Abstract
While it is increasingly recognized that voluntary movements are produced by an interaction between conscious and unconscious processes, the role of the latter in Parkinson's disease has received little attention to date. Here, we administered a subliminal masked prime task to 15 Parkinson's disease patients and 15 age-matched healthy elderly subjects. Compatibility effects were examined by manipulating the direction of the arrows and the interstimuli interval. Analysis of the positive compatibility effect revealed performance differences between the most and the least affected hand in Parkinson's disease patients. Additionally, patients did not show the same tendency toward a negative compatibility effect as compared to elderly controls. These novel findings provide evidence supporting the role of basal ganglia circuits in controlling the balance between automatic motor response facilitation and inhibition.
Similar content being viewed by others
Introduction
Parkinson's disease (PD) is the second most common adult-onset progressive neurodegenerative disorder. Severe striatal dopamine loss secondary to the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc)1 is considered as the main pathological hallmark of PD. The decrease in dopaminergic nigrostriatal terminals, which is typically more pronounced in the posterior aspects of the striatum in the hemisphere contralateral to the most clinically affected body side2, is believed to lead to an imbalance between cortico-basal circuits that regulate movement facilitation and inhibition3.
A related role of the basal ganglia is to implement response selection. Indeed, basal ganglia disorders such as PD are associated with response selection impairments. For example, in the Eriksen Flanker task4 in which participants are asked to respond to the direction of a target arrow, appearing with flanking arrows that point to the same or opposite direction, greater response interference effects were observed in PD patients than in healthy aged controls5,6. However, a number of studies using subliminal stimuli have questioned the assumption that the basal ganglia involvement in motor selection is restricted to situations in which the stimuli are perceived consciously7,8,9,10, by using a subliminal masked prime-arrow paradigm derived from the Eriksen Flanker Task. This task has been intensively used in order to study automatic and unconscious motor processes elicited by external stimuli presented below the threshold of awareness7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22. In this task, participants are asked to make rapid button presses with the left or right hand following leftward or rightward pointing arrows, which are preceded by a brief subliminal masked prime arrow. Two consecutive effects can be observed behaviorally when manipulating the direction compatibility between prime and target stimuli and the interstimuli interval (ISI): an initial positive compatibility effect (PCE) at ISI-0 (i.e., shorter RT for compatible than for incompatible trials), followed by a negative compatibility effect (NCE) at longer ISI (i.e., longer RT for compatible than incompatible trials). It is hypothesized that the former results from a prime-induced motor activation while the latter involves inhibition of the prime-induced activation23.
To the best of our knowledge, only two studies from the same group examined the effects of PD on the PCE and NCE9,10. Seiss and Praamstra observed an increased PCE in PD patients relative to healthy controls, while the time course of the NCE seemed to be normal9. However, the experimental paradigm in Seiss and Praamstra did not include neutral prime stimuli and did not allow prime-induced activation and inhibition processes to be disentangled from facilitation and conflict effects embedded in the task8,13. As it is known that PD patients characteristically show asymmetrical motor symptoms (see above), they also compared the PCE between the least and the most affected hand. By asking the subjects to respond in one block with the right hand and in another with the left hand, they failed to find any differences.
The goal of the present study was to further investigate these priming effects in PD patients using the subliminal masked prime task14,23 and three different interstimuli intervals (ISI: 0, 150, 300), which allowed the time course of activation/inhibition processes elicited by visual stimuli to be examined. As compared with the study of Seiss and Praamsta9, we used a longer ISI and added neutral trials to separate facilitation/inhibition from conflict effects across ISIs13.
First, we compared RT data between PD and age-matched controls (see Table 1 and Figure 1 for a summary and a graphical representation of expected compatibility effects). Second, in the patients, we looked for a relationship between RT data and the severity of motor impairment using two complementary approaches: i) by comparing the performance between the least and most clinically affected hand (LAH and MAH, respectively) and ii) by testing for a correlation between behavioral performance and the UPDRS III (Unified Parkinson's Disease Rating Scale) motor scores24.
Results
Identification task
Results confirmed that the prime stimuli were presented below the threshold of awareness. By taking into account the no-responses, overall participants correctly identified a 33 ms prime on 47% of all trials. This performance is lower than chance level. Accuracy rates for PD patients and age-matched controls was 0.42 (below the chance level) and 0.53 respectively, but the latter did not differ significantly from chance level, t(14) = 1.25; p = 0.23. No participant was able to reliably discriminate the prime. These results support the view that prime stimuli were unlikely to be consciously perceived by the subjects during the main task.
Compatibility effects in PD patients and age-matched controls
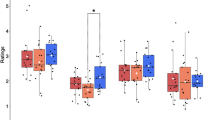
One PD subject was discarded from statistical analysis because of a large number of errors (more than 70% of errors in one condition). RT data analysis revealed a triple interaction between group, compatibility and ISI (Figure 2a), F(4,108) = 3.33; p = 0.022; η2 = 0.11. The ANOVA revealed a main effect of group characterized by longer global reaction time in PD than controls across all conditions, F(1,27) = 9.73; p = 0.004; η2 = 0.26 as well as a main effect of compatibility, F(2,54) = 18.73; p < 0.001; η2 = 0.41, accompanied by a significant ISI*compatibility interaction, F(4,108) = 6.34; p < 0.001; η2 = 0.19. The triple interaction suggests a tendency to an NCE in controls but not in PD patients when increasing ISI duration [interaction ISI*compatibility in elderly controls: F(4,56) = 9.38; p < 0.001; η2 = 0.40; interaction ISI*compatibility in PD patients: F(4,52) = 1.15; p = 0.34]. Both group showed the expected PCE at ISI-0, as revealed by the significant main effect of compatibility [Controls: F(2,28) = 22.66, p <0.001; PD: F(2,28) = 5.65, p = 0.009]. This compatibility effect persisted in PD patients [F(2,28) = 7.96, p = 0.002], but was attenuated (and did not reach the level of significance anymore) in elderly controls at ISI-150 [F(2,28) = 2.30, p = 0.14]. At ISI-300, none of the groups showed a significant main effect of compatibility [Controls: p = 0.38; PD: p = 0.15] (for a graphical representation of compatibility effects, see Figure 3). Statistically, no interaction was found between compatibility and group at ISI-0 (p = 0.12) and ISI-150 (p = 0.21). However, the compatibility*group interaction neared significance at ISI-300 (p = 0.06).
Mean RTs (a). and accuracy rates (b). for every conditions of each group.
A global PCE at ISI-0 in Parkinson's disease patients (PD) patients and age-matched controls (AC) and a change toward inhibition pattern for AC but not for PD at longer ISI are observed, suggesting an impaired motor inhibition process in PD. Vertical bars represent standard errors.
Concerning the accuracy rate, PD patients made more errors (+3%) than age-matched controls but this difference was not significant. A main effect of compatibility, with a worse accuracy rate in incompatible trials, F(2,54) = 3.93; p = 0.041; η2 = 0.13 and a main effect of ISI, F(2,54) = 10.27; p < 0.001; η2 = 0.27 were found, as well as a significant interaction between ISI and compatibility, F(2,54) = 8.92; p < 0.001; η2 = 0.25, but no three-way interaction (p = 0.39). However, accuracy rate analysis showed a change toward an NCE for age-matched controls when increasing ISI duration [controls: F(4,56) = 7.77; p < 0.001; η2 = 0.36] compared to PD patients. However, there was a trend toward significance in this group (p = 0.07) (Figure 2b). Additionally, no interaction was found between compatibility and group at ISI-0 (p = 0.96), both group showing the expected PCE.
Effect of lateralized motor impairment
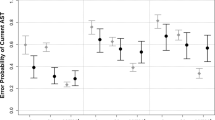
The repeated measures ANOVA showed a main effect of compatibility, F(2,26) = 9.79; p = 0.002; η2 = 0.43 and the three-way interaction approached significance, F(4,52) = 2.48; p = 0.06; η2 = 0.16. There was no main effect of hand. According to our a priori hypothesis of an asymmetric deficit in the automatic and unconscious motor activation in PD, the interaction should be especially marked in ISI-0. Indeed, the compatibility*hand interaction was significant, F(2,26) = 3.83; p = 0.03; η2 = 0.23 with an impaired facilitation effect and preserved conflict effect for the MAH (Facilitation = −2 ms; Conflict = 33 ms) in comparison to the LAH (Facilitation = 26 ms; Conflict = −4 ms) [Facilitation LAH > Facilitation MAH: t(13) = 2.13, p = 0.03; Conflict MAH > Conflict LAH: t(13) = 2.66, p = 0.01] (Figure 4). However, there was no significant difference in the PCE (incompatible – compatible) between the MAH and the LAH. In contrast, the control group did not show any differences in the facilitation and conflict effects between left and right hand responses [Facilitation right (13 ms) vs. left (22 ms): p = 0.36; Conflict right (37 ms) vs. left (23 ms): p = 0.35]. There was no compatibility difference between normal unilateral performances in PD patients and age-matched controls (i.e. facilitation for the LAH in PD vs. facilitation in controls, p = 0.61 and conflict for the MAH in PD vs. conflict in controls, p = 0.23). The accuracy rate showed a similar tendency but no significant effect was detected.
Correlation analysis in PD patients
We tested for a relationship between task performance (global RT, accuracy, compatibility effect) and severity of motor impairment at the time of testing as indexed by the total UPDRS III motor score assessed in the “on” state. We observed a negative correlation between disease severity and accuracy rate, R = −0.58; p < 0.05. However, disease severity did not correlate with global RT. Interestingly, the facilitation effect at ISI-0 was negatively correlated to disease severity, R = −0.68; p < 0.05, showing a stronger impairment of the automatic motor activation for more severe motor impairment. Additionally, the facilitation effect was negatively linked to the bradykinesia score of the MAH (R = −0.65; p < 0.05). Disease duration and levodopa equivalent daily dose did not correlate with any compatibility effects (p > 0.05).
Discussion
The purpose of the present study was to examine the effects of basal ganglia circuit disturbances on automatic and unconscious motor processes using a subliminal visuo-motor task. Age-matched controls showed a classical PCE at ISI-0. This PCE persisted at ISI-150, but its size decreased. At ISI-300, controls showed an NCE (i.e., longer RT and less accuracy for compatible than incompatible trials), albeit not statistically significant (Figure 2 and Figure 3). RT differed between PD and controls across ISIs as assessed by a three-way interaction analysis between groups, compatibility and ISIs. Whereas global RT was longer in PD than in controls, group differences in compatibility effects were observed at ISI-150 and ISI-300 during which PD patients showed no reduction in the magnitude of the PCE and no NCE, respectively. Additional group differences emerged when considering RT for neutral trials (Figure 3, middle and right panels). At ISI-0, controls but not PD patients showed evidence for motor response facilitation and conflict. Further analyses revealed differences between left and right hand motor performance in the patients but not in controls. Results showed that normal facilitation and conflict effects could still be observed in the patients for motor responses performed with the LAH and MAH, respectively (Figure 4). The facilitation effect at ISI-0 was negatively related to bradykinesia scores on the UPDRS III. Altogether, these results support the role of basal ganglia circuits in controlling the balance between automatic motor response facilitation and inhibition. The deficit in motor response facilitation elicited by unconsciously perceived visual stimuli may play a role in the pathophysiology of the motor disorder in PD.
We found that PD patients have longer global RT than age-matched controls in response to target stimuli whatever the physical properties of the prime stimulus. The slowness of PD patients in RT tasks, previously reported by different studies25,26,27,28,29, is mainly explained by a deficit in movement preparation (i.e. akinesia) rather than a slowness in execution of motor commands. Unlike healthy subjects, PD patients have difficulty in integrating different sources of stimuli for the selection of the most appropriate motor action (i.e., sensorimotor integration)30. The fact that PD patients are slower in both simple RT task (i.e., with the same response on every trial) and in a choice RT task, supports the hypothesis of a deficit at the level of response initiation26. At the prime level of the subliminal visuo-motor task, participants cannot consciously prepare the motor response associated with the prime stimulus. However, the motor preparation system is also activated by subliminal stimuli presentation even when no movement must be executed12. In daily life, various cues in the environment have the potential to trigger associated motor actions. Motor activation triggered by external stimuli is an automatic mechanism through which the simple view of an object is sufficient to partially select the corresponding movement if there is a well-established stimulus-response mapping, with the aim of facilitating motor execution31. This process is therefore essential for efficient interaction with our environment. PD patients showed an impaired automatic activation of the MAH and the facilitation effect was negatively related to disease severity. Therefore, this automatic and unconscious visuo-motor process may account for the difficulties PD patients have in initiating movements by efficiently integrating environmental stimulation and using this to assist motor program execution. Indeed, it is possible that one of the mechanisms through which the motor preparation system contributes to the control of movement is an efficient motor activation that can occur without conscious perception of the stimuli. However, it is well known that PD patients can take advantage of consciously perceived cues, notably when walking30, suggesting that impaired unconscious sensorimotor integration might be compensated by conscious perception of sensory information.
Basal ganglia circuit dysfunctions induced by PD alter the efficiency of interference control during action selection. This inefficiency stems from a reduced capacity to suppress the automatic activation of conflicting responses that increases interference in a traditional Eriksen Flanker Task5,6. When stimuli are presented below the threshold of awareness, PD patients still exhibit an enhanced susceptibility to interference9. We had recently underlined distinct roles for unconscious response facilitation and conflict in medial frontal areas13, notably the anterior cingulate cortex and the supplementary motor area, regions that are hypoactivated in PD patients32,33. However, the present results did not confirm the enhanced interference effect observed in previous studies when PD patients are compared with age-matched controls. For the MAH, the facilitation of the prime-induced response was reduced and the conflict was enhanced in comparison to the LAH, but was not significantly greater than in age-matched controls. The fact that there was no significant compatibility difference between normal unilateral performances in PD patients and controls suggests impairment of the automatic activation but not of the interference resolution process. In incompatible trials, response to target stimuli with the LAH was preceded by a deficient prime-induced activation of the MAH. By contrast, when target stimuli were responded to by the MAH, the subliminal prime stimulus efficiently activated the other hand, resulting in a normal conflict effect. An alternative explanation is that the unilateral preserved facilitation is caused by a shift of larger motor activation by subliminal stimuli in the LAH to equal out motor deficits in the MAH that can be generated by a shifted balance in the intercortical inhibition between motor cortices16. However, our data do not provide direct evidence for this because facilitation effects of the LAH in PD did not significantly differ from those of control participants.
The automatic and unconscious motor inhibition that follows the activation process has been previously investigated in PD patients. Indeed, Seiss and Praamstra10 have first showed that motor inhibition could not be observed at ISI-100 in PD patients or age-matched controls. When analyzing the time course of RT across several ISIs up to 200 ms, they observed a delayed NCE in elderly controls (at ISI-150 and ISI-200 but not at ISI-100). In PD patients, a PCE persisted across all ISIs but its size decreased at the longest ISIs9, resulting in an absent three-way interaction between the compatibility, ISI and group factors. The apparent discrepancy with our significant three-way interaction may be explained by the addition of a longer ISI in our experiment (i.e. 300 ms) in which the stronger between group differences appear. Here, a non-significant smaller PCE was found in ISI-300 for PD in comparison to other ISIs, suggesting maybe a relatively well-preserved pattern, but not as efficient as in age-matched controls who exhibited a tendency toward an NCE. Taken together, these results support the hypothesis of a deficit in automatic and unconscious activation/inhibition motor processes in PD subsequent to a disruption of cortico-basal circuits that influence the selection and the suppression of movements. However, the fact that compatibility effects were not significant at longer ISIs suggests these results should be interpreted with care. Indeed, it is possible that reduced PCEs are the result of some other mechanism such as a passive decay of primed response activation.
Interpretation of these results in terms of a tendency toward an NCE for ISI-150 and 300 is subject to the hypothesis of an inhibitory explanation of the reversed priming effect. However, some authors have suggested that the NCE may arise from processes that are entirely perceptual or even attentional34,35. Indeed, the mask-induced priming hypothesis proposes that the NCE may be produced by positive priming of the alternative response instead of motor inhibition34,36. For example, Lleras and Enns (2004) suggested that the mask contained elements similar to those that made up the primes and targets. Even if the debate is not completely over, it seems that when random line masks are employed, as in the present study, the major source of the NCE remains motor inhibition37.
A possible limitation of this study is that the visuomotor priming task was administered while PD patients were on their usual antiparkinsonian medications. By using the Simon task, Wylie et al.38 have recently shown that PD patients on agonist medication were less proficient at suppressing automatically activated responses compare to when they were tested off them. Additionally, doses of dopamine agonist were negatively linked to response suppression ability. If there is an overlap between conscious and unconscious response inhibition, it is therefore possible that dopamine agonists affect the inhibition of subliminal primed responses. In our study, 6 patients were treated with a dopaminergic agonist in addition to levodopa. Even if the levodopa equivalent daily dose does not correlate with compatibility effects, we cannot infer that medication has no effect on subliminal motor priming. Future studies are needed in order to investigate whether dopaminergic medications influence automatic and unconscious motor inhibition by comparing patients' performance between ‘on’ and ‘off’ states.
Methods
Study population
The study included 30 participants divided in two experimental groups, PD patients (n = 15) and controls (n = 15) matched for age [t(28) = 0.5, p = 0.62]. In PD, the mean age and disease duration was 67.7 ± 8 years and 11 ± 4 years, respectively. All PD patients were examined on their usual stable dopaminergic medications, that essentially consisted in levodopa monotherapy. They were tested in the morning, usually after having taken the first pill and all reported to be in an “ON” state at the time of testing. Six patients were treated with a dopaminergic agonist in addition to levodopa. Motor impairment was assessed using the motor part (Part III) of the UPDRS24. The median of the group was 19 (Table 2). The left and right upper limb bradykinesia scores (sum of scores from item 23: finger taps + item 24: hand movements + item 25: hand prosupination) were compared using the Wilcoxon test (median score LAH = 3, median score MAH = 5; p = 0.004). In order to screen patients for dementia, we administered the Mattis Dementia Rating scale39. The group mean score was 136 ± 5 (maximal score = 144). No patient was under the cut-off score of 123 that is specific to detect PD dementia40.
The 15 normal elderly subjects (6 men and 9 women) had an average age of 66.1 ± 9 years (Table 2). Elderly participants were non-institutionalized, alert and had no history of neurological problems, alcohol abuse or psychiatric disorders. This study was approved by the ethics committee of the University of Liège and all subjects provided informed written consent. They had normal or corrected vision and normal or corrected hearing.
Stimuli and procedures
The visuo-motor task was adapted from that reported in Eimer and Schlagecken14 and D'Ostilio and Garraux13. In this task, participants are asked to press a response button as accurately and as quickly as possible with their left or right index finger in response to the presentation of a left or right pointing arrow. In each trial, this target stimulus is briefly preceded by a central prime stimulus. Here, each trial started with a fixation dot presented for 2500 ms, immediately followed by a blank screen of 300 ms and then a prime stimulus displayed for 33 ms. The prime was followed by a mask stimulus consisting of 30 randomly oriented lines within a rectangular area centered on the prime display area on the center of the screen. A new random mask was constructed in each trial. Target stimuli consisted of two double arrows, which appeared for 150 ms, above and below the mask and presented either with the mask (ISI-0), or 150 ms (ISI-150), or 300 ms (ISI-300) after the mask. Trials were labeled as compatible when prime and target arrows pointed in the same direction (prime: ≪, target: ≪; prime: ≫, target: ≫) incompatible when they pointed in opposite directions (prime: ≫, target: ≪; prime: ≪, target: ≫) and neutral when the prime had no response assignment (prime: +, target: ≪ or ≫).
Each subject was trained on a practice block of 30 trials. The main experiment consisted in 432 trials divided into three experimental blocks (ISI-0–ISI-150–ISI-300, always presented in this order). Each block contained an equal number of trials with a compatible, incompatible or neutral prime-target relationship presented in a pseudorandomized order. After the completion of 48 trials, subjects were provided with a 20 sec. rest period during which the mean global reaction time during the last performed block was displayed. The outcome measures were the reaction time (RT) and accuracy in response to the target stimuli.
At the end of the experiment, participants were administered a prime identification task in order to assess whether or not the prime was consciously perceived8. The task comprised 60 trials. The task display was exactly the same as in the main experiment but participants were asked to guess the direction of the prime arrow stimuli presented before the mask. The percentage of correct responses were calculated and compared to chance level.
Participants were seated at a table in front of a laptop computer at a distance of 50 cm from the screen. They were instructed to maintain central eye fixation and to make a rapid button press with their left or right index finger according to the target arrows direction. Response keys were the Q key (for left responses) or the M key (for right responses) of a French computer keyboard. Depending on the mechanics of the buttons, there could be some delay between the key press/release and circuit switch on/off that might introduce some variability in RT recordings41. However, this variability is believed to be small and similar across conditions (compatible, incompatible and neutral) and ISIs and should not significantly bias compatibility effects reported in this study. Stimuli were displayed in black on a white background and subtended a visual angle of approximately 1.5° × 1°. Visual stimuli were generated and subject responses recorded by a personal computer using COGENT Cognitive interface software (COGENT 2000, Wellcome Department of Imaging Neuroscience, London, UK) implemented in Matlab 6.1 (Mathworks, Sherborn, MA).
Data analysis
The statistical analyses were carried out with SPSS version 20.0 (PC version. SPSS Inc., Chicago, IL, USA). Mean RT were calculated on a subject-by-subject basis for each condition (compatible, incompatible and neutral) using trials with correct responses and RT below 1500 ms. Statistical significance was assessed using Analysis of Variance (ANOVA) on mean RT and on accuracy of the within-subject factors ISI (0, 150, 300) and compatibility (compatible, incompatible, neutral) and the between-subjects factor group (PD patients, age-matched controls). Simple effects were calculated for each group. Significant effects were further analyzed using post hoc Newman-Keuls tests. Repeated measures ANOVAs on mean RT and accuracy were also performed only on PD patients with ISI, compatibility and hand (more affected, least affected) as within-subjects factors. Simple effects were also calculated in order to examine the interaction between compatibility and hand for each ISI. Planned comparisons were used to examine the facilitation effect (neutral – compatible) and the conflict effect (incompatible – neutral) for each hand with the a priori hypothesis of an impairment of these processes at ISI-0, related to a deficit of the automatic motor activation in PD patients. The data sphericity was tested using Mauchly's test. As sphericity was not respected, we used an adjustment (Huynh-Feldt) before considering the results of the ANOVAs. We estimated effect sizes by using partial eta squared (η2). A relationship between disease severity, measured by the UPDRS III scores at the time of the experiment and task performance was evaluated using Spearman's rank coefficients.
References
Greffard, S. et al. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol 63, 584–588 (2006).
Kish, S. J., Shannak, K. & Hornykiewicz, O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. N Engl J Med 318, 876–880 (1988).
Chou, K. L. & Hurtig, H. I. in Parkinson's Disease (eds Pfeiffer R. F., & Ebadi M.) 171–181 (CRC press, 2005).
Eriksen, C. W. The flankers task and response competition: A useful tool for investigating a variety of cognitive problems. Vis Cogn 2, 101–118 (1995).
Wylie, S. A., Stout, J. C. & Bashore, T. R. Activation of conflicting responses in Parkinson's disease: evidence for degrading and facilitating effects on response time. Neuropsychologia 43, 1033–1043 (2005).
Wylie, S. A. et al. The effect of Parkinson's disease on interference control during action selection. Neuropsychologia 47, 145–157 (2009).
D'Ostilio, K., Collette, F., Phillips, C. & Garraux, G. Evidence for a role of a cortico-subcortical network for automatic and unconscious motor inhibition of manual responses. Plos One 7, e48007 (2012).
Aron, A. R. et al. Inhibition of subliminally primed responses is mediated by the caudate and thalamus: evidence from functional MRI and Huntington's disease. Brain 126, 713–723 (2003).
Seiss, E. & Praamstra, P. Time-course of masked response priming and inhibition in Parkinson's disease. Neuropsychologia 44, 869–875 (2006).
Seiss, E. & Praamstra, P. The basal ganglia and inhibitory mechanisms in response selection: evidence from subliminal priming of motor responses in Parkinson's disease. Brain 127, 330–339 (2004).
Sumner, P. et al. Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron 54, 697–711 (2007).
D'Ostilio, K. & Garraux, G. Automatic Stimulus-Induced Medial Premotor Cortex Activation without Perception or Action. Plos One 6, e16613 (2011).
D'Ostilio, K. & Garraux, G. Dissociation between unconscious motor response facilitation and conflict in medial frontal areas. Eur J Neurosci 35, 332–340 (2012).
Eimer, M. & Schlaghecken, F. Effects of masked stimuli on motor activation: behavioral and electrophysiological evidence. J Exp Psychol Hum Percept Perform 24, 1737–1747 (1998).
Dehaene, S. et al. Imaging unconscious semantic priming. Nature 395, 597–600 (1998).
Praamstra, P. & Seiss, E. The neurophysiology of response competition: Motor cortex activation and inhibition following subliminal response priming. J Cogn Neurosci 17, 483–493 (2005).
Schlaghecken, F. & Sisman, R. Low-level motor inhibition in children: Evidence from the negative compatibility effect. Adv Cogn Psychol 2, 7–19 (2006).
Schlaghecken, F. & Eimer, M. Masked prime stimuli can bias "free" choices between response alternatives. Psychon Bull Rev 11, 463–468 (2004).
Schlaghecken, F., Klapp, S. T. & Maylor, E. A. Either or neither, but not both: locating the effects of masked primes. Proc Biol Sci 276, 515–521 (2009).
Schlaghecken, F. & Maylor, E. A. Motor control in old age: evidence of impaired low-level inhibition. J Gerontol B Psychol Sci Soc Sci 60, 158–161 (2005).
Sumner, P. & Brandwood, T. Oscillations in motor priming: positive rebound follows the inhibitory phase in the masked prime paradigm. J Mot Behav 40, 484–489 (2008).
Klotz, W. & Wolff, P. The effect of a masked stimulus on the response to the masking stimulus. Psychol Res 58, 92–101 (1995).
Eimer, M. & Schlaghecken, F. Response facilitation and inhibition in subliminal priming. Biol Psychol 64, 7–26 (2003).
Fahn, S., Elton, R. & Committee, U. D. in Recent development in Parkinson's disease (eds Fahn S., Marsden C. D., Calne D. B., & Goldstein M.) 153–164 (Florham Park, NJ: Macmillan, 1987).
Brown, V. J. et al. Dopamine dependent reaction time deficits in patients with Parkinson's disease are task specific. Neuropsychologia 31, 459–469 (1993).
Jahanshahi, M., Brown, R. G. & Marsden, C. D. Simple and choice reaction time and the use of advance information for motor preparation in Parkinson's disease. Brain 115(2), 539–564 (1992).
Stelmach, G. E., Worringham, C. J. & Strand, E. A. Movement preparation in Parkinson's disease. The use of advance information. Brain 109(6), 1179–1194 (1986).
Pullman, S. L., Watts, R. L., Juncos, J. L., Chase, T. N. & Sanes, J. N. Dopaminergic effects on simple and choice reaction time performance in Parkinson's disease. Neurology 38, 249–254 (1988).
Ballanger, B., Gil, R., Audiffren, M. & Desmurget, M. Perceptual factors contribute to akinesia in Parkinson's disease. Exp Brain Res 179, 245–253 (2007).
Abbruzzese, G. & Berardelli, A. Sensorimotor integration in movement disorders. Mov Disord 18, 231–240 (2003).
Sumner, P. & Husain, M. At the Edge of Consciousness: Automatic Motor Activation and Voluntary Control. Neuroscientist 14, 474–486 (2008).
Jenkins, I. H. et al. Impaired activation of the supplementary motor area in Parkinson's disease is reversed when akinesia is treated with apomorphine. Ann neurol 32, 749–757 (1992).
Playford, E. D. et al. Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann neurol 32, 151–161 (1992).
Lleras, A. & Enns, J. T. Negative compatibility or object updating? A cautionary tale of mask-dependent priming. J exp psychol Gen 133, 475–493 (2004).
Sohrabi, A. & West, R. L. Positive and negative congruency effects in masked priming: a neuro-computational model based on representation, attention and conflict. Brain Res 1289, 124–132 (2009).
Verleger, R., Jaskowski, P., Aydemir, A., van der Lubbe, R. H. & Groen, M. Qualitative differences between conscious and nonconscious processing? On inverse priming induced by masked arrows. J exp psychol Gen 133, 494–515, 10.1037/0096-3445.133.4.494 (2004).
Sumner, P. Mask-induced priming and the negative compatibility effect. Exp Psychol 55, 133–141 (2008).
Wylie, S. A. et al. Dopamine agonists and the suppression of impulsive motor actions in Parkinson disease. J Cogn Neurosci 24, 1709–1724 (2012).
Mattis, S. in Geriatric Psychiatry: a Handbook for Psychiatrist and Primary Care Physicians. (eds Bellack L., & Karusu T. B.) (Grune & Straton, New York, 1976).
Matteau, E. et al. Mattis Dementia Rating Scale 2: screening for MCI and dementia. Am J Alzheimers Dis Other Demen 26, 389–398 (2011).
Li, X., Liang, Z., Kleiner, M. & Lu, Z. L. RTbox: a device for highly accurate response time measurements. Behav res methods 42, 212–225 (2010).
Acknowledgements
This work was supported by FRS-FNRS (Grant 1.5.047.06). The authors would like to thank Ms Luci Crook for skillful manuscript editing.
Author information
Authors and Affiliations
Contributions
K.D. was implied in conception, organization and execution of the research project, design and execution of the statistical analysis, as well as in drafting the manuscript. J.C., V.D. & B.S. were implied in the execution of the research project and gave revision of the manuscript. G.G. was implied in conception, organization and execution of the research project and gave critical revision of the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
D'Ostilio, K., Cremers, J., Delvaux, V. et al. Impaired automatic and unconscious motor processes in Parkinson's disease. Sci Rep 3, 2095 (2013). https://doi.org/10.1038/srep02095
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02095
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.