Abstract
Human vascular wall-resident CD44+ multipotent stem cells (VW-MPSCs) within the vascular adventitia are capable to differentiate into pericytes and smooth muscle cells (SMC). This study demonstrates HOX-dependent differentiation of CD44(+) VW-MPSCs into SMC that involves epigenetic modification of transgelin as a down-stream regulated gene. First, HOXB7, HOXC6 and HOXC8 were identified to be differentially expressed in VW-MPSCs as compared to terminal differentiated human aortic SMC, endothelial cells and undifferentiated pluripotent embryonic stem cells. Silencing these HOX genes in VW-MPSCs significantly reduced their sprouting capacity and increased expression of the SMC markers transgelin and calponin and the histone gene histone H1. Furthermore, the methylation pattern of the TAGLN promoter was altered. In summary, our findings suggest a role for certain HOX genes in regulating differentiation of human VW-MPSC into SMCs that involves epigenetic mechanisms. This is critical for understanding VW-MPSC–dependent vascular disease processes such as neointima formation and tumor vascularization.
Similar content being viewed by others
Introduction
New vessel formation by both, angiogenesis and post-natal vasculogenesis is a prerequisite for tissue regeneration but also for several diseases, including tumor progression and atherosclerosis. The active cellular component in these processes is granted by endothelial lineage cells, but neovascularization does not only depend on endothelial cell migration and proliferation with subsequent formation of endothelial tubes, it also requires pericyte coverage of vascular sprouts for vessel stabilization and survival1. Until some years ago bone marrow cells and endothelial cells lining the lumen of quiescent blood vessels were thought to be the only sources providing vascular progenitor cells or mature endothelial cells forming new vessels. Remarkably, recently published results identified the wall of adult blood vessels itself as a niche for stem cells2,3,4,5. Furthermore, organ-specific stem cell types are associated with the vessel wall, i.e. within the so called ”vasculogenic zone” of the vascular adventitia6,7. Together with stem cell supporting functions of endothelial cells these findings suggest that the vascular wall provides different somatic stem cell types within the sub-endothelial space and the vascular adventitia.
Consistent with the niche function of the adventitial vasculogenic zone the presence of Sca-1(+) smooth muscle cell progenitors have been shown within this zone8. It also was reported that a subset of CD34(+) cells within the vascular adventitia has the capacity to differentiate into pericytes9. More recently, our group identified CD44(+)CD90(+)CD73(+)CD34(−)CD45(−) cells within the adult human arterial adventitia, which we named vascular wall-resident multipotent stem cells (VW-MPSCs); these cells were capable to differentiate into vascular smooth muscle cells (SMC) and pericytes under in vitro and in vivo conditions. Furthermore, these cells reside predominantly in the vasculogenic zone of adult human blood vessels and contribute to maturation of newly formed vessels10. VW-MPSCs have the capacity to differentiate into chondrocytes, osteocytes and adipocytes suggesting a mesenchymal stem cell (MSC)-like behavior of VW-MPCs. MSCs may represent an important source of pericytes and smooth muscle cells during angiogenesis under physiological and pathological conditions. Classical MSC marker proteins show highly overlapping expression profiles with human aortic smooth muscle cells (hAoSMC), as well as vascular endothelial cells (EC)7,11.
HOX genes encode homeodomain-containing master regulators of regional specification and organ development in the embryo and are widely expressed in the adult12. In humans 39 HOX transcription factors reside in four separate clusters, HOXA through -D, that are located on four different chromosomes. Commonly HOX proteins require co-factors for binding to specific DNA sequences in order to activate or repress target genes13. HOX genes orchestrate cell differentiation during embryonic development in many different lineages and developmental pathways14. Acknema et al. investigated HOX gene expression profiles of individual colony forming unit-fibroblasts (CFU-F) derived from various organs and revealed that CFU-F have characteristic HOX expression signatures that are heterogeneous but highly specific for their anatomical origin15. The topographic specificity of the HOX code is maintained during differentiation, suggesting that they are an intrinsic property of MSC. Furthermore, specific HOX gene expression profiles of stem and progenitor cells from mesodermal tissues, the so called "biological fingerprint" can be used to distinguish functionally distinct MSC populations derived from bone marrow and cord blood16.
We hypothesized that determining the expression pattern and the potential role of HOX genes in VW-MPSCs would help to discriminate them from mature vascular cells such as mature EC and SMC and would provide new insight into the molecular mechanisms governing the differentiation of VW-MPSCs into SMC. We identified HOXB7, HOXC6 and HOXC8 to be differentially expressed in VW-MPSCs as compared to hAoSMC, EC and embryonic stem cells (ES), suggesting that HOXB7, HOXC6 and HOXC8 expression in VW-MPSCs can be used to distinguish these cells from other vascular wall cells, e.g. SMC or EC and to manipulate their differentiation. Indeed, our data show that the aforementioned HOX genes are essentially involved in the differentiation of VW-MPSCs into SMCs by influencing the expression of calponin (CNN1) and TAGLN, apparently through alterations in the methylation status of the promotor regions of these genes. Moreover, these HOX genes affect Histone 1 (H1), which in turn also influences the expression of CNN1 and TAGLN in VW-MPSCs and thus might be involved in the regulation of their differentiation into SMC.
Results
HOXB7, HOXC6 and HOXC8 are differentially expressed in human VW-MPSCs
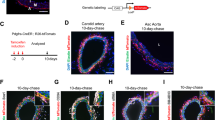
A principal goal of these studies was to determine whether HOX genes play a role in the biology of stem and progenitor cells that reside in the vascular adventitia. As a first step in addressing this we screened HOX expressions in VW-MPSCs in comparison to undifferentiated pluripotent human embryonic stem (hES) cells and mature aortic smooth muscle cells (hAoSMCs) by quantitative Real-Time RT-PCR (qRT-PCR). In addition, we included mature vascular endothelial cells (ECs) in these studies, namely human umbilical vein endothelial cells (HUVECs). Subsequently hierarchical clustering analysis for the 39 HOX genes whose signals were derived from qRT-PCR analysis for each sample was performed (Fig. 1A and Supplementary Table S2). The qRT-PCR analyses revealed nearly no HOX gene expression in undifferentiated hES cells, only a slight expression of HOXC12, -D13, -B1, -C13 and -D12. In VW-MPSCs on the other hand, HOXC8 and HOXC6 (p < 0.005), were strongly expressed in comparison to all other cell types tested, whereas HOXB7 (p < 0.005) was also highly expressed in HUVECs (Fig. 1B). To a lower extent HOXA2, -A3, -B5, -B6, -B8, -C4 and -C5 were selectively expressed in VW-MPSC, whereas expression of HOXA1, -A7, -A4, -A10, -B3, -B4, -C9, -C10 and -D3 was present in VW-MPSC and in HUVECS. Like in hES cells nearly no HOX gene expression was detected in hAoSMC cells, only a slight expression of HOXD3, -A1 and -A2. (Fig. 1A). As HOX genes may be expressed in many different lineages of very different embryonic origins the related hierarchical clustering analyses revealed that a couple of HOX genes were expressed in VW-MPSC and HUVEC cells suggesting a close proximity of both lineages. In contrast, expression of classical EC marker genes was not detected in VW-MPSCs (Supplementary Fig. S1 online) and VW-MPSCs failed to differentiate into endothelial lineage cells upon growth factor treatment, including the treatment with VEGF (Supplementary Fig. S2 online). Discriminating between different types of vascular wall cells using classical MSC marker proteins such as CD90, CD105 and CD73 (Fig. 1C) is difficult. The expression of ACTA2 and more prominent that of TAGLN was highly detectable in hAoSMC confirming TAGLN as a good marker to distinguish undifferentiated VW-MPSCs from mature SMC.
HOXB7, HOXC6 and HOXC8 mRNA is expressed in human VW-MPSCs at a high level.
(A) HOX expressions in VW-MPSCs was done as compared to undifferentiated pluripotent human embryonic stem (hES) cells and mature aortic smooth muscle cells (hAoSMCs) and human umbilical cord endothelial cells (HUVEC) by quantitative Real-Time RT-PCR (qRT-PCR). Subsequently hierarchical clustering analysis for the 39 HOX genes whose signals were derived from qRT-PCR analysis for each sample was performed. (B) Diagram of expressed HOX genes in VW-MPSCs (purple bars) as compared to hES (blue bars), SMC (dark blue bars) and HUVEC (white bars). (C) MSC as well as SMC marker gene expressions in VW-MPSCs as compared hAoSMC. Obtained expression levels were normalized by division through the mean expression value of the reference gene (Actin) and are shown as relative quantification units (RQ). Data are presented as mean ± SD from five (MSC), three (human ES), four (SMC) and three (HUVEC) independent experiments measured two times each. *, p < 0.05; **, p < 0.005 (MSC vs. SMC).
HOX gene silencing reduced the invasive capacity of cultured VW-MPSCs
By using siRNA transfection to silence HOX genes that were identified to be overexpressed in CD44(+) VW-MPSCs, we tested their effects on proliferation and differentiation. First, we studied the effect of HOX gene silencing on VW-MPSC proliferation. This was achieved by assessing the number of VW-MPSCs transfected with HOXB7, HOXC6 and HOXC8 siRNA alone or in indicated combinations in comparison to the number of VW-MPSCs cultured in normal growth media (NGM) alone or transfected with control (non-silencing) siRNA 4 days after treatment. The data show that the proliferation of VW-MPSCs was not affected by HOX gene silencing (Fig. 2A, Supplementary Fig. S3A online). To further evaluate the functional consequences of HOXB7, HOXC6 and HOXC8 gene silencing we analysed the capacity of VW-MPSCs to invade Matrigel (in-gel sprouting) when these cells were cultured as spheroids following transfection with siRNAs specific for these HOX genes, either individually or in combination and with control siRNA (Fig. 2B). The results show that VW-MPSCs cultured in NGM alone and those transfected with control siRNA exhibit a higher invasive capacity in comparison to VW-MPSCs with gene silencing for HOXB7, HOXC6 and HOXC8. Moreover, treatment of VW-MPSCs after silencing of these HOX genes with angiogenic growth factors including VEGF, FGF2, PDGF, TGFβ1 did not restore the reduced in-gel sprouting (not shown).
Silencing over expressed HOX genes in cultured vascular wall-derived MPSCs reduces in-gel sprouting while proliferation is not affected.
(A) Equal cell numbers of VW-MPSCs were seeded and cultured in NGM and transfected with Control (non-silencing) or HOXB7, HOXC6 and HOXC8, siRNA. After 4 days cell numbers were analysed. Data are presented as mean ± SD from 3 independent experiments performed in duplicates each. (B) For analysing the sprouting capacity transfected cells were embedded in GFR-Matrigel as 3D-spheroids. In-gel sprouting was quantified 48 hours after embedding. The data represent the mean cumulative length of all cord-like sprouts growing from 10 individual spheroids per experimental group. The figure shows the results of 1 of 3 independent experiments with similar results. (B) ** p ≤ 0.005.
TAGLN is a down-stream regulated gene of HOXB7, HOXC6 and HOXC8 activity in VW-MPSCs
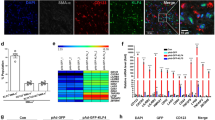
Because inhibition of HOX genes expressed in VW-MPSCs altered important functions of these cells as exemplified by in gel-sprouting, we performed cDNA microarray analysis using Affymetrix® DNA chips in order to identify potential down-stream regulated genes of HOXB7, HOXC6 and HOXC8 activity (Fig. 3 and Supplementary Table S1 online). To this aim CD44(+) VW-MPSCs were transfected with HOXB7, HOXC6 and HOXC8-specific siRNAs both individually or in defined combinations using non-specific siRNAs as controls. After 4 days cells were harvested and subjected for total RNA isolation with subsequently performed microarray analysis using Affymetrix® DNA chips according to Croner et al.17. Results are presented schematically by presenting the numbers of genes which were significantly altered upon HOX gene silencing (Fig. 3). For each probe set three (control, HOXC6, HOXB7) and two (HOXC8) Affymetrix® DNA chips were used. Upon silencing the expression of 1666, 1609 and 2075 genes was affected by HOXC8, HOXC6 and HOXB7 siRNAs, respectively. Comparing all three groups of differentially expressed genes, expression of 373 genes was significantly altered: 172 genes were up-regulated and 201 genes were down-regulated in comparison to VW-MPSCs transfected with control siRNA. The genes differentially expressed in response to HOX siRNA-mediated inhibition included several histone genes and perhaps most interestingly TAGLN, which is a well-known SMC differentiation marker (Fig. 3; for complete list see Supplementary Table S3 online). Next we performed qRT-PCR analyses using RNA extracts from VW-MPSCs transfected with control siRNA and with siRNA for HOXB7, HOXC6 and HOXC8 to validate the expression differentials of histone genes (Fig. 4A). In agreement with the microarray data most histone isoforms were up-regulated after siRNA treatment. Interestingly, histone H1 expression was up-regulated upon HOXC6 and HOXC8 gene silencing but the most prominent increase of H1 expression was detectable in VW-MPSCs silenced for HOXB7 expression. Up-regulation of H1 protein expression was confirmed at the protein level by Western blot analysis (Fig. 4B) of VW-MPSCs silenced for HOXB7, HOXC6 and HOXC8 both individually and in combination. As expected, H1 could also be detected at the protein level in the nuclei of cultured VW-MPSC and was found to be partially co-localized with the HOXC6 and HOXC8 protein (Fig. 4C, Supplementary Fig. S4 online).
HOXB7, HOXC6 and HOXC8 signalling down-stream regulated genes were analyzed in human VW-MPSCs by gene expression profiling using Affymetrix® DNA chips.
Results are presented as scheme depicting numbers of genes significantly altered upon siRNA silencing (mean signal control (average) compared to mean signal HOXC6 (average) as well as HOXB7 (average) and HOXC8 (average)). For each probe set three (Control, HOXC6, HOXB7) and two (HOXC8) Affymetrix® DNA chips were used. Histone genes as well as TAGLN which were represented on the chips are listened and fold induction (siRNA treatment/control) was calculated. For complete list see Supplementary Table S1 online.
Altered histone expression in human VW-MPSCs upon HOX inhibition by siRNA treatment.
(A) VW-MPSCs were transfected with Control (non-silencing) or HOXC8, HOXC6 and HOXB7 siRNA in single or in indicated combinations. After 4 days cells were harvested and subjected for mRNA isolation and Real-Time RT-PCR quantification of histone gene expression. Data are presented as mean ± SEM from 4 independent experiments performed in duplicates each. (B) Histone H1, H2B, H2A protein levels were detected by Western blot. Equal protein amounts (50 μg, whole cell lysate) were loaded. Beta-actin was included as a loading control. (C) Double-immunofluorescence analyses on freshly isolated CD44(+) cells plated on glass cover slips were performed for HOXC6 and HOXC8 (green) and H1 (red). H1 was detected in the nuclei of cultured VW-MPSC and was found to be partially co-localized with the HOXC6 and HOXC8 protein. Hoechst 33242 iodide was used for nuclei staining.
TAGLN expression is down-regulated in human VW-MPSCs upon knock-down of HOXB7, HOXC6 and HOXC8 expression
Among the genes up-regulated in response to the silencing of specific HOX genes, we focused our attention on TAGLN, one of the earliest and most reliable markers of vascular SMC differentiation. TAGLN showed a general tendency to be up-regulated in our microarray analyses after gene silencing for HOXB7, HOXC6 and HOXC8 (Fig. 5). To confirm these results we performed qRT-PCR analysis and determined the induced expression levels of TAGLN upon HOXB7, HOXC6 and HOXC8 gene silencing (Fig. 5A). The data show that the most prominent increase of TAGLN expression was found upon HOXC6 silencing in VW-MPSCs. Additional support for the apparent induction of TAGLN expression upon HOXC6 silencing was obtained at the protein level by Western blot analyses (Fig. 5B) and immunofluorescence staining of cultured VW-MPSCs (Supplementary Fig. S3B online). Furthermore, the protein expression levels of calponin1 (CNN1), an additional marker for mature SMCs, were also enhanced in response to HOXB7, HOXC6 and HOXC8 silencing (Fig. 5B).
Increased TAGLN expression in human VW-MPSCs upon HOXB7, HOXC6 and HOXC8 and inhibition by siRNA treatment.
(A) VW-derived MPSCs were transfected with Control (non-silencing) or HOXB7, HOXC6 and HOXC8, siRNA. After 4 days cells were harvested and subjected for mRNA isolation and qRT-PCR quantification of the late SMC differentiation marker TAGLN. (B) Increased TAGLN as well as CNN1 expression were confirmed at the protein level after gene silencing of HOXB7, HOXC6 and HOXC8 in VW-MPSCs. (C, D) Silencing H1 mRNA expression directly decreases gene expression of TAGLN and CNN1. Data are presented as mean ± SEM from 4 independent experiments performed in duplicates each * p ≤ 0.05.
Interestingly, silencing of these HOX genes also resulted in an up-regulation of histone H1, both at transcriptional and translational levels (Supplementary, Fig. S3B online). Thus, we were curious to know whether H1 knockdown might affect TAGLN or CNN1 expression. Using specific siRNA we could effectively silence H1 in VW-MPSCs (Fig. 5C) with concomitant reductions of TAGLN and CNN1 expression (Fig. 5C,), with the latter being confirmed at the protein level (Fig. 5D).
This resulted in substantially reduced expression of the analyzed HOX genes (Fig. 5C). To further rule out that the changes in Histone gene expression observed upon silencing of the selected HOX genes may not simply be a consequence of affecting the cell cycle, we determined the stages of the cell cycle phases of cultured VW-MPSCs upon silencing of HOX genes and H1 (Fig. 6, Supplementary Fig. S5 online). As demonstrated by the proliferation marker Ki67 most of the cells were proliferating, meaning that they were not in G0. Furthermore no changes of Cyclin D1 (G1-cyclin) and Cyclin B1 (mitotic cyclin) could be detected (Fig. 6B) upon HOX gene silencing. Finally Nicoletti staining and flow cytometric analysis were used to determine the number of cells actively synthesizing DNA and their position in G0/1, S, G2/M phase of the cell cycle (Fig. 6C, Supplementary Fig. S5 online). Again, no changes in the cell cycle phases were detectable upon HOX gene silencing.
Cell cycle analysis after silencing over-expressed HOX genes and H1 in cultured vascular wall-derived MPSCs.
VW-derived MPSCs were transfected with Control (non-silencing, HOXC8, HOXC6, HOXB7 or H1 siRNA alone or in indicated combinations and subjected for immunofluorescence staining for the proliferation marker Ki67 (A). Cell cycle distribution was analysed by Western blot analysis of Cyclin B1 and D1 expression (B) as well as by Nicoletti staining (C). Two-color flow cytometric analysis was used to quantify cells actively synthesizing DNA and their position in G1, G0/1, S, G2/M phase of the cell cycle. Representative images for three independent experiments are shown. Data are presented as mean from 3 independent experiments.
Gene silencing of HOXB7 and HOXC6 in VW-MPSCs altered the CpG methylation status of the TAGLN promoter
To assess potential changes in the methylation status of the TAGLN promotor region upon HOX gene silencing, genomic DNA was isolated from VW-MPSCs transfected with control RNA (non-silencing) and with siRNAs specific for HOXB7, HOXC6 and HOXC8 and then subjected to bisulfite conversion. The subsequently performed PCR and sequence analysis using specific primers (Fig. 7, Supplementary Figure S6 online) revealed methylated CpG sites at positions 408, 476 and 485 that were present in the DNA of VW-MPSCs transfected with control siRNA but not in the native, non-methylated TAGLN sequence. Thus, at these methylated CpG sites cytosine residues were not converted into thymine (Fig. 7, arrows). Upon HOXB7 mRNA silencing all three sites and upon HOXC6 mRNA silencing only cytosine residues on position 408 became unmethylated indicating an alteration of TAGLN promotor status in response to the silencing of these HOX genes. These changes are consistent with the observed changes in TAGLN expression (Fig. 5), thus suggesting that HOX activity patterns play a role in VW-MPSC differentiation through epigenetic mechanisms. HOXC8 silencing in VW-MPSCs apparently did not alter the methylation status of cytosine residues in TAGLN promoter. In addition, two sites at position 425 and 499 displaying cytosine residue in the control sequence were changed into thymine upon HOXB7 and HOXC6 siRNA treatment, which then showed the same sequence as in the native, non-methylated TAGLN sequence.
HOXB7, HOXC6 and HOXC8 gene expression silencing alters methylation pattern of the TAGLN promoter sequence.
Genomic DNA was isolated from control (non-silencing siRNA) and silencing siRNA (HOXC8, HOXC6, HOXB7) transfected VW-MPSC, subjected for bisulfite conversion and finally sequenced using specific primers for the modified DNA which do not contain any CpG sites in their sequence and were generated around the predicted CpG islands. Predicted promoter regions of the human gene TAGLN (ENSG00000149591) were emphasized. Org: Original sequence; BSC bisulfite modified sequence (MethPrimer results); HOXB7, HOXC6, HOXC8, Con (non-silencing control) sequence analysis after bisulfite conversion of siRNA treated genomic DNA isolates; ++ CpG sites (for display, assume all CpG sites are methylated); non-CpG 'C' converted to ‘T’; * similar to BSC (original); # C vs T; TATA box (at position 368) in bold red; promoter start at position 500 encircled. Arrows indicate positions which were altered in non silencing siRNA treated cells as compared to original sequence (BSC), whereas siRNA (HOXB7 and HOXC6) treatment results in sequences similar to original sequence (BSC). Underlined nucleotides of control sequence indicate additional CpG sites.
Discussion
Accumulating data published in recent years suggests that the vessel wall, in particular the vascular adventitia, is a niche for different types of stem and progenitor cells with the capacity to differentiate into both vascular and non-vascular cells. Here we demonstrated that HOXB7 and HOXC6 are involved in the differentiation and morphogenetic events of CD44(+) MSC-like vascular wall-resident multipotent stem cells (VW-MPSCs). We showed that expression of TAGLN, an early and potent marker of SMC differentiation and CNN1, a structural component of differentiated mature SMCs, is dependent on the activity patterns of HOXB7, HOXC6 and HOXC8. Our current data show that silencing the HOX genes in VW-MPSCs leads to an alteration of CpG methylation in the TAGLN promotor region and increased TAGLN expression. This suggests that these HOX genes play a role in the epigentic regulation of VW-MPSC differentiation, although the underlying mechanisms are currently unknown. Finally, we were able to show that gene silencing of HOXB7 and HOXC6 in VW-MPSCs enhanced the expression of histone 1 (H1) at both mRNA and protein level. Gene silencing of H1 in turn suppressed the expression of TAGLN and CNN1. Interestingly, H1 gene silencing in VW-MPSCs reduced the expression of HOXB7, HOXC6 and HOXC8, thus suggesting that H1 influences the expression of these HOX genes, which might represent an additional regulatory mechanism involved in the VW-MPSC differentiation.
We recently showed that VW-MPSCs exhibit MSC-like behaviour10. Basically, MSCs are multipotent cells giving rise to both mesenchymal and non-mesenchymal tissues in vitro and in vivo18,19. They are commonly characterized by their ability to adhere to plastic and the expression of a panel of typical MSC surface markers, as well as their ability to differentiate into different cell types such as osteocytes, chondrocytes and adipocytes under specific in vitro differentiating conditions. We found that the wall of adult human blood vessels harbours not only EPCs but also CD44(+)CD34(−)CD45(−) multipotent MSC-like stem cells, which are capable to differentiate into pericytes/SMC and cover endothelial cell layers of newly formed blood vessels in vitro and in vivo10. These cells predominantly reside in the so called “vasculogenic zone” of vascular adventitia. This zone was identified in human adult vessels as a niche for CD34 + CD31− EPCs and for progenitors of macrophages6 earlier. Later, it was shown that CD34(+)Sca1(+) cells cluster in a domain of Sonic hedgehog signalling which was restricted to the inner part of mouse arterial adventitia similar to the vasculogenic zone. These cells reside in this zone from the developmental embryonic to adult phase and have the capacity to differentiate into SMC and pericytes8. More recently, it could be shown that a subset of adventitial CD34(+)CD31(−) progenitors of human saphenous vein have the capacity to differentiate into pericytes and to improve the neovascularisation and blood flow after intramuscular injection9. Finally, confirming the results of Zengin et al. it was shown that mature murine aorta contains hematopoietic progenitors capable of self-renewal and multilineage differentiation that are localized primarily in the vascular adventitia6,20. In accordance to embryonic development where hematopoietic and endothelial progenitors co-exist within the same niche, the so called “blood islands”, adult vascular adventitia seems also to harbor cells with the capacity to differentiate into both endothelial and blood cells. These data conclusively suggest the adult vascular adventitia, in particular the vasculogenic zone of the vascular adventitia, as a niche for different types of progenitor and stem cells including the CD44(+) VW-MPSCs.
In order to identify molecular mechanisms governing the differentiation of the vascular wall-resident MPSCs into SMC, we first performed qRT-PCR analyses on CD44(+) cells isolated from hITA fragments in comparison to mature SMC of human aorta, hES cells and human EC. Among several genes being differentially expressed in VW-MPSCs the HOX genes HOXB7, HOXC6 and HOXC8 were found to be expressed in VW-MPSCs on a clearly higher level compared to mature hAoSMC. HOX genes encode for homeodomain proteins which are transcription factors that regulate lineage-specific gene expression and thus control cell and organ differentiation. Moreover, through a Hox expression code21,22 they specify positional identities during embryonic development of organs including the vascular system23. Already in the 90ies it was reported that restricting expression of HOX genes allows distinguishing fetal from adult human SMC24. Among HOX genes being identified in this work HOXB7 was found to be preferentially expressed in human fetal SMC. More interestingly, it was shown that over-expression of HOXB7 in the multipotent cell line C3H10T1/2 induced the expression of early SMC markers such as calponin and SM22a, but not the intermediate SMC marker myosin heavy chain25,26.
Mammalian promotors can be readily classified into sets with high (HCPs), intermediate (ICPs) or low (LCPs) CpG-content27,28. Promotors containing a 500 bp interval within −0.5 kb to +2 kb with a GC content ≥ 0.55 and a CpG observed to expected ratio ≥ 0.6 were classified as high CpG promotors (HCPs). Low CpG promotors (LCPs) contain no 500 bp interval with CpG observed to expected ≥ 0.4. All others were classified as intermediate promotors (ICPs). Based on these criteria the TAGLN promotor region is classified as an IPC with a (G + C) fraction of 0.64 and a CpG observed to expected ratio of 0.47. We show here that HOXB7 silencing in CD44+ VW-MPSCs induces the expression of SMC differentiation and maturation markers such as TAGLN and CNN1, which clearly indicates a differentiation of VW-MPSCs to a more mature SMC phenotype.
HOXA7, HOXB2, HOXB5, HOXB7 and HOXC4, have previously been demonstrated to act as transcripts regulating self-renewal and differentiation of these stem cells29. Furthermore, distinct HOX expression patterns have been reported for cord blood derived MSC (CB-MSC). These CB-MSCs behave almost like MSC from bone marrow (BM-MSC) and unrestricted somatic stem cells (USSC), which show a distinct differentiation potential for lineages of all three germ layers16,30. RT-PCR analysis of HOX expression revealed a high similarity between BM-MSC and CB-MSC which were HOX-positive, whereas USSC are closely related to HOX-negative H9 ES cells. HOXA9, HOXB7, HOXC10 and HOXD8 are candidate markers to discriminate MSC from USSC. Interestingly, no classical EC marker genes are detectable in our VW-MPSCs and these cells failed to differentiate into endothelial lineage cells. An even closer relationship seems to exist between VW-MPSCs and mature SMC. SMC differentiation is accompanied by enhanced ACTA2 and TAGLN and CNN1 expression. TAGLN is expressed exclusively in smooth muscle-containing tissues of adult mammals and is one of the earliest markers of differentiating SMC31. While the expression of these markers seems to be common feature of SMCs regardless of their anatomical position it has been shown that SMC of different parts of adult arteries, e.g. aortic arch, abdominal aorta and femoral artery exhibit distinct HOX expression profiles; this suggests the existence of a topographic Hox code in SMCs that specifies positional identities23,32 and that might be independent of Hox activity patterns in MSCs residing in the vessel wall. More recently, it was reported that ectopic expression of HOXC11, which is normally restricted to the SMC of lower limbs vessels, in carotid arteries, aortic arch and descending aorta results in drastic vessel wall remodelling including elastic laminae fragmentation, SMC loss and intimal lesion formation32. Since our data are obtained from analyses on VW-MPSCs isolated from hITA the intriguing question is, whether also the adventitial VW-MPSCs differ in their HOX code depending on vessel type and body part where the blood vessels are located and if this might have an impact on the development of vascular diseases e.g. neointima formation in certain adult arterial segments. Indeed, as summarized in recent reviews, adventitial cells are involved in the formation of atherosclerotic lesions and neointima formation4,33,34.
We provide evidence that H1 is involved in the regulation of VW-MPSC differentiation into SMC. H1 expression in VW-MPSCs is significantly enhanced at both mRNA and protein level after HOXB7, HOXC6 and HOXC8 gene silencing. While H1 is not involved in the primary steps of nucleosome packaging, it is involved in the packaging of the beads on a string sub-structure into a high order structure, binding to the “linker DNA” and stabilizing the chromatin structure whose details have not yet been solved35. Cellular studies in the past which aimed to address H1 function showed that H1 can alter the chromatin structure and serves as both a positive and negative regulator of transcription depending on the gene36. H1 influences DNA methylation and regulates specific gene expression37,38,39. Although a 50% reduction of histone H1 levels in ES cells affects chromatin structure globally, the expression of only few genes was found to be altered40. Terme et al. showed that pluripotent cells have decreased levels of H1.0 but increased levels of H1.1, H1.3 and H1.5 compared to differentiated cells41. The knockdown of H1.0 in human ES did not affect self-renewal but impaired their differentiation. Moreover, H1 has been shown to enhance wound healing and increase interleukin -6 expressions of adult stem cells42. Thereby, H1 treatment showed an up-regulation of mitogen-activated protein kinases (MAPKs), extracellular-regulated kinase 1/2 (ERK1/2) and sequential NF-κB translocation. A very recent study showed that depletion of H1 in ES cells and in mouse embryos with knock-out of H1c/H1d/H1e decreased the expression of many HOX genes significantly43. In line with these findings our results also show that H1 depletion in VW-MPSCs results in transcriptional suppression of selected HOX genes. In contrast to Zhang et al.43, who showed that HOXB7 expression was not altered after H1 depletion in ES cells, we observed a reduced HOXB7 expression after H1 silencing in VW-MPSCs. This finding suggests that the interaction of H1 and HOXB7 might be a more specific mechanism regulating gene expression and differentiation of VW-MPSCs. While it is still unclear how (direct or indirect) these both factors might interact with each other previous studies showed that HOXB7 is expressed at a markedly higher level in embryonic vascular SMC compared with mature SMC of adult vessels24. Moreover, in human atherosclerotic lesions, where mature SMC revert to a more immature and less contractile phenotype, HOXB7 mRNA was detected at a higher level than in normal artery walls26. More intriguingly, our data showed that H1 silence in VW-MPCs suppresses the expression of HOXB7 while HOXB7 silencing in these cells significantly up-regulates the expression of H1. Thus, the data presented here suggest that both HOXB7 and H1 are involved in the epigenetic controlling of the differentiation of VW-MPSCs into mature SMC in physiological remodeling processes of the vessel wall and might also be relevant for vascular diseases. However, whether or not they interact with each other in these processes cannot be drawn from our analyses and needs to be studied by future studies.
Moreover, we are well aware of the fact, that HOX genes acting as epigenetic regulators are known to involve non coding RNAs (ncRNAs), which can be described from HOX gene clusters. Long ncRNAs may have diverse roles in gene regulation, especially in epigenetic control of chromatin, but the prevalence of different mechanism by which they act is not known44,45. NcRNAs encoded in HOX clusters have been thought to regulate HOX gene expression by mRNA cleavage or interference between mRNA and the translational machinery46. A large number of HOX ncRNAs, their complex clustering on the chromosomes and potentially diverse modes of action suggest that ncRNAs play a significant role in HOX regulation45. Finally we cannot rule out if ncRNAs are critically involved in HOX gene downstream signaling and may contribute to cross-regulation among HOX genes. Whether or not, or how HOX ncRNAs might interfere with the presented regulation of TAGLN as a downstream target of HOX signaling in VW-MPSCs should be addressed in future studies.
In summary, activated CD44(+) VW-MPSCs enter the differentiation into mature SMC and pericytes which can be recruited for stabilization of new vessels or repair of pre-existing vessels under physiological conditions. But they also can contribute to neointima formation and tumor vascularization (Fig. 8). Very recently, sirolimus, a drug frequently used in stent therapy to prevent coronary artery restenosis, has been reported to induce migration of adventitial stem/progenitor cells of from the adventitia into the intima as well as their differentiation into SMCs suggesting that they could contribute to restenosis, often observed even after drug diluting stent therapy47. Our CD44+ MPSCs might serve a candidate of adventitial cell types being activated by sirolimus. Future pre-clinical in vivo experiments should focus on this issue in order to clarify how the differentiation of VW-MPSCs in SMCs can be prohibited therapeutically. Hypothetically VW-MPSCs can be mobilized from adventitia to the media and differentiate to SMC in cases of injury or damage of the arterial wall cells in order to replace them. Localized within the vascular adventitia, which serves as an interface between the inner parts of vessel wall inclusively blood flow and the surrounding tissue, the VW-MPSCs might serve as an important therapeutic target. Since our data suggest a role for certain HOX genes in regulating VW-MPSCs differentiation it will be critical to define the underlying HOX-dependent mechanisms.
HOX genes are involved in the differentiation of CD44(+) VW-MPSCs into SMC.
(A) CD44(+) VW-MPSCs are preferentially resident in the adventitial “vasculogenic zone” of the vessel wall. Without mobilization from the niche they express HOXB7 and HOXC6 at high level compared to hAoSMCs. These HOX genes suppress the expression of TAGLN in VW-MPSCs, an essential factor of early SMC differentiation. This mechanism probably accounts for keeping the VW-MPSCs quiescent in the adventitial niche. In contrast, silencing of HOX genes alter the CpG methylation of TAGLN promotor resulting in increased TAGLN expression which induces the VW-MPSC differentiation into SMC/pericytes. HOX gene silencing also results in increased expression of H1 in VW-MPSCs. H1 silencing in turn results in reduced expression of both, TAGLN and CNN1 as well as HOXB7 and HOXC6. Whether H1 directly enhances the expression TAGLN and CNN1 remains to be investigated. (B) Signals released, e.g. by endothelial dysfunction, may hypothetically induce the mobilization of CD44(+) cells from the niche toward the intima accompanied by a modification of HOX gens regulating their differentiation into mature SMC. VW-MPSCs differentiation into SMC by mechanisms identified here might be of clinical relevance contributing to new vessel stabilization, neointima formation and tumor vascularization.
Methods
Reagents and antibodies
Control A and B siRNA, HOXC8, HOXC6 and HOXB7 siRNA were from Santa Cruz (Santa Cruz, USA). Mouse anti-human CD44 antibody was from Antibodies Online (Aachen, Germany), TAGLN antibody was from Acris Antibodies (Herford, Germany), HOXC8, HOXC6 and HOXB7, αSMA, H1, H2B, H2A and H4 antibodies were from Santa Cruz (Santa Cruz, USA). Human embryonic stem cells (hES cells) used for RNA isolation had been cultured according to standard protocols and as previously described48,49 (hES cells; lines H9, H9.2 passages 32–61 and I3 passages 55–82). All peroxidase- and fluorescently-labelled secondary antibodies were from Jackson IR Laboratories (West Grove, USA).
Tissue and cells
Human internal thoracic artery (hITA) samples were obtained during surgery (sparse material) according to local ethical and biohazard regulations and provided from the Clinics of Thoracic and Cardiovascular Surgery, University Hospital Essen for our institute. All these studies including human tissue samples were approved by the local ethic committee. Informed consent (written form, Nr.10-4363) was obtained from the Ethik-Kommission, University Medical Faculty, Essen, Germany. hAoSMC and HUVEC were from Lonza (Walkersville, USA) and cultivated as recommended by the manufactures in complete SmGM-2 and EGM-2 medium.
Isolation and purification of VW-MPSCs and EC
Specimens of hITA were excised as previously described10. In brief, contaminating fatty and muscle tissue was removed from the vessel fragment. After several washes vessels were mechanically minced and dissociated for 30–40 minutes at 37°C in OptiMEM I medium (GIBCO) containing 0.2% type 2-collagenase (Worthington, Lakewood, USA). On dissociation cells were washed and cellular suspensions were passed through 70 μm pore size filters. Highly pure VW-MPSCs were generated using a CD44 antibody and MACS technology (Miltenyi Biotec). MACS was used according to the manufacturer's instructions and as described previously10. Primary VW-MPSCs were cultivated on plastic cell culture plates using complete human MSC-GM media (PromoCell, Heidelberg, Germany). Medium was removed 24 hours after initial plating, non-adherent cells were washed away and fresh medium was replaced. For EC isolation vessel sections were incubated in OptiMEM I containing 0.2% type 2-collagenase for 10 min. Crude cell extracts were plated on fibronectin-coated cell culture plastics. Medium was removed 30–45 min after initial plating, non-adherent cells were washed away and fresh medium (EGM-2 medium) was replaced.
siRNA transfection
For siRNA treatment VW-MPSC were transfected with non-silencing (control) and HOX siRNA using PrimeFect siRNA transfection reagent (Lonza) according to the manufactures instructions. Cells were analysed for downstream effects 48 hrs or 4 days after transfection.
In-vitro angiogenesis assay
VW-MPSCs were used to generate spheroids of defined cell number (500 cells/spheroid) and used for in-gel sprouting angiogenesis experiments as previously described50. In brief, defined cell numbers of VW-MPSCs were mixed in methocell medium and plated onto non-adherent plastic square petri dishes in 25 μl drops containing 500 MPSCs each. Spheroids were harvested as described above, resuspended in pre-cooled GFR-Matrigel (20–30 spheroids/250 μl Matrigel) and plated in 48-well cell culture plates. Data are presented as mean ± SD from three independent experiments.
RNA isolation and cDNA synthesis
For RNA isolation the cells were lysed directly in the plastic Petri dishes as previously described and the RNA was isolated using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instruction51.
Real-time RT-PCR analysis
Analysis was carried out using the oligonucleotide primers listed in Supplementary Table 2 online. The PCR program consisted of initial denaturation at 95°C for 30 s, annealing at 58°C for 40 s and extension at 72°C for 30 s for 25–30 cycles. Specificity of all PCR reactions was tested by parallel reactions using water instead of cDNA. Real-time PCR analysis was performed using SYBR®Green PCR Master Mix (Applied Biosystems, Darmstadt, Germany) and standard conditions. The experiments were performed on ABI PRISM® 7000 sequence detection system (Applied Biosystems). Expression levels of analysed genes were normalized to beta actin mRNA expression.
Immunohistochemistry and immunofluorescence
Paraffin embedded tissue sections were hydrated using a descending alcohol series, incubated for 10–20 minutes in target retrieval solution (Dako) and incubated with blocking solution (2% FCS/PBS). After permeabilisation sections were incubated with primary antibodies over night at 4°C. Antigen was detected with an anti-rabbit Alexa488 and anti-mouse Alexa555-conjugated secondary antibody (1/500). Hoechst 33242 iodide was used for nuclei staining. Specimens were analyzed by confocal microscopy.
Western blot
Whole cell lysates were generated by scraping cells into ice-cold RIPA-P buffer (150 mmol/L NaCl, 1% NP40, 0.5% sodium-desoxycholate, 0.1% sodium-dodecylsulfate, 50 mmol/L Tris/HCL pH 8, 10 mmol/L NaF, 1 mmol/L Na3OV4), supplemented with a complete Protease-Inhibitor-Cocktail (Roche) and performing 2–3 freeze-thaw cycles. Protein samples (50–100 μg total protein) were subjected to SDS-PAGE electrophoresis and Western blots were done as previously described using H2A, H2B, H1 (all 1/200)or βActin (1/5000) antibodies51.
Analysis of cell cycle distribution
VW-derived MPSCs were transfected with Control (non-silencing) or HOXC8, HOXC6, HOXB7 siRNA alone or in indicated combinations and subjected for Nicoletti staining. In brief, cells were then fixed, permeabilized and stained for total DNA by 7-amino-actinomycin D (7-AAD). Two-color flow cytometric analysis was used to determine cells actively synthesizing DNA and their position in G0/1, S, G2/M phase of the cell cycle.
Statistical analysis
Paired or unpaired two-tailed t-tests were performed using GraphPad InStat3 software depending on effective matching of the analyzed data. The SD and SEM are indicated by error bars. Significance was assumed for P values < 0.05.
cDNA microarray analysis
RNA quality and quantity were determined by the “Lab-on-a-Chip” method (Bioanalyzer 2100; Agilent Technologies, Palo Alto, CA) according to the manufacturer's instructions. The 3′:5′ ratios for the housekeeping genes glycerinaldehyde-3-phosphatase (GAPDH) and beta-actin supplied by the GeneChip were used as further measures of RNA quality and to exclude partial degradation. A 3′:5′ ratio < 3.0 was regarded as an indicator of good RNA quality according to the manufacturer's protocol (Affymetrix, Santa Clara, CA)52. Gene expression was examined using GeneChip technology (Affymetrix). Biotin-labeled cyclic RNA (cRNA) was generated by in vitro transcription, as described previously and was hybridized to the GeneChips (HG-U133A) according to the manufacturer's instructions53. First-strand cDNA synthesis and labeled cRNA was generated as previously described17. Fragmentation of cRNA, hybridization to GeneChips, washing, staining and scanning of the arrays in the GeneArray scanner (Agilent) were performed as recommended by the Affymetrix gene expression analysis technical manual. Background correction and the extraction of the expression measure were performed according to Irizarry et al. using the robust multichip average (rma) expression measure as described previously17,54,55. Gene expression values were normalized according to instructions in the Affymetrix user manual. The reported signal of array n and gene i were normalized by multiplying a quotient of the target signal set to 500 and the average value of all genes after removing the values in the lowest and highest 2% of genes.
Bisulfite conversion of DNA and sequence analysis
Genomic DNA was isolated from control (non-silencing siRNA) and silencing siRNA (HOXC8, HOXC6, HOXB7) transfected VW-MPSC using QIAamp DNA Micro Kit (Qiagen) according to the manufactures instructions. Bisulfite conversion of DNA was performed using the EZ DNA Methylation-Direct™ Kit (Zymo Research Corporation, Irvine, USA). The human gene transgelin TAGLN (ENSG00000149591; Chromosome 11: 117,070,037-117,075,503 forward strand) was analyzed for promoter prediction sites using the Promotor 2.0 prediction server (www.cbs.dtu.dk/services/Promoter/) and SoftBerry NSITE (http://linux1.softberry.com). Primer for bisulfite sequencing PCR that are specific for the modified DNA but do not contain any CpG sites in their sequence were generated around the predicted CpG islands using the program MethPrimer (http://www.urogene.org/methprimer/index1.htm): BC Primer 2 (sense) GGGTAGGGTTTTGTTTATAAAAGGT, BC Primer 2 (antisense) CAATAACTCCACACAAACTCCATATT; BC Primer 1 (sense) GGTTAGAGAATAGTGAAGTAGGAGTAGT, BC Primer 1 (antisense) AAACACCAAATTAAAACAATCTACC56,57. Amplification of bisulfite-treated DNA for methylation detection was done using ZymoTaq™ DNA Polymerase according to the manufactures instructions. Resulted PCR products were directly sequenced according to standard protocols.
References
Gaengel, K., Genove, G., Armulik, A. & Betsholtz, C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29, 630–638 (2009).
Pasquinelli, G. et al. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells 25, 1627–1634 (2007).
Invernici, G. et al. Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol 170, 1879–1892 (2007).
Ergun, S., Tilki, D. & Klein, D. Vascular wall as a reservoir for different types of stem and progenitor cells. Antioxid Redox Signal 15, 981–995 (2011).
Majesky, M. W., Dong, X. R., Hoglund, V., Daum, G. & Mahoney Jr, W. M. The adventitia: a progenitor cell niche for the vessel wall. Cells Tissues Organs 195, 73–81 (2012).
Zengin, E. et al. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development 133, 1543–1551 (2006).
Bogart, L. M. et al. Preliminary healthy eating outcomes of SNaX, a pilot community-based intervention for adolescents. J Adolesc Health 48, 196–202 (2011).
Passman, J. N. et al. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A 105, 9349–9354 (2008).
Campagnolo, P. et al. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation 121, 1735–1745 (2010).
Klein, D. et al. Vascular wall-resident CD44+ multipotent stem cells give rise to pericytes and smooth muscle cells and contribute to new vessel maturation. PLoS One 6, e20540 (2011).
Dominici, M. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317 (2006).
Wang, K. C., Helms, J. A. & Chang, H. Y. Regeneration, repair and remembering identity: the three Rs of Hox gene expression. Trends Cell Biol 19, 268–275 (2009).
Lumsden, A. & Krumlauf, R. Patterning the vertebrate neuraxis. Science 274, 1109–1115 (1996).
Akam, M. Hox genes: from master genes to micromanagers. Curr Biol 8, R676–678 (1998).
Ackema, K. B. & Charite, J. Mesenchymal stem cells from different organs are characterized by distinct topographic Hox codes. Stem Cells Dev 17, 979–991 (2008).
Liedtke, S. et al. The HOX Code as a "biological fingerprint" to distinguish functionally distinct stem cell populations derived from cord blood. Stem Cell Res 5, 40–50 (2010).
Croner, R. S. et al. Microarray versus conventional prediction of lymph node metastasis in colorectal carcinoma. Cancer 104, 395–404 (2005).
da Silva Meirelles, L., Caplan, A. I. & Nardi, N. B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26, 2287–2299 (2008).
da Silva Meirelles, L., Chagastelles, P. C. & Nardi, N. B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119, 2204–2213 (2006).
Psaltis, P. J. et al. Identification of a monocyte-predisposed hierarchy of hematopoietic progenitor cells in the adventitia of postnatal murine aorta. Circulation 125, 592–603 (2012).
Kessel, M. & Gruss, P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 67, 89–104 (1991).
McGinnis, W. & Krumlauf, R. Homeobox genes and axial patterning. Cell 68, 283–302 (1992).
Pruett, N. D. et al. Evidence for Hox-specified positional identities in adult vasculature. BMC Dev Biol 8, 93 (2008).
Miano, J. M. et al. Restricted expression of homeobox genes distinguishes fetal from adult human smooth muscle cells. Proc Natl Acad Sci U S A 93, 900–905 (1996).
Tintut, Y., Parhami, F., Bostrom, K., Jackson, S. M. & Demer, L. L. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem 273, 7547–7553 (1998).
Bostrom, K., Tintut, Y., Kao, S. C., Stanford, W. P. & Demer, L. L. HOXB7 overexpression promotes differentiation of C3H10T1/2 cells to smooth muscle cells. J Cell Biochem 78, 210–221 (2000).
Fouse, S. D. et al. Promoter CpG methylation contributes to ES cell gene regulation in parallel with Oct4/Nanog, PcG complex and histone H3 K4/K27 trimethylation. Cell Stem Cell 2, 160–169 (2008).
Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature 448, 553–560 (2007).
Phinney, D. G., Gray, A. J., Hill, K. & Pandey, A. Murine mesenchymal and embryonic stem cells express a similar Hox gene profile. Biochem Biophys Res Commun 338, 1759–1765 (2005).
Mahdipour, E. & Mace, K. A. Hox transcription factor regulation of adult bone-marrow-derived cell behaviour during tissue repair and regeneration. Expert Opin Biol Ther 11, 1079–1090 (2011).
Potta, S. P. et al. Functional characterization and transcriptome analysis of embryonic stem cell-derived contractile smooth muscle cells. Hypertension 53, 196–204 (2009).
Pruett, N. D. et al. Changing topographic Hox expression in blood vessels results in regionally distinct vessel wall remodeling. Biol Open 1, 430–435 (2012).
Hu, Y. & Xu, Q. Adventitial biology: differentiation and function. Arterioscler Thromb Vasc Biol 31, 1523–1529 (2011).
Majesky, M. W., Dong, X. R., Hoglund, V., Mahoney Jr, W. M. & Daum, G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol 31, 1530–1539 (2011).
Bustin, M., Catez, F. & Lim, J. H. The dynamics of histone H1 function in chromatin. Mol Cell 17, 617–620 (2005).
Gunjan, A., Alexander, B. T., Sittman, D. B. & Brown, D. T. Effects of H1 histone variant overexpression on chromatin structure. J Biol Chem 274, 37950–37956 (1999).
Shen, X., Yu, L., Weir, J. W. & Gorovsky, M. A. Linker histones are not essential and affect chromatin condensation in vivo. Cell 82, 47–56 (1995).
Jedrusik, M. A. & Schulze, E. A single histone H1 isoform (H1.1) is essential for chromatin silencing and germline development in Caenorhabditis elegans. Development 128, 1069–1080 (2001).
Lu, X. et al. Linker histone H1 is essential for Drosophila development, the establishment of pericentric heterochromatin and a normal polytene chromosome structure. Genes Dev 23, 452–465 (2009).
Rupp, R. A. & Becker, P. B. Gene regulation by histone H1: new links to DNA methylation. Cell 123, 1178–1179 (2005).
Terme, J. M. et al. Histone H1 variants are differentially expressed and incorporated into chromatin during differentiation and reprogramming to pluripotency. J Biol Chem 286, 35347–35357 (2011).
Hsu, L. W. et al. The effect of exogenous histone H1 on rat adipose-derived stem cell proliferation, migration and osteogenic differentiation in vitro. J Cell Physiol 227, 3417–3425 (2012).
Zhang, Y., Liu, Z., Medrzycki, M., Cao, K. & Fan, Y. Reduction of Hox gene expression by histone H1 depletion. PLoS One 7, e38829 (2012).
Bernstein, E. & Allis, C. D. RNA meets chromatin. Genes Dev 19, 1635–1655 (2005).
Rinn, J. L. et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129, 1311–1323 (2007).
Shah, N. & Sukumar, S. The Hox genes and their roles in oncogenesis. Nat Rev Cancer 10, 361–371 (2010).
Wong, M. M. et al. Sirolimus Stimulates Vascular Stem/Progenitor Cell Migration and Differentiation Into Smooth Muscle Cells via Epidermal Growth Factor Receptor/Extracellular Signal-Regulated Kinase/beta-Catenin Signaling Pathway. Arterioscler Thromb Vasc Biol (2013).
Thomson, J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Koch, P., Opitz, T., Steinbeck, J. A., Ladewig, J. & Brustle, O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci U S A 106, 3225–3230 (2009).
Klein, D. et al. Wnt2 acts as an angiogenic growth factor for non-sinusoidal endothelial cells and inhibits expression of stanniocalcin-1. Angiogenesis 12, 251–265 (2009).
Klein, D. et al. Wnt2 acts as a cell type-specific, autocrine growth factor in rat hepatic sinusoidal endothelial cells cross-stimulating the VEGF pathway. Hepatology 47, 1018–1031 (2008).
Hsiao, L. L. et al. A compendium of gene expression in normal human tissues. Physiol Genomics 7, 97–104 (2001).
Durig, J. et al. Expression of ribosomal and translation-associated genes is correlated with a favorable clinical course in chronic lymphocytic leukemia. Blood 101, 2748–2755 (2003).
Irizarry, R. A. et al. Exploration, normalization and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Irizarry, R. A. et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31, e15 (2003).
Li, L. C. & Dahiya, R. MethPrimer: designing primers for methylation PCRs. Bioinformatics 18, 1427–1431 (2002).
Li, L. C. Designing PCR primer for DNA methylation mapping. Methods Mol Biol 402, 371–384 (2007).
Acknowledgements
We thank Prof. Brüstle, Institute of Reconstructive Neurobiology, Life and Brain Center, University of Bonn and Hertie Foundation, Germany for the provision of cDNA from hESC lines H9, H9.2, I3. The authors are grateful to Prof. A. Awgulewitsch, Dept. of Medicine Director - MUSC Transgenic Mouse Core, Medical University of South Carolina (MUSC), Charleston, for his great help in discussing our data regarding HOX genes.
Author information
Authors and Affiliations
Contributions
D.K. and M.B. performed experiments, V.K. was involved in critical discussions; D.K. supervised and analysed results and made the figures; H.G.J. provided materials; D.K. and S.E. designed research and wrote the paper. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-Non-Commercial-ShareAlike 3.0 Unported licence. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Klein, D., Benchellal, M., Kleff, V. et al. Hox genes are involved in vascular wall-resident multipotent stem cell differentiation into smooth muscle cells. Sci Rep 3, 2178 (2013). https://doi.org/10.1038/srep02178
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02178
This article is cited by
-
Single-cell analysis reveals the spatial-temporal expression of genes associated with esophageal malformations
Scientific Reports (2024)
-
Altered DNA methylation pattern reveals epigenetic regulation of Hox genes in thoracic aortic dissection and serves as a biomarker in disease diagnosis
Clinical Epigenetics (2021)
-
Switching of vascular cells towards atherogenesis, and other factors contributing to atherosclerosis: a systematic review
Thrombosis Journal (2020)
-
HoxC6 Functions as an Oncogene and Isoform HoxC6-2 May Play the Primary Role in Gastric Carcinogenesis
Digestive Diseases and Sciences (2020)
-
Direct conversion of human fibroblasts into therapeutically active vascular wall-typical mesenchymal stem cells
Cellular and Molecular Life Sciences (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.