Abstract
Chlorinated aliphatic hydrocarbons and chlorinated aromatic hydrocarbons (CHCs) are toxic and carcinogenic contaminants commonly found in environmental samples and efficient online detection of these contaminants is still challenging at the present stage. Here, we report an advanced Fourier transform infrared spectroscopy-attenuated total reflectance (FTIR-ATR) sensor for in-situ and simultaneous detection of multiple CHCs, including monochlorobenzene, 1,2-dichlorobenzene, 1,3-dichlorobenzene, trichloroethylene, perchloroethylene and chloroform. The polycrystalline silver halide sensor fiber had a unique integrated planar-cylindric geometry and was coated with an ethylene/propylene copolymer membrane to act as a solid phase extractor, which greatly amplified the analytical signal and contributed to a higher detection sensitivity compared to the previously reported sensors. This system exhibited a high detection sensitivity towards the CHCs mixture at a wide concentration range of 5~700 ppb. The FTIR-ATR sensor described in this study has a high potential to be utilized as a trace-sensitive on-line device for water contamination monitoring.
Similar content being viewed by others
Introduction
Chlorinated aliphatic hydrocarbons and chlorinated aromatic hydrocarbons (CHCs) are a class of highly toxic and persistent contaminants commonly found in the environment1, which pose a substantial threat to the ecological system and human health2. Thus, effective monitoring and control of these contaminants are essential. For the detection of CHCs in acqueous environment, a variety of chromatographic (e.g., high-performance liquid chromatograhphy (HPLC)3, gas chromatography (GC)4, gas chromatography and mass spectrometry (GC/MS)5) and spectroscopic (e.g., ultraviolet-visible (UV)6, Raman7 and fluorescence8 spectrometry) methods have been proposed and used. These techniques generally show high a detection sensitivity, however, usually involve complex and time-consuming pre-concentration/extraction steps9 and are mostly confined to laboratory use. At present, sensitive online detection techniques for in-situ and continuous monitoring of CHCs in water are still lacking. This gap has inspired the present study to develop more suitable online detection techiques through taking advantage of the inherent molecular selectivity of infrared attenuated total reflectance (ATR) spectroscopy.
Fourier transform infrared spectroscopy (FTIR) spectroscopy is known as a rapid and non-destructive detection technology for organic constituents due to the generated fingerprint spectra. Especially, the combined use of ATR with FTIR enables a direct examination of liquid phase samples without further preparation and has gained increasing polularity in recent years. ATR uses the principle of internal total reflection to generate an evanescent field, which emanates at the waveguide surface and penetrates a few micrometers into the adjacent sample volume10. However, a major challenge of FTIR-ATR is its relatively low detection sensitivity, which limits its practical implementation. The detection sensitivity of FTIR-ATR is largely governed by the properties of the ATR waveguide. Typically, crystalline ATR elements are used, which may be divided into three main categories according to the material properties: glass-like fibers (e.g., chalcogenides and heavy metal fluorides), polycrystalline (e.g., silver halides) or crystalline fibers (e.g., sapphire) and hollow waveguides (e.g., hollow silica or sapphire tubes)11. Among these, silver halide (AgClxBr1−x) fibers are considered the most practical ATR waveguide material11, which provides access to the entire mid-infrared (MIR) spectral range of interests (3–18 μm)12 and allows flexible tuning of its geometry13. AgClxBr1−x fibers have been demonstrated to offer direct and accurate detection of crude oil in a deionized water matrix14 and of water in hexane at ppm levels15. Hence, directly probing water contaminants may be achieved by immersing a ATR-based fiber into an aqueous solution serving as the active transducer16. However, ppm-level detection sensitivity is still insufficient to meet the detection requirements for CHCs in real water samples, as most constituents are usually present at ppb levels. Moreover, the considerable background noise signals in real water samples presents an additional challenge. The detection senstivity may be signficantly lowered by reducing the spectral noise arising from the interfering absorbance of water (O-H stretching vibration at 3300 cm−1; O-H bending vibration at 1640 cm−1; combination vibration at 2100 cm−1; and libration vibration at 750 cm−1)14 and other background molecules15. Thus, to improve the detection sensitivity, efforts are needed to further increase the signal-to-noise ratio of such fiberoptic ATR transducers.
Here, we describe an advanced FTIR-ATR sensor with ppb-level sensitivity towards detection of water contaminants like CHCs. This high sensitivity was enabled by the adoption of an unique fiber geometry, as well as an efficient enrichment coating at the fiber surface, which greatly enhanced the analytical signal for CHCs while reducing the background noise levels. First of all, a planar AgClxBr1−x fiber segment serving as active transducer with cylindrical extensions at both ends was used. It has been reported that the intensity of the evanescent field in ATR spectroscopy may be substantially enhanced by tuning the waveguide geometry17. In this context, decreasing the fiber diameter or tapering a section of the fiber are both effective strategies to improves the analytical sensitivity18. Especially, the geometry of a planar sensing region with fiberoptic couplers at both ends has been reported to substantially increase the number of internal reflections, thereby leading to enhanced signals - up to one order of magnitude - compared to conventional cylindrical fibers13. In addition, to further amplify the detection signal and reduce the noise, a polymer membrane was coated onto the sensor surface. Polymeric enrichment coatings have been proven as an effective strategy to enhance the detection sensitivity and robustness of IR fiberoptic sensors19. On the one hand, particular chemical recognition membranes (e.g., polyisobutylene, ethylene-propylene copolymer and Teflon AF) have been reported to selectively enrich the targeted species at the fiber surface and within the evanescent field facilitating their detection20. On the other hand, the interference of water and other species can be dramatically reduced using a hydrophobic membrane; furthermore, to a certain degree the sensor may also be protected from harsh environments by such a coating19. Thus, in our system a hydrophobic ethylen/propylene copolymer (E/P-co) coating, which is ideally suited for enrichment of hydrophobic hydrocarbons21 including CHCs2,22 was used.
In this study, the combined use of the integrated planar-cylinder fiber geometry and of a highly-sensitive E/P-co coating substantially enhanced the detection sensitivity of the FTIR-ATR sensor for CHC monitoring, thereby far exceeding the state-of-art performance of such sensors. Furthermore, simultenous and in-situ detection of six common CHCs pollutants including monochlorobenzene (MCB), 1,2-dichlorobenzene (o-DCB), 1,3-dichlorobenzene (m-DCB), trichloroethylene (TCE), perchloroethylene (PCE) and chloroform (CF) at ppb levels was successfully achieved. Consequently, the present study may guide the design and implementation of highly sensitive FTIR-ATR sensors for online monitoring of CHCs in complex aqueous environments and pollution scenarios.
Results
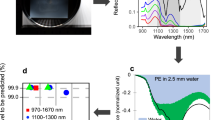
A mixture of PCE, TCE, CF, o-DCB, MCB, m-DCB (each with a concentration of 500 ppb (v/v)) was prepared and used for testing the sensor. The ATR spectrum of the mixture after 30-min enrichment is shown in Figure 1. The characteristic absorption bands of each CHC could be readily identified according to molecule-specific C-H vibrations in the fingerprint region of the IR spectrum. To reduce the measurement interference caused by peak overlapping at between 740 and 770 cm−1, several strategies were adopted, e.g., using the peak height as the indicator for analyte concentration calibration; processing the spectrum data using baseline correction and curve-fitting. The peak at 776 cm−1 is ascribed to PCE, which has two feature peaks. Therefore, to avoid interference, the other peak of PCE at 910 cm−1 was chosen for its concentration calibration. Thus, all CHCs could be simultaneously detected, discriminated and quantified within a single measurement. The characteristic bands for quantitative determination of these CHCs are: TCE at 930 cm−1, PCE at 910 cm−1, m-DCB at 867 cm−1, CF at 760 cm−1, o-DCB at 748 cm−1 and MCB at 740 cm−1.
Although the sensor performance might be affected by many factor, e.g., flow velocity, flow channel height and analyte diffusion properties within the aqueous phase22, in our system only the flow velocity was tunable. This parameter was set to 0.2 mL/min to enable measurements in the time regime of several minutes without using excessive amounts of anlyte solution.
For maximizing the analytical signal, a certain period of time is needed to allow sufficient enrichment of the analytes. The infrared aborbance of selected CHCs vs. the enrichment time in a typical measurement scenario is illustrated in Figure 2. It is clearly evident that for all analytes a diffusion equilibirum is reached after approximately 30 min, which was adopted during the subsequent experiments.
To validate the performance of the as-prepared sensor for CHC detection, the absorbance vs. concentration calibration functions for each analyte in the mixture in different concentration ranges were experimentally determined (Figs. 3 and 4) confirming the excellent analytical accuracy of the developed sensor system for simultaneous detection of multiple CHCs both at moderate (100~700 ppb) and low (5 to 70 ppb) concentration ranges (R2 values above 0.9 for all analytes except for TCE). The slighlty poorer detection performance for TCE is likely attributed to the reduced enrichment of this compound and a lower absorbance coefficient2 compared to other CHCs. The difference in the calibration slope/sensitivity between different concentration ranges was originated from the different rates of CHCs diffusing into the polymer, which is governed by both thermodynamics and dynamics. At a higher concentration, more time is needed to achieve diffusion equilibrium. Therefore, the insufficient enrichment of CHC at a higher analyte concentration might have led to a lower sensitivity compared with that at a lower concentration. In addition, the higher range of concentration variation (i.e., 600 ppb) under the high concentration conditions might have also contributed to the lower R2 compared with that under the low concentration conditions when a linear correlation is not strictly followed. The obtained results confirm that the IR-ATR fiberoptic sensor could serve as an efficient device to probe trace amounts of multiple CHCs in aqueous environments with high sensitivity, wide detection ranges and reduced background interferences.
Discussion
The utility of ATR techniques has greatly advanced IR spectroscopy as a flexible and tunable in-situ detection technology. IR-ATR spectroscopy has proven to be an effective method for directly probing water contaminants, yet, to date its detection resolution remains insufficient to meet practical implementation requirements. Here, we demonstrate that the detection capability of such sensor systems could be remarkedly enhanced toward practically relevant detection levels by combining a unique transducer geometry with an appropriate enrichment coating.
The combined planar-cylinder geometry of the transducer facilitated an increased number of internal reflections and led to an amplified absorbance signal (Fig. 5a and c). According to Snell's law, total internal reflection occurs at the interface of two dielectric media, if light is incident from the optically denser medium at the interface at an angle large than the critical angle θc. Here, θc is defined as23

where n1 and n2 are the refractive indices of the waveguide and the surrounding medium, respectively.
By interaction of the incident and reflected light wave, an exponentially decaying evanescent field is established, which penetrates exponentially decaying a certain distance into the surrounding medium24. Theoretically, the number of internal reflections (n) in a waveguide can be calculated as

where L is the length of the total sensing length, d is the waveguide thickness, a is the distance between each reflection.
Thus, with the same L level and given the same incident light angle θ, the waveguide with a planar-cylinder integrated geometry allows more internal reflections compared to a purely cylindrical waveguide without flattened segment. It is estimated that there are hundreds of internal reflections present within this comfiguration, i.e., approximately 5 times more compared to a conventional fiberoptic waveguide and approximately 50 times more compared to a commercial trapezoidal ZnSe waveguide element with only one usable detection surface2
In an IR-ATR system, the amplitude of the evanescent field E(x) within a medium decreases exponentially with the distance x to the fiber-medium interface following24

where E0 represent the intensity and dp is the light penetration depth.
Thus, after n internal reflections within the waveguide, a total Etotal(x) may be obtained (usually: x1 = x2 = … = xn):

Therefore, the absorbance increases with n, i.e., more internal reflections led to higher absorbance signals.
In addition to the geometric effect, the use of an E/P-co coating in the present system also considerably amplified the detection signal via the selective enrichment of CHCs. It has been reported that E/P-co not only facilitates the selective enrichment of non-polar analytes24, but also decreases the interference signals of water due to its hydrophobic nature. Hence, CHCs are preferentially partitioning into the polymer via Van der Waals forces or dipole-dipole interactions. The degree of enrichment, i.e, the amount of a target analyte absorbed by the membrane, is highly dependent on its equilibrium-state distribution between the polymer and water phase, which is expressed by the partition coefficient (K)25. A higher value of K indicates that the analyte more efficiently enriches into the polymer. In our case, the K values of E/P-co for MCB, PCE and CF were 258, 846 and 23 respectively21, thereby indicating the excellent enrichment capability for most CHCs. The relatively low value of CF is mainly due to its high water solubility26.
However, it should be noted that for efficient detection via the evanescent field it is essential that the absorbed constituents rapidly diffuse into the polymer matrix toward the waveguide surface. Thus, the diffusion of CHCs within the E/P-co coating as well as the coating thickness are also critical factors to determine the detection performance19.
The diffusion rate of hydrocarbons within polymers may be affected by a variety of factors including the degree of crystallinity, the glass transition temperature Tg of the polymer and the size and structure of the hydrocarbon molecules. In the present system, amorphous E/P-co (i.e., 0% crystallinity)21 was adopted. The comparatively long distances between the disordered chains of amorphous E/P-co provide sufficient void volume, which facilitates rapid diffusion of CHCs. Additionally, the small molecular dimensions of CHCs in the range of 100–200 Å3 (calculated via ChemWindow by Roy and Mielczarski2) also favor rapid diffusion.
The coating thickness is a critical parameter that affects the diffusion time27. An appropriate coating thickness may be estimated according to the penetration depth (dp) of the evanescent field, which can be expressed by

where λ is the wavelength of the incident light. For our system, the typical values of these variables are: n1 = 2.2 (AgClxBr1−x); n2 = 1.48 (E/P-co); θ = 75°; λ = 10 μm (i.e., 1000 cm−1).
Hence, an approximated value of dp was calculated as:

It is recommended that the coating thickness should be roughly three times of the penetration depth to ensure sufficient detection sensitivity via enrichment enhancement, yet an acceptable short response time21. Thus, in the present studies a coating thickness of 2.91 μm was selected as optimal. In addition, the E/P-co coating not only enables an augmentation of the analytical signal, but also offers protection to the waveguide via isolation from corrosive or fouling substances, therefore extending the long-term stability of the sensor.
Nowadays, a number of detection techniques are been used for the analysis of CHCs and other volatile organic compounds (VOCs). Table 1 shows a comparative evaluation of their performance. For conventional chromatography (including GC/FID, GC/MS and HPLC )28,29,30,31, off-line sample pretreatment and preconcentration steps are required. Although latest attempts circumventing this step via e.g., membrane inlet proton transfer reaction mass spectrometry (MI-PTRMS) techniques32 and coupling of membrane inlets GC/MS33, the rather complex operating procedures and high instrumental costs still limit their widespread application. Other techniques such as quartz-crystal microbalance (QCM) sensors and enzyme-linked immunosorbent assay (ELISA) also allow for direct and in-situ measurements. However, the operation of QCMs in liquid is complicated and time-consuming, as the the film coated on the gold electrode of an QCM needs to be exposed to hot air after analyte absorption and detection may not start/continue until the initial resonance frequency of the quartz crystal has been recovered34, while ELISA may only detect one specific target at a time and is fairly costly35. In comparison, spectroscopic methods are relatively simple and allow rapid detection. However, UV spectroscopy may not discriminate individual compounds in a mixture of VOCs6, while Raman spectroscopy7 may only reach detectivities at the ppm level for VOCs even when combined with a solid-phase microextraction preconcentration step. Fluorescence spectroscopy8 allows simultaneous detection of multiple species, but it may only detect luminescent contaminants and suffers from severe interference by the chlorine atom within the CHCs and other fluorescence quenching substances in water.
Hence, IR-ATR sensors appear to be among the most promising methods to directly detect multiple CHCs and other VOCs with high sensitivity and accuracy. Using appropriate polymer matrices for in-situ enrichment at the fiber surface along with advanced sensor geometries, limits of detection at mid- to low-ppb levels may be achieved, thus suggesting a high potential for practical implementation in water contamination monitoring.
More importantly, our previous studies have demonstrated that such detection processes are not interfered by salinity, humic acids and turbidity36, thus implying a substantial potential for the detection of other organic pollutants in environmental matrices37. However, despite these prospects, currently there are still some challenges remaining toward real-world implementations. For example, the detection accuracy and sensitivity for selected contaminants - such as TCE in the present study - are yet to be improved to meet the practical demands; the long-term performance may be affected by long-term diffusion of water and other substances into the polymer coating. Therefore, future efforts are needed to further improve the detection senstivity and stability of such sensing systems. This can be achieved by a further optimization of the fiber design, material and operations (e.g., solvent flow rate and enrichment time) as well as a combined use of this method with other high-precise techniques (e.g., tunable quantum cascade lasers in lieu of FTIR).
In summary, CHC detection by using an advanced IR-ATR sensor system with a dedicated transducer geometry and an E/P-co enrichment coating was evaluated. This sensor enables sensitive, rapid and simultaneous detection of several CHCs at ppb concentration levels in one-step measurement procedure without any sample pretreatment. Furthermore, a substantial improvement in both the detection range (5~700 ppb) and sensitivity over previously reported IR-ATR sensors has been obtained. Thus, this IR-ATR sensor may be used as a powerful tool for on-line, in-situ and sensitive probing of multiple water pollutants and has a substantial potential for practical water contamination monitoring.
Methods
Methanol, PCE, TCE, CF, o-DCB, MCB, m-DCB, n-hexane (all of analytical grade) and E/P-co (60:40) were purchased from Aldrich (Milwaukee, USA). All solutions were prepared using deionized water. A minute amount of methanol was added serving as solubility mediator to ensure full dissolution the CHCs in water, which causes no inference to the sensor readings according to previous studies36.
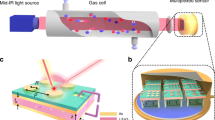
An integrated planar-cylinder fiber was used as active transducer, which consisted of a flatten silver halide fiber segment (refractive index of the waveguide n1 = 2.237, length 45 mm, thickness 150 μm) with fiberoptic extensions (length 15 mm, thickness 700 μm) at both ends (Fig. 6).
E/P-co solution (1%, v/v) was prepared by dissolving E/P-co into n-hexane. Then, the solution was dip coated onto the fiber surface to form a thin film38. The film thickness was controlled at 2.9 μm, which approximately matched the information depth of the evanescent field.
Evanescent field measurements were performed by focusing the external collimate IR beam from a Bruker Vertex 70 spectrometer (Bruker Optics, Billerica, USA) onto the facet of the cylindrical extension connected to the flattened silver halide fiber segment using a gold-coated, off-axis, parabolic mirror (OAPM, focal length: 25.4 mm; Janos Optics, Townshend, USA). Light from the distal end of the transducer was focused onto a liquid nitrogen-cooled photoconductive mercury-cadmium-telluride detector (detector element 1 × 1 mm, Infrared Associates, USA). Spectra were recorded in a frequency window of 4000–400 cm−1 averaging 128 scans at a spectral resolution of 2 cm−1. A schematic along with an image of the experimental setup is shown in Figure 6.
References
Acha, V. et al. ATR-FTIR sensor development for continuous on-line monitoring of chlorinated aliphatic hydrocarbons in a fixed-bed bioreactor. Biotechnol. Bioeng. 68, 473 (2000).
Roy, G. & Mielczarski, J. A. Infrared detection of chlorinated hydrocarbons in water at ppb levels of concentrations. Water Res. 36, 1902 (2002).
Liska, I. Fifty years of solid-phase extraction in water analysis - historical development and overview. J. Chromatogr. A 885, 3 (2000).
Wilkins, A. L., Singh-Thandi, M. & Langdon, A. G. Pulp mill sourced organic compounds and sodium levels in water and sediments from the Tarawera River, New Zealand. Bull. Environ. Contam. Toxicol. 57, 434 (1996).
Martinez, E. et al. Multicomponent analysis of volatile organic compounds in water by automated purge and trap coupled to gas chromatography-mass spectrometry. J. Chromatogr. A 959, 181 (2002).
Wittkamp, B. L., Hawthorne, S. B. & Tilotta, D. C. Determination of aromatic compounds in water by solid phase microextraction and ultraviolet absorption spectroscopy. 1. Methodology. Anal. Chem. 69, 1197 (1997).
Wittkamp, B. L. & Tilotta, D. C. Determination of BTEX compounds in water by solid-phase microextraction and raman spectroscopy. Anal. Chem. 67, 600 (1995).
Carr, J. W. & Harris, J. M. In situ fluorescence detection of polycyclic aromatic hydrocarbons following preconcentration on alkylated silica adsorbents. Anal. Chem. 60, 698 (1998).
Lin, W. & Li, Z. Detection and quantification of trace organic contaminants in water using the FT-IR-attenuated total reflectance technique. Anal. Chem. 82, 505 (2010).
Hanick, N. J. Internal Reflection Spectmscopy. Interscience Publishers: New York, 1967.
Mizaikoff, B. Mid-IR fiber-optic sensors. Anal. Chem. 75, 258A (2003).
Griffiths, P. R. & Christopher, C. C. Handbook of Vibrational Spectroscopy. John Wiley & Sons: New York, 2002.
Kosower, E. M. et al. Surface-enhanced infrared absorption and amplified spectra on planar silver halide fiber. J. Phys. Chem. B 108, 12633 (2004).
Luzinova, Y. I. et al. In-situ trace analysis of oil in water with mid-infrared fiberoptic chemical sensors. Anal. Chem. 84, 1274 (2012).
Luzinova, Y. et al. Detecting trace amounts of water in hydrocarbon matrices with infrared fiberoptic evanescent field sensors. Analyst 137, 333 (2012).
Küpper, L. et al. Novel developments in mid-IR fiber-optic spectroscopy for analytical applications. J. Mol. Struct. 563–564, 173 (2001).
Simhony, S. et al. Evanescent wave infrared spectroscopy of liquids using silver halide optical fibers. J. Appl. Phys. 64, 3732 (1988).
Vongsvivut, J. et al. Characterization of supported cylinder-planar germanium waveguide sensors with synchrotron infrared radiation. Appl. Spectrosc. 58, 143 (2004).
Flavin, K. et al. A comparison of polymeric materials as pre-concentrating media for use with ATR/FTIR sensing. Int. J. Environ. Anal. Chem. 86, 401 (2006).
Walsh, J. E. et al. Sensing of chlorinated hydrocarbons and pesticides in water using polymer coated mid-infrared optical fibres. Analyst 121, 789 (1996).
Göbel, R. et al. Investigation of different polymers as coating materials for IR/ATR Spectroscopic trace analysis of chlorinated hydrocarbons in water. Appl. Spectrosc. 48, 678 (1994).
Fedorov, A. G. et al. Model-based optimal design of polymer-coated chemical sensors. Anal. Chem. 75, 1106 (2003).
Walsh, J. E. et al. Midinfrared fiber sensor for the in-situ detection of chlorinated hydrocarbons. SPIE Proceeding 233 (1995).
Mizaikoff, B. Mid-infrared evanescent wave sensors - a novel approach for subsea monitoring. Meas. Sci. Technol. 10, 1185 (1999).
Jakusch, M. & Mizaikoff, B. Selective polymer materials: Absolute determination of their sorption properties. SPIE Proceedings 4205, 93 (2001).
Haynes, W. M. CRC Handbook of chemistry and physics. CRC Press/Taylor and Francis: Boca Raton, FL. 2012.
Langenfeld, J. J., Hawthorne, S. B. & Miller, D. J. Quantitative analysis of fuel-related hydrocarbons in surface water and wastewater samples by solid-phase microextraction. Anal. Chem. 68, 144 (1996).
Hennion, M. C. Solid-phase extraction: method development, sorbents and coupling with liquid chromatography. J. Chromatogr. A 856, 3 (1999).
Zhang, Z. & Pawliszyn, J. Quantitative extraction using an internally cooled solid phase microextraction device. Anal. Chem. 67, 34 (1995).
Silva, F. C., de Carvalho, C. R. & de Cardeal, Z. L. Solid-phase microextraction method for the quantitative analysis of styrene in water. J. Chromatogr. Sci. 38, 315 (2000).
Chen, M. et al. Analyses of nitrobenzene, benzene and aniline in environmental water samples by headspace solid phase microextraction coupled with gas chromatography-mass spectrometry. Chin. Sci. Bull. 51, 1648 (2006).
Boscaini, E. et al. Membrane inlet proton transfer reaction mass spectrometry (MI-PTRMS) for direct measurements of VOCs in water. Int. J. Mass Spectrom. 239, 171 (2004).
Chang, C. C. & Her, G. R. On-line monitoring trihalomethanes in chlorinated water by membrane introduction-fast gas chromatography mass-spectrometry. J. Chromatogr. A 893, 169 (2000).
Ayad, M. M., El-Hefnawey, G. & Torad, N. L. Quartz crystal microbalance sensor coated with polyaniline emeraldine base for determination of chlorinated aliphatic hydrocarbons. Sens. Actuator B-Chem. 134, 887 (2008).
Sanvicens, N. et al. Determination of haloanisols in white wine by immunosorbent solid-phase extraction followed by enzyme-linked immunosorbent Assay. J. Agric. Food Chem. 54, 9176 (2006).
Kraft, M. & Mizaikoff, B. A mid-infrared sensor for monitoring of chlorinated hydrocarbons in the marine environment. Int. J. Environ. Anal. Chem. 78, 367 (2000).
Karlowatz, M., Kraft, M. & Mizaikoff, B. Simultaneous quantitative determination of benzene, toluene and xylenes in water using mid-infrared evanescent field spectroscopy. Anal. Chem. 76, 2643 (2004).
Mizaikoff, B. et al. Water monitoring using infrared fiber optic sensors. Proceedings of OCEANS' 98 Conference 1401 (1998).
Acknowledgements
The authors wish to thank the National Basic Research Program of China (2011CB933700), the Natural Science Foundation of China (51129803 and 21261160489) and the Program for Changjiang Scholars and Innovative Research Team in University, China for the partial support of this work.
Author information
Authors and Affiliations
Contributions
R.L., B.M. and H.Q.Y. designed the experiments; R.L., C.Q. and Y.R. conducted the experiments; R.L., B.M., A.K. and H.Q.Y. contributed to the planning and coordination of the project; R.L., B.M., W.W.L., G.P.S. and H.Q.Y. wrote and edited the manuscript. All authors contributed to discussion about the results and the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Lu, R., Mizaikoff, B., Li, WW. et al. Determination of Chlorinated Hydrocarbons in Water Using Highly Sensitive Mid-Infrared Sensor Technology. Sci Rep 3, 2525 (2013). https://doi.org/10.1038/srep02525
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02525
This article is cited by
-
IR absorption spectra for chlorinated ethenes in water, calculated using density functional theory
Multiscale and Multidisciplinary Modeling, Experiments and Design (2019)
-
Enhanced Fenton-like Degradation of Trichloroethylene by Hydrogen Peroxide Activated with Nanoscale Zero Valent Iron Loaded on Biochar
Scientific Reports (2017)
-
Separation and determination of estrogen in the water environment by high performance liquid chromatography-fourier transform infrared spectroscopy
Scientific Reports (2016)
-
High-sensitivity infrared attenuated total reflectance sensors for in situ multicomponent detection of volatile organic compounds in water
Nature Protocols (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.