M., Shul, Y. G. & Choy, J. H. DNA Core@Inorganic Shell. J. Am. Chem. Soc. 132, 16753–16753 (2010).
Li, S. et al. Soot trapping and combustion on nanofibrous perovskite LaMnO3 catalysts under a continuous flow of soot. Appl. Catal. B-Environ. 93, 383–386 (2010).
Nuraje, N. et al. Biotemplated Synthesis of Perovskite Nanomaterials for Solar Energy Conversion. Adv. Mater. 24, 2885 (2012).
Blanco, J. et al. Novel One-Step Synthesis of Porous-Supported Catalysts by Activated-Carbon Templating. Adv. Mater. 18, 1162–1165 (2006).
Civera, A., Pavese, M., Saracco, G. & Specchia, V. Combustion synthesis of perovskite-type catalysts for natural gas combustion. Catal. Today 83, 199–211 (2003).
Kim, H. J., Nam, K. H. & Shul, Y. G. Preparation of TiO2 fiber and its photocatalytic properties. Mater. Sci. Forum 439, 271–276 (2003).
Mota, N. et al. Insights on the role of Ru substitution in the properties of LaCoO3-based oxides as catalysts precursors for the oxidative reforming of diesel fuel. Appl. Catal. B-Environ. 113–114, 271–280 (2012).
Mawdsley, J. R., Vaughey, J. T. & Krause, T. R. Neutron Diffraction Studies of Nickel-Containing Perovskite Oxide Catalysts Exposed to Autothermal Reforming Environments. Chem. Mater. 21, 4830–4838 (2009).
Rida, K. et al. Effect of calcination temperature on the structural characteristics and catalytic activity for propene combustion of sol–gel derived lanthanum chromite perovskite. Appl. Catal. A-Gen. 327, 173–179 (2007).
Fino, D., Russo, N., Cauda, E., Saracco, G. & Specchia, V. La–Li–Cr perovskite catalysts for diesel particulate combustion. Catal. Today 114, 31–39 (2006).
Matatov-Meytal, Y. & Sheintuch, M. Catalytic fibers and cloths. Appl. Catal. A-Gen. 231, 1–16 (2002).
Sunarso, J., Torriero, A. J., Zhou, W., Howlett, P. C. & Forsyth, M. Oxygen Reduction Reaction Activity of La-Based Perovskite Oxides in Alkaline Medium: A Thin-Film Rotating Ring-Disk Electrode Study. J. Phys. Chem. C 116, 5827–5834 (2012).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A32, 751–767 (1976).
Gaur, S., Pakhare, D., Wu, H., Haynes, D. J. & Spivey, J. J. CO2 Reforming of CH4 over Ru-Substituted Pyrochlore Catalysts: Effects of Temperature and Reactant Feed Ratio. Energ. Fuel. 26, 1989–1998 (2012).
Qiao, L. & Bi, X. Direct observation of Ni3+ and Ni2+ in correlated LaNiO3−δ films. EPL 93, 57002p1–57002p6 (2011).
Ertl, G., Knozinger, H. & Weitkamp, J. Handbook of Heterogeneous Catalysis, Wiley VCH, Weinheim (1997).
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2009-C1AAA001-2009-0092926 and No. 2012R1A2A2A02011268) and we thank GS Caltex Corporation for partial support.
Author information
Authors and Affiliations
Contributions
Y.K.J., D.H.P. and J.I.P. carried out the experiments, analyzed the data and wrote the manuscript. S.H.Y., I.M. and J.H.C. contributed to critical discussions on gas oil analyses and material characterizations. Y.G.S. and J. I. P. conceived the original idea, designed and supervised the whole study and made critical comments on the manuscript. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supporting Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Jeon, Y., Park, DH., Park, JI. et al. Hollow Fibers Networked with Perovskite Nanoparticles for H2 Production from Heavy Oil. Sci Rep 3, 2902 (2013). https://doi.org/10.1038/srep02902
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02902
This article is cited by
-
Platinum incorporation into titanate perovskites to deliver emergent active and stable platinum nanoparticles
Nature Chemistry (2021)
-
Magnetic and optical properties of LaCr1-xGaxO3: the effect of Ga doping
Applied Physics A (2021)
-
La-enhanced Ni nanoparticles highly dispersed on SiC for low-temperature CO methanation performance
Rare Metals (2021)
-
Synthesis and microstructures of La1−xCaxCrO3 perovskite powders for optical properties
Journal of Materials Science: Materials in Electronics (2019)
-
Ferromagnetism in LaMnO3 Nanoparticles Prepared by Sol–Gel Method Combined with Polyvinyl Alcohol
Journal of Electronic Materials (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.