Abstract
Evaluation of circadian phenotypes is crucial for understanding the pathophysiology of diseases associated with disturbed biological rhythms such as circadian rhythm sleep disorders (CRSDs). We measured clock gene expression in fibroblasts from individual subjects and observed circadian rhythms in the cells (in vitro rhythms). Period length of the in vitro rhythm (in vitro period) was compared with the intrinsic circadian period, τ, measured under a forced desynchrony protocol (in vivo period) and circadian/sleep parameters evaluated by questionnaires, sleep log and actigraphy. Although no significant correlation was observed between the in vitro and in vivo periods, the in vitro period was correlated with chronotype, habitual sleep time and preferred sleep time. Our data demonstrate that the in vitro period is significantly correlated with circadian/sleep preference. The findings suggest that fibroblasts from individual patients can be utilized for in vitro screening of therapeutic agents to provide personalized therapeutic regimens for CRSD patients.
Similar content being viewed by others
Introduction
Behavioral and physiological processes such as sleep/wakefulness and hormone secretion exhibit circadian rhythms1. Individual differences in daily activity/sleep time, known as the diurnal preference/chronotype, are commonly assessed using the conventional self-reported Horne-Östberg Morningness-Eveningness Questionnaire (MEQ)2 and/or the recently developed online self-reported Munich ChronoType Questionnaire (MCTQ)3. The morning (early) chronotype manifests earlier timings for sleep and physiological rhythms such as core body temperature and melatonin secretion than the intermediate chronotype and still earlier than the evening (late) chronotype4,5,6. The various daily behavioral and physiological rhythms are regulated by a system of self-sustained clocks and are entrained to environmental cues, such as light exposure, food intake and work schedules, enabling us to adapt to changes in the external environment7,8. In mammals, the circadian clock system is hierarchically organized such that the central oscillator in the suprachiasmatic nuclei (SCN) of the hypothalamus integrates environmental information and synchronizes the phase of oscillators in peripheral cells, tissues and organs9,10. The molecular mechanism of the circadian clock system involves a complex set of transcription-translation negative feedback loops that regulate multiple clock genes including Bmal1, Clock, Cry, Per, Ror and Rev-Erb11,12.
Circadian rhythm sleep disorders (CRSDs) are characterized by the inability to fall asleep and awaken at a desired time13. There are several subtypes of CRSDs: advanced sleep phase type (ASPT), delayed sleep phase type (DSPT) and free-running type (FRT). ASPT patients show extremely advanced involuntary timing of sleep and wake, DSPT patients show significantly delayed sleep onset and wake times and FRT patients have sleep times that occur with ~1-h delay each day and are not able to adapt to the external 24-h day. CRSDs are attributed etiologically to malfunction and/or maladaptation of the circadian clock system14,15,16,17 and therefore evaluation of circadian phenotypes is crucial for understanding the pathophysiology of CRSDs. The intrinsic circadian period, τ (the free-running period of circadian rhythms in the absence of external cues), is considered to be a critical factor in the pathophysiology of CRSDs. Indeed, we recently demonstrated that τ was significantly prolonged in FRT patients under a strict forced desynchrony (FD) protocol compared to healthy subjects with the intermediate chronotype18. However, although the FD protocol is regarded as the most reliable and valid method for the assessment of τ in humans, it is laborious and costly to perform in clinical settings19,20. A more convenient evaluation of circadian phenotypes is therefore required both to reduce burden on the subjects and to increase the feasibility of examination.
To this end, Brown et al. developed a luminescence rhythm assay system using biopsy samples to evaluate an individual’s circadian phenotype21. In this system, the biopsy-derived fibroblasts are transfected with a circadian reporter, the Bmal1 promoter-driven luciferase gene (Bmal1-luc), using a lentiviral system. Luciferase activity under the control of the Bmal1 promoter was found to show daily rhythms in cultured fibroblasts (in vitro rhythm). Moreover, by monitoring the luciferase activity level for 4–6 cycles and evaluating the rhythmic characteristics of luminescence expression in these fibroblasts, Brown et al. found that cultured fibroblasts from morning-type subjects had a shorter period length than those from evening-type subjects22. Additionally, the period length of the in vitro rhythm is proportionally related to that of the physiological rhythm as assessed under a constant routine (CR), multiple nap, or nearly dark protocol23. On the other hand, Hasan et al. recently reported that neither chronotype nor τ (the period of melatonin rhythm assessed under a 9-day FD protocol) were significantly correlated with in vitro period length24. It is unclear then whether surrogate measurements using cultured fibroblasts derived from an individual’s biopsy samples are in fact useful for assessing circadian phenotype. Furthermore, exactly what in vitro rhythms represent is not yet fully understood.
To address these issues, in this study we measured clock gene expression in primary fibroblasts derived from subjects’ skin biopsy samples using a non-viral luminescence assay system and compared the period of in vitro rhythms with τ measured under a strict FD protocol and circadian/sleep parameters evaluated by questionnaires, sleep log and actigraphy in a real-life setting.
Results
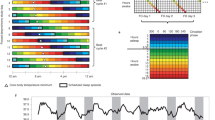
Circadian rhythms were sustained in cultured cells for several cycles, as indicated by luminescence levels (Fig. 1a). The in vitro period length of Bmal1-luc rhythm (in vitro period) varied among fibroblasts from different individuals (Fig. 1b, Table 1). The in vivo period length of melatonin rhythm (in vivo period) had been determined for each subject in our previous study18. The average in vitro period was significantly shorter than the average in vivo period in our subjects (23.46 ± 0.76 h vs. 24.17 ± 0.20 h; t = −3.80, df = 16, P = 0.002).
An individual’s circadian rhythm in vitro and the period length of in vitro rhythms.
(a) Representative detrended data of Bmal1-luc rhythm in cultured fibroblasts from subject I4. Primary fibroblasts were obtained from a skin biopsy sample and were transfected with the circadian reporter Bmal1-luc utilizing an electroporation system. After the cultured cells were synchronized by treatment with dexamethasone for 2 h, bioluminescence rhythms from the cells were continuously measured for 5 cycles. (b) In vitro period length of Bmal1-luc rhythms in 9 intermediate type subjects (green circles) and 8 evening type subjects (blue circles). Data are presented as mean value ± standard error of the mean.
Next, a comparison of the in vitro and in vivo periods for each subject (9 intermediate types and 8 evening types) revealed a significant correlation between the two periods in intermediate types (R = 0.750, P = 0.020) but not in evening types (R = −0.336, P = 0.416) or in all subjects (R = 0.093, P = 0.723) (Fig. 2). The in vivo period did not differ between intermediate and evening types (24.12 ± 0.12 h vs. 24.22 ± 0.27 h; t = −0.98, df = 9.31, P = 0.353). By contrast, the in vitro period did differ significantly between the two types (23.09 ± 0.55 h vs. 23.87 ± 0.77 h; t = −2.42, df = 15, P = 0.028).
Comparison of in vitro and in vivo rhythms between intermediate (I, green circles) and evening (E, blue circles) types.
Dots represent the period length of the in vitro (horizontal axis) or in vivo (vertical axis) rhythm for each subject. No significant correlation was found between in vitro and in vivo periods when all subjects were examined. A longer in vitro period was observed in evening types compared to intermediate types (P = 0.028).
MEQ scores indicate morningness-eveningness preference (chronotype). As anticipated, the in vitro period was significantly correlated with individual MEQ score (R = −0.570, P = 0.017) (Fig. 3a). Additionally, there was a significant correlation between the in vitro period and habitual sleep time (R = 0.632, P = 0.007) (Fig. 3b). By contrast, the in vivo period was not associated with MEQ score (R = −0.046, P = 0.860) (Fig. 3a) or habitual sleep time (R = −0.060, P = 0.819) (Fig. 3b). Correlations between the in vitro or in vivo period and circadian/sleep parameters were assessed using mid-sleep timings on work days (MSW), mid-sleep timings on free days (MSF) and sleep-corrected MSF (MSFsc; another indicator of chronotype) obtained by the MCTQ. No significant correlation was found between MSW and the in vitro period (R = 0.343, P = 0.178) or the in vivo period (R = −0.249, P = 0.336) (Fig. 4a). By contrast, MSF, which represents the preferred sleep timing free of social constraints, was strongly correlated with the in vitro period (R = 0.617, P = 0.008) (Fig. 4b), as was MSFsc, which represents chronotype (R = 0.592, P = 0.012) (Fig. 4c). Evening preference was associated with a longer in vitro period. There was no association between MSF (R = −0.037, P = 0.889) or MSFsc (R = −0.108, P = 0.680) and the in vivo period (Fig. 4b and 4c).
Correlations between the in vitro (circles) or in vivo (triangles) period and (a) MEQ score or (b) habitual sleep timing.
A strong correlation was seen between in vitro period and MEQ score (R = −0.570, P = 0.017) and habitual sleep time (R = 0.632, P = 0.007) when all subjects (9 intermediate types denoted by green circles and 8 evening types, blue circles) were examined.
Correlations between the in vitro (circles) or in vivo (triangles) period and (a) MSW, (b) MSF, or (c) MSFsc.
Strong correlations were observed between the in vitro period and MSF (R = 0.617, P = 0.008) or MSFsc (R = 0.592, P = 0.012) when all subjects (9 intermediate types denoted by green circles and 8 evening types, blue circles) were examined.
Discussion
Despite the fact that only a limited number of subjects were assessed in this study, the results demonstrate that an individual’s in vitro circadian period is significantly correlated with circadian/sleep preference.
Consistent with previous reports21,22,24, primary fibroblast cells derived from individuals showed rhythmic expression of the circadian reporter Bmal1-luc. However, despite Hasan et al. finding that in vitro periods were longer than in vivo periods24, we observed that in vitro periods were in fact shorter. There are a number of differences in the experimental conditions between the present study and previous studies, such as the reporter constructs utilized, transfection methods and recording media. Serum factors, pH levels and Ca2+ concentration are known to alter the circadian characteristics of in vitro rhythms25,26,27. Consequently, the differences in experimental conditions between the present and past studies might account for the differences observed in in vitro period length.
When in vitro and in vivo circadian rhythms were compared, a moderate but significant correlation between the two rhythms was observed in intermediate types, but not in evening types or in all subjects. Additionally, Hasan et al. found no correlation between in vitro and in vivo periods in their subjects24. Pagani et al. reported that the in vitro period was proportional to the in vivo period in their subjects, although they did not observe a longer in vitro period in blind subjects who are known to have a significantly longer in vivo period than sighted subjects23. In vivo rhythms such as core body temperature and melatonin secretion are known to be affected by the after-effects of entrainment28. Long-term effects of previous long sleep-wake cycles might cause the long in vivo period in blind individuals. These data suggest that in vitro rhythms are not strongly correlated with in vivo rhythms.
The relationship between the central and peripheral oscillators has been studied by measuring luminescence rhythms in cultured SCN cells and peripheral tissues explanted from circadian reporter transgenic animals9,29,30. The period and the phase of SCN rhythm are different from those of peripheral rhythms even under the same condition (i.e., in organotypic slice culture)31,32. This implies that individual tissues show distinct circadian characteristics under conditions in which the tissues are dissociated. Primary fibroblasts used in this study are a group of dissociated cells established from skin biopsy samples. Unlike tissues in vivo, cultured cells do not receive environmental information or any circadian signals from other tissues (SCN and periphery). On the contrary, almost all of the in vivo tissues are co-regulated or are interdependent even when masking effects are minimized. It was recently reported that age-related differences are observed for numerous characteristics of behavioral and physiological rhythms but not in the molecular machinery of peripheral circadian clocks25. These findings imply that in vitro rhythms reflect the molecular mechanism of circadian clock components in peripheral cells, whereas in vivo rhythms reflect the physiological mechanism of the circadian clock system of an individual.
The longer in vitro period observed in evening types compared to intermediate types in this study is in agreement with a previous report that extreme evening types had a longer in vitro period than extreme morning types22. Furthermore, in vitro period length, but not in vivo period length, was significantly correlated with MEQ score (chronotype), habitual sleep time, MSF (preferred sleep time) and MSFsc (chronotype). Our data strongly support the notion that the period length of circadian rhythms in fibroblasts from individuals represents their circadian/sleep preference. By contrast, the in vivo period was not correlated with any of these parameters. The in vivo period converges to a nearly 24-h period20,33 and does not vary greatly among individuals. This characteristic of in vivo rhythms might weaken the correlation between the in vivo period and circadian/sleep preference. Given that genetic factors (individual traits) have a significant effect on the determination of circadian/sleep preference34, these findings suggest that the properties of in vitro rhythms might reflect individual differences in circadian clock traits better than those of in vivo rhythms. Evaluating rhythmic expression of clock genes in isolated fibroblast cells might therefore be an appropriate method to assess an individual’s circadian clock phenotype.
Hasan et al. reported that MEQ score is correlated with the in vivo period but not with the in vitro period24, which is inconsistent with our findings. Most of their subjects were intermediate types, whereas 8 of our 17 subjects were evening types. In the present study, in vitro period length varied greatly between intermediate and evening types and a significant correlation was observed between the in vitro period and chronotype. These differences in subjects might explain the discrepancy between the two sets of results. Further validation using larger cohorts should be performed to accurately determine whether the period length of the in vitro rhythm can predict an individual’s circadian clock phenotype.
In the age of personalized medicine, one of our goals is to tailor therapies to individuals based upon their specific disorders. However, sleep disorders, like many of the conditions for which therapeutic intervention would be useful, are extremely complex genetically. Even if genomic or single nucleotide polymorphism analysis were to be performed in patients with these conditions, the data obtained would not provide sufficient information to test effective new pharmaceutical agents, let alone prescribe them for treatment. To overcome this, effective in vitro screens to test therapeutic agents are required. To this end, we have now shown that fibroblasts in culture have circadian periods correlated with the circadian clock phenotype of the individual. Thus, we believe that these isolated fibroblasts could be utilized for in vitro screening of therapeutic agents to modify circadian disruption (e.g. altered period, phase and amplitude of circadian rhythms) to develop personalized therapies for patients with CRSDs.
Methods
Subjects
Subjects were 17 healthy males aged 19–39 years (mean age ± standard deviation (SD), 22.6 ± 4.4 years) who participated in our previous study18. None had sleep disorders (as assessed by clinical polysomnography and the Pittsburgh Sleep Quality Index questionnaire), psychiatric disorders (assessed by a semi-structured interview with a psychiatrist and the Center for Epidemiology Studies Depression Scale questionnaire) or severe physical diseases. None had traveled across time zones or had been on any medication over the past 6 months. MEQ score was used to determine each subject’s chronotype, where a score of 16–41 denoted evening type and that of 42–58 denoted intermediate type. Accordingly, 9 subjects were classed as intermediate type (subjects I1, I2, I3, I4, I5, I6, I7, I8 and I9) and 8 as evening type (subjects E1, E2, E3, E4, E5, E6, E7 and E8).
The protocol was approved by the Institutional Ethics Committee of the National Center of Neurology and Psychiatry and written informed consent was obtained from all subjects.
In vivo rhythm assay
A total of 17 subjects participated in a 13-day FD protocol in a sleep laboratory free from external time cues in our previous study18. Briefly, the FD protocol was composed of 3 experiments: 1) initial assessment of circadian phase under CR35 (1st CR); 2) a 28-h sleep-wake schedule (9.33 h of sleep and 18.67 h of wakefulness) for 7 days; and 3) a second assessment of circadian phase under CR (2nd CR). Throughout the experiments, lights were maintained at a low intensity (<15 lx) during the wake period and turned off (0 lx) during the sleep period. Ambient temperature and humidity in the laboratory were maintained at 25 ± 0.5°C and 50 ± 5% relative humidity, respectively. During the periods of CR, subjects were required to lie on a reclining chair in a semi-recumbent position and stay awake for 34 h. Water was available at all times and a 200-kcal meal was provided every 2 h. Blood samples were collected every hour using an intravenous catheter placed in a forearm vein. Plasma was immediately separated by centrifugation (15 min at 1600 × g and 4°C) and stored at −80°C until analysis. Concentrations of plasma melatonin were measured by radioimmunoassay. Dim light melatonin onset (DLMO) time was defined as the time when plasma melatonin concentration rose from a low background level to above 10 pg/mL36. To calculate intrinsic circadian period, τDLMO, the difference in the DLMO time measured during the 1st CR and 2nd CR conducted at the beginning and end of the FD protocol, respectively, was divided by the number of experimental days. τDLMO was used as the period length of in vivo rhythm (in vivo period) in this study (Table 1).
Habitual sleep time
For 7 days prior to laboratory admission, subjects maintained their daily routines and slept at a regular time every night at home under a dim light condition. Their regular sleep-wake routine was verified by sleep log and actigraphy. Wrist activity was monitored with an Actiwatch (Philips Respironics) around the non-dominant wrist. Activity data were analyzed using computer-calculated sleep-wake determinations37. Average sleep onset and wake times during these 7 days were used as the habitual sleep onset time and wake time, respectively. Habitual sleep time was designated as the midpoint between habitual sleep onset time and wake time.
Munich chronotype questionnaire3
The MCTQ was administered on the admission day to assess the subjects’ sleep onset and wake times separately on work days or free days. MSW and MSF were calculated as the midpoint between sleep onset time and wake time on work days and free days, respectively. MSFsc was used as another indicator of chronotype.
Skin biopsy, cell culture and in vitro rhythm assay
A skin biopsy of the dorsal region was performed using a biopsy punch (2 mm in diameter and 7 mm in length) with a plunger system (Kai Industries) on the first day of the 28-h sleep–wake schedule (i.e., Day 4 of the FD protocol). Primary fibroblast cultures, derived from the skin biopsy samples, were established by culturing in DMEM/F12 (GIBCO/Life Technologies) supplemented with 20% FBS (NICHIREI BIOSCIENCES), 1% FUNGIZONE (GIBCO, Life Technologies), 0.5 μg/mL MC-210 (DS Pharma Biomedical Co., Ltd.) and 1% penicillin/streptomycin (GIBCO/Life Technologies) at 37°C and 5% CO2. For each measurement, 3 × 105 primary cells were transfected with 3 μg of the Bmal1-luc construct Bp/527-LUC38 using Neon (Life Technologies) and were plated in a 35-mm dish containing DMEM/F12 supplemented with 20% FBS without penicillin/streptomycin (Day 0). After 3 days (Day 3), the medium was changed to DMEM/F12 supplemented with 5% FBS and 1% penicillin/streptomycin and on Day 10 was changed to fresh medium (DMEM/F12 supplemented with 5% FBS and 1% penicillin/streptomycin). On Day 17, 0.1 μM dexamethasone (Sigma-Aldrich) was applied and the cells were incubated for 2 h to synchronize rhythms in the fibroblasts. Luminescence from the cells was measured for at least 5 cycles in recording medium (DMEM #D-2902; Sigma-Aldrich) supplemented with 19.4 mM glucose (final concentration 25 mM), 10 mM HEPES (Sigma-Aldrich), 0.25% penicillin/streptomycin and 0.1 mM beetle luciferin potassium salt (Promega), using photomultiplier tubes (Hamamatsu) in a dark box at 37°C as previously described39. The data were detrended by subtracting the 24-h running average from the raw data and then smoothed with a 2-h running average using Origin7.0 (OriginLab) as previously described40. The period length of the Bmal1-luc rhythm (in vitro period) was determined by regression analysis using the second to fourth peak times of the luminescence rhythm. The in vitro period for each subject is presented as the mean of 6 to 10 independent measurements ± SD (Table 1).
Statistical analysis
Kolmogorov–Smirnov tests were performed and frequencies for the parameters tested in this study were normally distributed. Paired t-tests were used to compare in vivo and in vitro period length and unpaired t-tests were used to compare the in vivo and in vitro periods between the intermediate and evening types. Correlations between the parameters were assessed by Pearson’s correlation analysis. Unpaired Student’s t-tests were used to compare the physiological in vivo period and the fibroblast in vitro period between the intermediate and evening types. P < 0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS ver. 11 (SPSS Japan Inc.). Data are presented as mean ± SD.
References
Pittendrigh, C. S. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol 55, 16–54 (1993).
Horne, J. A. & Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4, 97–110 (1976).
Roenneberg, T., Wirz-Justice, A. & Merrow, M. Life between clocks: daily temporal patterns of human chronotypes. Journal of biological rhythms 18, 80–90 (2003).
Kerkhof, G. A. The 24-hour variation of mood differs between morning- and evening-type individuals. Percept Mot Skills 86, 264–266 (1998).
Duffy, J. F., Dijk, D. J., Hall, E. F. & Czeisler, C. A. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med 47, 141–150 (1999).
Baehr, E. K., Revelle, W. & Eastman, C. I. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. Journal of sleep research 9, 117–127 (2000).
Gachon, F., Nagoshi, E., Brown, S. A., Ripperger, J. & Schibler, U. The mammalian circadian timing system: from gene expression to physiology. Chromosoma 113, 103–112 (2004).
Foster, R. G. & Roenneberg, T. Human responses to the geophysical daily, annual and lunar cycles. Curr Biol 18, R784–R794 (2008).
Yamazaki, S. et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288, 682–685 (2000).
Yoo, S. H. et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America 101, 5339–5346 (2004).
Reppert, S. M. & Weaver, D. R. Coordination of circadian timing in mammals. Nature 418, 935–941 (2002).
Takahashi, J. S., Hong, H. K., Ko, C. H. & McDearmon, E. L. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9, 764–775 (2008).
ICSD-2. The International Classification of Sleep Disorders 2nd ed.: Diagnostic and Coding Manual, (Westchester, IL, 2005).
Okawa, M. & Uchiyama, M. Circadian rhythm sleep disorders: characteristics and entrainment pathology in delayed sleep phase and non-24-h sleep-wake syndrome. Sleep Med Rev 11, 485–496 (2007).
Barion, A. & Zee, P. C. A clinical approach to circadian rhythm sleep disorders. Sleep medicine 8, 566–577 (2007).
Hida, A., Kitamura, S. & Mishima, K. Pathophysiology and pathogenesis of circadian rhythm sleep disorders. Journal of physiological anthropology 31, (2012).
Ebisawa, T. Analysis of the molecular pathophysiology of sleep disorders relevant to a disturbed biological clock. Molecular genetics and genomics : MGG (2013).
Kitamura, S. et al. Intrinsic circadian period of sighted patients with circadian rhythm sleep disorder, free-running type. Biological psychiatry 73, 63–69 (2013).
Klerman, E. B., Dijk, D. J., Kronauer, R. E. & Czeisler, C. A. Simulations of light effects on the human circadian pacemaker: implications for assessment of intrinsic period. The American journal of physiology 270, R271–282 (1996).
Czeisler, C. A. et al. Stability, precision and near-24-hour period of the human circadian pacemaker. Science 284, 2177–2181 (1999).
Brown, S. A. et al. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol 3, e338 (2005).
Brown, S. A. et al. Molecular insights into human daily behavior. Proceedings of the National Academy of Sciences of the United States of America 105, 1602–1607 (2008).
Pagani, L. et al. The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PloS one 5, e13376 (2010).
Hasan, S. et al. Assessment of circadian rhythms in humans: comparison of real-time fibroblast reporter imaging with plasma melatonin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology (2012).
Pagani, L. et al. Serum factors in older individuals change cellular clock properties. Proceedings of the National Academy of Sciences of the United States of America 108, 7218–7223 (2011).
Lee, S. K. et al. Extracellular low pH affects circadian rhythm expression in human primary fibroblasts. Biochemical and biophysical research communications 416, 337–342 (2011).
Noguchi, T., Wang, C. W., Pan, H. & Welsh, D. K. Fibroblast circadian rhythms of PER2 expression depend on membrane potential and intracellular calcium. Chronobiology international 29, 653–664 (2012).
Scheer, F. A., Wright, K. P., Jr, Kronauer, R. E. & Czeisler, C. A. Plasticity of the intrinsic period of the human circadian timing system. PloS one 2, e721 (2007).
Yoo, S. H. et al. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proceedings of the National Academy of Sciences of the United States of America 102, 2608–2613 (2005).
Noguchi, T. et al. Dual-color luciferase mouse directly demonstrates coupled expression of two clock genes. Biochemistry 49, 8053–8061 (2010).
Pendergast, J. S., Friday, R. C. & Yamazaki, S. Endogenous rhythms in Period1 mutant suprachiasmatic nuclei in vitro do not represent circadian behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 14681–14686 (2009).
Pendergast, J. S., Niswender, K. D. & Yamazaki, S. Tissue-specific function of Period3 in circadian rhythmicity. PloS one 7, e30254 (2012).
Wright, K. P., Jr, Hughes, R. J., Kronauer, R. E., Dijk, D. J. & Czeisler, C. A. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proceedings of the National Academy of Sciences of the United States of America 98, 14027–14032 (2001).
Koskenvuo, M., Hublin, C., Partinen, M., Heikkila, K. & Kaprio, J. Heritability of diurnal type: a nationwide study of 8753 adult twin pairs. Journal of sleep research 16, 156–162 (2007).
Mills, J. N., Minors, D. S. & Waterhouse, J. M. Adaptation to abrupt time shifts of the oscillator(s) controlling human circadian rhythms. The Journal of physiology 285, 455–470 (1978).
Lewy, A. J., Cutler, N. L. & Sack, R. L. The endogenous melatonin profile as a marker for circadian phase position. Journal of biological rhythms 14, 227–236 (1999).
Hyde, M. et al. Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. Journal of sleep research 16, 213–216 (2007).
Yu, W., Nomura, M. & Ikeda, M. Interactivating feedback loops within the mammalian clock: BMAL1 is negatively autoregulated and upregulated by CRY1, CRY2 and PER2. Biochemical and biophysical research communications 290, 933–941 (2002).
Yamazaki, S. & Takahashi, J. S. Real-time luminescence reporting of circadian gene expression in mammals. Methods in enzymology 393, 288–301 (2005).
Abe, M. et al. Circadian rhythms in isolated brain regions. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 350–356 (2002).
Acknowledgements
We thank Junko Takei for training in primary cell culture and Dr. Hajime Tei for technical support. A part of this study is the result of “Understanding of molecular and environmental bases for brain health” carried out under the Strategic Research Program for Brain Sciences from the Ministry of Education, Culture, Sports, Science and Technology of Japan. This study was supported by Grants-in-Aid for Scientific Research (#21390335, #22791161 and #24621015) from Japan Society for the Promotion of Science, an Intramural Research Grant (#23-3) for Neurological and Psychiatric Disorders of National Center of Neurology and Psychiatry, a Grant-in-Aid (H22-SeisakuSouyaku-Ippan-013) from the Ministry of Health, Labour and Welfare and a Grant from Takeda Research Foundation.
Author information
Authors and Affiliations
Contributions
A.H., S.K. and K.M. designed research. A.H., S.K., Y.O., M.E., Y.K., Yu, M., Yo, M., K.N., M.W., S.A., S.H., M.K., Y.K. and K.M. performed research. A.H., S.K., Y.O. and M.E. analyzed the data. S.Y., Y.G. and M.I. contributed reagents/materials/analysis tools. A.H., S.K. and K.M. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Hida, A., Kitamura, S., Ohsawa, Y. et al. In vitro circadian period is associated with circadian/sleep preference. Sci Rep 3, 2074 (2013). https://doi.org/10.1038/srep02074
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02074
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.