Abstract
The objective of this study was to explore the potential of CO2 single contrast in-line phase contrast imaging (PCI) for pre-clinical small intestine investigation. The absorption and phase contrast images of CO2 gas production were attained and compared. A further increase in image contrast was observed in PCI. Compared with CO2-based absorption contrast imaging (ACI), CO2-based PCI significantly enhanced the detection of mucosal microstructures, such as pits and folds. The CO2-based PCI could provide sufficient image contrast for clearly showing the intestinal mucosa in living mice without using barium. We concluded that CO2-based PCI might be a novel and promising imaging method for future studies of gastrointestinal disorders.
Similar content being viewed by others
Introduction
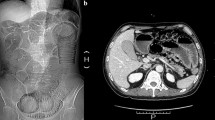
For a long time, the small intestine has been the most difficult segment of the gastrointestinal tract to examine1,2,3. Endoscopy is a valuable tool in the evaluation of small intestinal disease, but the procedure usually produces discomfort. Moreover, most part of the small intestine is inaccessible to endoscopy4. Capsule endoscopy is a good way to noninvasively visualize the entire small bowel mucosa2,5. However, the lesions are usually detected by accident, so they may be missed if the capsule endoscopy does not take an image as it moves through an area of disease1. CO2 -barium double contrast gastrointestinalgraphy is a common clinical tool to assess gastrointestinal conditions. Before the examination, some aerogenic powders are taken orally by the patient. Aerogenic powders mainly contain a mixture of citric acid and NaHCO3. Reactions between the dry powders won't take place until they encounter water. The chemical reaction equation is as follows: citric acid + NaHCO3 +water → sodium citrate + CO2 + water. The gastrointestinal tract will be expanded by the produced CO2 gas. The CO2 -barium double contrast x-ray imaging can not only provide optimal visualization of mucosal abnormalities, but also evaluate the intestinal peristaltic function. However, barium will be forbidden if a patient is suspected of gastrointestinal perforation or complete obstruction. In this case, CO2 single contrast x-ray imaging seems to be a safe method to be chosen. Still, conventional radiography always provides poor image contrast for soft tissues which have low differential absorption of x-rays. Because the gastrointestinal tract is the soft tissue tube and the density difference between the mucosa and CO2 is small, the mucosal structures were hardly detected by CO2 single contrast gastrointestinalgraphy (Fig. 1).
The gastrointestinalgraphy of a 50-year-old female patient with abdominal discomfort after administration of CO2 gas only (a), or CO2 gas and barium (d).
(b–c) and (e–f) are magnified images of the red boxed regions in (a) and (d), respectively. Note that CO2-barium double contrast gastrointestinalgraphy (e–f) provided clear visualization of mucosal folds (asterisks) and intermucosal grooves (arrowheads), which were hardly shown on CO2 single contrast gastrointestinalgraphy (b–c).
It has been demonstrated that synchrotron radiation phase contrast imaging (SR-PCI) has outstanding potential for medical applications6,7. By utilizing the phase shift, SR-PCI can offer superb image contrast for soft tissues8,9,10,11,12. Because the gas has a different refractive index from the surrounding soft tissues, the gas–tissue interfaces create significant phase shifts to make the boundaries highly visible in PCI8,13. By means of the detection of the phase shift generated by intestinal mucosa and CO2, PCI may enable to provide clear visualization of CO2-filled mucosa of small intestine without using barium.
In this study, we first report the pre-clinical applications of CO2-based in-line PCI for small intestine research. CO2-based SR absorption contrast imaging (ACI) and PCI were performed and compared. CO2 gas produced by aerogenic powders was imaged real-timely by PCI. Additionally, the potential of CO2 single contrast PCI for showing small intestinal mucosal microstructures in living mice was investigated.
Results
Comparison between CO2 gas-based ACI and PCI
No CO2 bubbles could be obtained before physiological saline injection (Fig. 2c,g). After physiological saline was injected, aerogenic powders reacted with water to produce CO2 gas. However, the difference in the x-ray absorption of the CO2 gas and physiological saline is small, so the CO2 bubbles could hardly shown in ACI (Fig. 2e). Since CO2 has a different refractive index from physiological saline, the gas–physiological saline interfaces created significant phase shifts to make the boundaries extremely visible in PCI (Fig. 2i).
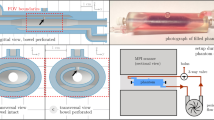
ACI and PCI of CO2 gas production.
(a) The experimental setup for generating CO2 gas. Aerogenic powders were placed in a polyethylene tube which was perpendicular to the x-ray beam. Physiological saline was injected to the aerogenic powders through a PE-50 tube connected to a syringe pump. ACI (b,d) and PCI (f,h) of aerogenic powders before (b,f) and after (d,h) the physiological saline injection. (c), (e), (g) and (i) are magnified images of the red boxed regions in (b), (d), (f) and (h), respectively. Note that the production of CO2 bubbles could be clearly displayed in phase contrast image (i), but not in absorption contrast image (e). The pixel size was 9 μm × 9 μm; the exposure time was 3 ms. AP indicates aerogenic powders; PS indicates physiological saline. Asterisks indicate CO2 bubbles. Scale bars: 1 mm (b,d,f,h) and 200 μm (c,e,g,i).
Real-time PCI of CO2 gas production
The characteristic of high-temporal resolution for PCI contributes to clearly capturing the CO2 bubbles production process real-timely. CO2 gas was clearly shown to be continuously produced, meanwhile, the aerogenic powders gradually dissolved in the physiological saline (Fig. 3, Supplementary movie 1).
Representative, sequential PCI of CO2 gas production and aerogenic powders solution.
CO2 gas was continuously produced and the aerogenic powders gradually dissolved in the physiological saline. Images were obtained after the physiological saline injection at 0 s (a), 1 s (b), 30 s (c) and 4 min (d), respectively. The pixel size was 9 μm × 9 μm; the exposure time was 3 ms. AP indicates aerogenic powders. Scale bars: 1 mm.
ACI and PCI of small intestine ex vivo and in vivo
Because the difference between the absorption coefficients of intestinal mucosa and CO2 was small, no evident contrast could be detected by ACI (Fig. 4c, 5b). In comparison, PCI exploited the differences in the refractive index and enabled clear visualization of the intestinal microstructures (Fig. 4e, 5d). The CO2-based PCI could provide adequate image contrast for clearly showing the intestinal mucosa in living mice without using barium.
Ex vivo SR images of the same mouse duodenum with two sample-to-detector distances of 1 cm (b) and 60 cm (d).
(a) Schematic of the CO2 single contrast preparation. (c) and (e) are magnified images of the region in a red box in (b) and (d), respectively. Note that duodenal mucosal microstructures could be more visibly revealed on phase contrast image (e) than on absorption contrast image (c). The pixel size was 3.7 μm × 3.7 μm. The exposure time was 1 s. Asterisks indicate mucosal folds.
In vivo SR images of the same mouse duodenum with two sample-to-detector distances of 1 cm (a) and 60 cm (c).
(b) and (d) are magnified images of the region in a red box in (a) and (c), respectively. Note that duodenal mucosal microstructures could be more visibly revealed on phase contrast image (d) than on absorption contrast image (b). The pixel size was 9 μm × 9 μm. The exposure time was 5 ms.
Discussion
Conventional absorption imaging principally employs the absorption characteristics of x-rays and discriminates different tissues through the linear attenuation coefficient (μ), which is proportional to the density of the tissue14. By taking barium orally, the surface of the gastrointestinal mucosa will be coated with this strong x-ray absorption material. The image contrast can be highly increased to indirectly depict the mucosa. However, barium will be forbidden if bowel perforation or complete obstruction is suspected. Here, we introduce a pre-clinical imaging method to show the mouse mucosal microstructures without using barium. The characteristic of high temporal resolution for PCI is quite suitable for real-timely imaging and evaluating the intestinal peristaltic function. Also, PCI can offer high spatial resolution to clearly show several ten microns mouse mucosal folds or pits. Besides high resolution, high sensitivity for soft tissues is another important advantage for PCI. Compared with ACI, PCI can highly enhance image contrast between CO2 gas and water, indicating a good application of CO2 as phase contrast agent. CO2-based PCI utilizes the phase shifts created by the CO2–mucosa interfaces and makes the borders highly detectable. Therefore, the microstructures of the mucosa can be directly and noticeably depicted without using barium.
In summary, PCI is characterized by high spatial-temporal resolution, greatly enhanced contrast and good soft tissue discrimination. Compared with ACI, PCI is capable of creating notable visibility of CO2-filled intestinal mucosa. The CO2-PCI technique has great potential for showing small intestinal mucosal microstructure in living mice. It may become a novel in vivo imaging tool for future studies of gastrointestinal disorders.
Methods
Patient
A 50-year-old female patient with abdominal discomfort was enrolled for gastrointestinalgraphy with CO2 single contrast and CO2-barium double contrast. Informed consent was obtained from the patient before the examinations. The Ethics Committee for Clinical Research at Shanghai Jiao Tong University, China approved the study protocol.
SR parameters
Images were captured at the BL13W1 beamline in Shanghai Synchrotron Radiation Facility (SSRF, China). X-rays were produced from a 3.5 GeV electron storage ring and were monochromatized using a double-crystal monochromator with Si(111) and Si(311) crystals. The beamline covered an energy range of 8 to 72.5 keV. The energy resolution (△E/E) was less than 5 × 10−3. The transmitted x-rays were captured by a 100-μm thick CdWO4 cleaved single-crystal scintillator and converted to a visible image. Samples were placed on 34 m downstream of the synchrotron source. The distance between the sample stage and the detector had a changeable range of 8 m (Fig. 6).
ACI and PCI of CO2 gas production
Aerogenic powders (East Wind Chemicals, China) were placed in a polyethylene tube which was perpendicular to the x-ray beam (Fig. 2a). 100 μl physiological saline was injected to the aerogenic powders through a PE-50 tube connected to a computer-controlled syringe pump. ACI and PCI of CO2 gas production were performed at the energy of 19 keV with two different sample-to-detector distances (d = 1 and 60 cm, respectively). Images were consecutively captured by a CCD camera (Photonic Science, Britain) with the resolution of ~9 μm.
Animals
All experiments were performed in accordance with guidelines for the care and use of laboratory animals of Shanghai Jiao Tong University. BALB/c mice were purchased from the Animal Center, CAS, Shanghai, China. Animals were housed in a temperature-controlled room with a 12-h light/dark cycle and acclimatized for at least 7 days before use. Prior to imaging, food was withheld overnight with free access to water. Mice were anesthetized using an intraperitoneal injection of ketamine (100 mg kg−1) and xylazine (10 mg kg−1).
PCI of CO2 single contrast preparation
For imaging small intestine with CO2 single contrast, duodenum was chosen and gently exteriorized by a mid-line upper abdominal incision after anesthesia. An incision was made on the wall of proximal duodenum. The distal small intestine was ligated at 5 cm from the incision. The intestinal mucosa was fully dried with cotton. Subsequently, 200 μl CO2 gas was injected to inflate the duodenum by a PE-10 tube through the incision. Then the proximal duodenum near the incision was ligated to prevent CO2 gas leaking from the incision. The distance between two ligations was 2 cm. For in vivo imaging, mice were positioned to set the abdominal cavity perpendicular to the x-ray beam and then they were imaged by the 9-μm CCD camera. For ex vivo imaging, the CO2 gas-filled duodenum was harvested and imaged using a CCD camera (Photonic Science, Britain) with the resolution of ~3.7 μm. Schematic of the duodenum preparation was shown in Fig 4a. ACI and PCI were performed at the energy level of 19 keV, with the sample-to-detector distance of 1 cm and 60 cm, respectively.
References
Federle, M. P. CT of the small intestine: Enterography and angiography. Applied Radiology 36, 55 (2007).
Hara, A. K., Leighton, J. A., Sharma, V. K., Heigh, R. I. & Fleischer, D. E. Imaging of small bowel disease: comparison of capsule endoscopy, standard endoscopy, barium examination and CT1. Radiographics 25, 697–711 (2005).
Zhang, L.-H. et al. Multi-detector CT enterography with iso-osmotic mannitol as oral contrast for detecting small bowel disease. World J Gastroenterol 11, 2324–2329 (2005).
Sunada, K. et al. Clinical outcomes of enteroscopy using the double-balloon method for strictures of the small intestine. World J Gastroenterol 11, 1087–1089 (2005).
Ali, A., Santisi, J. M. & Vargo, J. Video capsule endoscopy: a voyage beyond the end of the scope. Cleveland Clinic journal of medicine 71, 415–425 (2004).
Lewis, R. Medical phase contrast x-ray imaging: current status and future prospects. Physics in medicine and biology 49, 3573 (2004).
Zhou, S.-A. & Brahme, A. Development of phase-contrast X-ray imaging techniques and potential medical applications. Physica medica: PM: an international journal devoted to the applications of physics to medicine and biology: official journal of the Italian Association of Biomedical Physics (AIFB) 24, 129 (2008).
Davis, T., Gao, D., Gureyev, T., Stevenson, A. & Wilkins, S. Phase-contrast imaging of weakly absorbing materials using hard X-rays. Nature 373, 595–598 (1995).
Tang, R. et al. Microbubble-based synchrotron radiation phase contrast imaging: basic study and angiography applications. Physics in Medicine and Biology 56, 3503 (2011).
Xi, Y., Tang, R., Wang, Y. & Zhao, J. Microbubbles as contrast agent for in-line x-ray phase-contrast imaging. Applied Physics Letters 99, 011101–011103 (2011).
Tang, R., Chai, W.-M., Yang, G.-Y., Xie, H. & Chen, K.-M. X-ray Phase Contrast Imaging of Cell Isolation with Super-Paramagnetic Microbeads. PloS one 7, e45597 (2012).
Tang, R. et al. Anti-VEGFR2-conjugated PLGA microspheres as an x-ray phase contrast agent for assessing the VEGFR2 expression. Physics in Medicine and Biology 57, 3051 (2012).
Wilkins, S., Gureyev, T., Gao, D., Pogany, A. & Stevenson, A. Phase-contrast imaging using polychromatic hard X-rays. Nature 384, 335–338 (1996).
Tang, L., Li, G., Sun, Y.-S., Li, J. & Zhang, X.-P. Synchrotron-radiation phase-contrast imaging of human stomach and gastric cancer: in vitro studies. Journal of Synchrotron Radiation 19, 319–322 (2012).
Acknowledgements
This work was supported by the Nation Basic Research Program of China (973 Program 2010CB834305) and Natural Science Foundation of China, Grant no. 81271740. We thank Honglan Xie and Yanan Fu for technical assistance.
Author information
Authors and Affiliations
Contributions
R.T. designed and performed experiments, analysed data and wrote the paper; W.X.L. and W.H. performed experiments; F.Y. and W.M.C. analysed data; G.Y.Y. and K.M.C. designed experiments and analysed data.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Real-time PCI of CO2 gas production.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Tang, R., Li, WX., Huang, W. et al. CO2-based in-line phase contrast imaging of small intestine in mice. Sci Rep 3, 2313 (2013). https://doi.org/10.1038/srep02313
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02313
This article is cited by
-
Molecular evaluation of thrombosis using X-ray phase contrast imaging with microbubbles targeted to P-selectin in mice
European Radiology (2016)
-
Three-dimensional visualization of the microvasculature of bile duct ligation-induced liver fibrosis in rats by x-ray phase-contrast imaging computed tomography
Scientific Reports (2015)
-
Microdissection of Human Esophagogastric Junction Wall with Phase-contrast X-ray CT Imaging
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.