Abstract
An ‘anomalous’ negative flux, in which carbon dioxide (CO2) enters rather than is released from the ground, was studied in a saline/alkaline soil. Soil sterilization disclosed an inorganic process of CO2 dissolution into (during the night) and out of (during the day) the soil solution, driven by variation in soil temperature. Experimental and modeling analysis revealed that pH and soil moisture were the most important determinants of the magnitude of this inorganic CO2 flux. In the extreme cases of air-dried saline/alkaline soils, this inorganic process was predominant. While the diurnal flux measured was zero sum, leaching of the dissolved inorganic carbon in the soil solution could potentially effect net carbon ecosystem exchange. This finding implies that an inorganic module should be incorporated when dealing with the CO2 flux of saline/alkaline land. Neglecting this inorganic flux may induce erroneous or misleading conclusions in interpreting CO2 fluxes of these ecosystems.
Similar content being viewed by others
Introduction
Projections of climatic change and its relationship with carbon dioxide (CO2) have attracted a great deal of scientific and public attention concerning carbon (C) cycling1. As one of the major determinants of the C balance in terrestrial ecosystems2, soil CO2 flux, is considered an important regulator of climate change3. Conventionally, the soil CO2 flux is interpreted in the context of an unstated hypothesis that biotic processes, including microbial oxidation of soil organic matter and litter fall and root respiration, largely determine the processes of CO2 release from soil4. Not surprisingly, most research has centered on defining empirical relationships between soil CO2 fluxes and specific climatic parameters, such as temperature5,6, precipitation7 and soil water content – in order to create basic regressions to predict fluxes in changing environments5,7,8,9.
However, in some arid and semi-arid systems, both positive and negative CO2 fluxes (including negative fluxes at night), which could not be attributed to conventional biological processes, have been observed with both chambers and open- or close-path eddy systems10,11,12. Possible explanations have been proposed to rationalize these ‘anomalous fluxes’. Growth of cryptobiotic crust organisms (lichens, mosses and cyanobacteria) were proposed to account for a significant portion of the C uptake in the Mojave Desert11. Night time uptake of CO2 by CAM plants is another biological explanation for low or negative night time flux rates13. Abiotic processes such as leaching14,15, photo-degradation16,17,18 and CO2 dissolution19,20, can also contribute to CO2 flux, which had been largely neglected in the global C cycle11. Globally, soils contain huge amounts of inorganic C (750–950 Pg C)19,21 that could potentially be an active participant in short-term CO2 fluxes, particularly in arid areas with large amounts of soil carbonate19,22. Large daily CO2 emissions in association with rainfall events during the dry season were proposed to result from equilibrium reactions occurring in carbonate soils19,22. More importantly, carbonate or soil CO2 dissolution could cause CO2 flow into soil (i.e. negative flux) on short time scales (hourly). Such negative fluxes have been reported under certain conditions, such as in a dry valley23 and hot desert soils12,13 and might temporally dominate the soil CO2 flux12. For example, large CO2 uptake was observed either in situ or after sterilization in the Gubantonggut Desert, western China and was attributed to absorption by saline/alkaline soil12. This provoked a debate on deserts as the location of the long-sought ‘missing sink’ for C10,24. Namely, there is strong evidence suggesting that inorganic processes can contribute significantly to the total soil CO2 flux20. However, the mechanisms and magnitude of such fluxes are still a matter of controversy10,24.
Using data from a field, laboratory and modeling study, we propose that the flux measured after sterilization of a saline/alkaline soil was predominately the effusion-dissolution of CO2 entering the soil solution during the night or leaving the soil solution during the day (hereafter: ‘inorganic flux’). We show that this inorganic process can contribute significantly to the total soil CO2 flux for saline/alkaline soils and dominates the soil CO2 flux when soils are dry, with carbonate in the soil not significantly involved. After experimentally partitioning the total soil CO2 flux into organic (conventional soil CO2 flux) and inorganic fluxes, a process-based soil CO2 equilibrium-transport model was built to quantify this inorganic flux. This model could easily be incorporated into C flux models for soils of differing degrees of salinity/alkalinity. Neglecting this inorganic flux could induce erroneous or misleading interpretations of the C fluxes of arid and semi-arid ecosystems that make up nearly half the land surface24,25.
Results
Field measurement of under-canopy and inter-plant CO2 fluxes
Soil CO2 fluxes under the canopy were significantly higher than those in inter-plant spaces (Fig. 1), with rates ranging from −0.28 to 1.76 and −0.42 to 1.24 μmol m−2s−1 for under the canopy and inter-plant spaces, respectively. Both under the canopy and in inter-plant spaces, the soil CO2 fluxes exhibited similar diurnal pattern over the growing season. Most noteworthy was that the soil CO2 fluxes were always negative during night in inter-plant spaces over the whole growing season, which was unexpected according to the conventional definition of soil respiration. Any biological uptake of CO2 (such as photosynthesis of CAM plants or cryptobiotic crusts) can be ruled out as we were measuring on bare soil.
Laboratory verification of the diurnal flux
To rule out the possibility of instrumental error and be sure the negative flux was real, we checked the instrument and measuring procedure carefully by using inert quartz sand as the soil matrix to verify the flux. When pure dry quartz sand was used as a replacement for soil, the fluxes were always close to zero (Fig. 2); when distilled water was added into quartz sand, there were small fluxes in a range of ± 0.1 μmol m−2s−1. In contrast, flux rates of approximately ± 1 μmol m−2s−1 were observed when sterilized soil solution (pH = 8.97, EC = 6 dS m−1) was added to the quartz sand. Hence, the lack of detectable variations of flux for the quartz sand alone indicated that the instrument was reliable. The strong diurnal pattern of the flux that related to saline soil solution was not an artifact – a process other than oxidation of organic carbon (either biotic or abiotic) must have occurred in soil, which should be the cause of the negative flux (Fig. 1).
Partitioning the total soil CO2 flux into organic and inorganic components
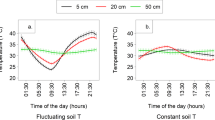
To partition the total soil CO2 flux into inorganic (Rinorganic) and organic (Rorganic) fluxes, we measured the fluxes of sterilized and unsterilized soils with 10% water content synchronously. The sterilized and unsterilized soils were considered inorganic and total fluxes respectively, with the difference between the two representing the organic CO2 flux, which exhibited a single peak and was in the range of 0.08–0.93 μmol m−2s−1 (Fig. 3). We use the term “inorganic” flux to differentiate between abiotic flux (which could include abiotic C oxidation processes) and the effusion/dissolution process for reasons that will become apparent during the results and discussion sections.
The contribution of inorganic CO2 flux to total soil CO2 flux in soils varying in pH and EC at soil moisture content of 10% and the overall contribution of inorganic flux at different pHs [pHs for (a)–(e) were 7.19, 7.80, 8.30, 8.55 and 8.85, respectively].
The shaded parts indicate the period in which the inorganic CO2 fluxes are positive and the instantaneous ratio of inorganic CO2 flux to total CO2 flux were calculated (f). Error bars represent standard error of the mean.
Total soil CO2 flux was positive in the daytime and peaked during 12:00–16:00. More important, the total soil CO2 flux could be negative under the influence of the inorganic flux (Fig. 3d and e), which had parallel diurnal patterns of a single peak at 12:00–14:00 similar to organic flux and varied in a diurnal sinusoidal waveform. Diurnal variations of Rinorgnaic, Rorganic and Rtotal were all highly associated with variations in soil temperature (r2 values were within 0.49–0.87 for all measurements, n = 48, P < 0.01). In addition, the amplitude of the inorganic flux increased with increased pH, which climbed from 0.14 at pH 7.19 to 0.65 at pH 8.55. To evaluate the contribution of the inorganic flux to total soil CO2 flux, mean instantaneous (e.g. hourly) ratios were calculated for the five different soils when the inorganic flux was positive, which ranged within 0.14–0.39 in a positive relationship with pH (r2 = 0.89, P < 0.01) (Fig. 3f). By contrast, there was no significant difference between the total soil CO2 flux and the inorganic flux for the air-dried soils (Fig. 4) – when the soil was sufficiently dry, total soil CO2 flux all came from the inorganic flux. Regardless of being dry or wet, the inorganic flux was driven by soil temperature on a daily scale and its magnitude was mainly determined by soil pH (Figs. 3 and 4).
Model validation
In general, the inorganic CO2 flux was negative (i.e. into the soil) at night and positive (or outward from soil to atmosphere) during the day, with greater diurnal amplitude for soils with high EC and pH (Fig. 5). The amplitude of the inorganic flux varied from about 0.10 μmol m−2s−1 for EC = 5 dS m−1 and pH 7.80, to as high as 0.51 μmol m−2s−1 for EC = 126 dS m−1 and pH 8.30. There was good agreement between measured and simulated inorganic fluxes both in the magnitude and diurnal pattern (Fig. 5). Linear regressions between simulated and measured inorganic fluxes yielded regression coefficients close to one (Supplementary Fig. S1).
The inorganic CO2 flux was positive during the day and negative at night with a zero sum over 24 h (Figs. 3–5). To quantify the inward and outward amounts of CO2, exchange rates were modeled for a range of pH and EC with 10% water content (Fig. 6). The amount of CO2 exchange was in the range of 35.0–1749.4 mg CO2 m−2d−1 for the given range of pH and EC (Fig. 6).
Sensitivity analysis of the model
Table 1 presents the sensitivity of the magnitude of inorganic CO2 exchange to an increase of 5% in the model parameters over a day. Each change in an input parameter of the model was simulated under real environmental conditions. The parameters were listed in order of importance of their impact on the inorganic processes (Table 1) – pH was the dominant parameter for inorganic processes, with a more significant impact than others parameters: a 5% increase in pH produced an increase of 138% inorganic CO2 exchange. This is easily explained by an exponential increase in solubility of CO2 in soil solution with a pH increase26.
Discussion
The integration of these results provides new insight into the ‘anomalous’ negative fluxes observed in saline/alkaline soils. Thus, when slow changes in soil organic or inorganic C storage, pools of C in biological soil crusts or CAM plants cannot explain the observed large net uptake10,13,24, the observations are not necessarily wrong10,24,27,28. An inorganic process − the effusion and dissolution of CO2 into and out of the soil solution (Figs. 3–5) − can make the nighttime soil CO2 flux negative and accounts for the ‘anomalous’ flux.
The inorganic flux, driven by diurnal temperature variation, was derived from the change in the size of the reservoir of dissolved inorganic carbon (DIC) in the soil solution as determined by Henry's Law29,30,31. The size of this reservoir increases during the day as temperature increases and decreases at night as temperature decreases (Figs. 3–5).
For the positive CO2 fluxes, it may be argued that these fluxes could derive from other abiotic oxidation processes, such as thermal degradation and photo-degradation16,17,18. While we can not rule out a contribution from abiotic oxidation processes to the positive CO2 flux occurring during the day, the organic matter content in saline/alkaline soils of the arid zone is very low6,7,32. More important, these other abiotic processes can not explain the negative CO2 fluxes occurring at night and the modeling suggests that CO2 effusion/dissolution adequately explains the magnitude of both the positive and negative fluxes observed in the field and laboratory experiments (Figs. 3–5). In addition, the zero sum total flux (Figs. 3–5) would also suggest that other biotic and abiotic processes are not a significant source of positive C flux in these soils. Therefore, within the context of the current study, we assume the measured CO2 fluxes from the sterilized soil are predominantly derived from the inorganic effusion/dissolution of CO2 into and out of the soil. In acid soils and/or soils rich in organic matter, the effusion/dissolution process would likely be insignificant in comparison with biotic (or other abiotic sources of) soil C flux, but the nature of the fluxes can still be easily distinguished by the diurnal pattern.
Although the diurnal inorganic effusion/dissolution process was zero sum (Figs. 3–5), the DIC itself could still be a significant C reservoir in saline/alkaline soils29, mainly determined by pH and soil moisture (Fig. 6, Table. 1). According to the simulation, the amount of DIC contained in the 100 cm × 100 cm × 40 cm soil pillars in the present experiment was in the range of 0.178–0.183 mol under normal saline conditions (for parameters listed in Table 1), which is similar to the C contained in up to 11 m3 of air with CO2 concentration of 380 ppm. Assuming a soil moisture content of similar value, DIC in the soil could be considerably greater in the field, as soil CO2 partial pressures (pCO2) are 10–100 times higher than those of the atmosphere33. In the absence of carbonate, dissolution from soil pCO2 would account for all C present in DIC34. Thus, this inorganic flux can occur with or without carbonate in the soil, provided the soils are alkaline. Dissolution or precipitation of carbonates implies CO2 consumption or emission20,35, which affects the concentration of DIC and total alkalinity (AT)31, but the direct contribution to DIC has been shown to be relatively small31. Karberg et al.31 used an isotopic mixing model to partition the source of DIC and revealed that approximately 90% of DIC-C was from soil pCO2 rather than carbonate salts. Other salts (whether carbonate or non-carbonate) play a similar role in changing the ionic strength and AT of the soil solution and therefore also affect the DIC content in the soil solution20.
We suggest there could also be a seasonal inorganic flux variation from winter to summer, as soil temperature varies as it does on a daily scale (Figs. 3–5). Rainfall or irrigation processes that change the soil moisture would also certainly alter this inorganic CO2 flux, as it not only changes the porosity occupied by soil air or soil solution, but also changes the EC or AT of the soil solution. Although in our experiment inorganic flux on a diurnal basis resulted in no net gain or loss of C, DIC could be lost from the soil by leaching, runoff or fluctuations in groundwater. Losses of DIC through such process would be an additional source of C in Net Ecosystem Exchange (NEE)1,14,15.
We refrain from going into further detail concerning the effect of soil moisture because of its complex and multiple impacts on this inorganic process. However, our data indicate that the drier the saline/alkaline soils are, the more predominant the inorganic process will be in determining the soil CO2 flux (Figs. 3 and 4). If this inorganic C flux is significant for the approximately one-fourth of the global land that comprises arid saline/alkaline soils, why has such a significant flux been neglected for so long? In most ecosystems, the soil CO2 flux is positive during both day and night and the negative flux resulting from this inorganic process is hidden by a measurable positive CO2 flux at night. Under certain extreme conditions, such as saline deserts12 or Antarctic soils36, where flux rates are inherently low due to low biotic activity, the inorganic flux is more pronounced and is then comparable to and even exceeds the magnitude of the organic flux, resulting in a negative CO2 flux at night (Fig. 1). The relative contribution of inorganic flux to total CO2 flux (Rinorganic/Rtotal) is the key to discerning where this inorganic process will be important. When the transient rate of the inorganic flux is lower than the organic flux, as in most ecosystems, the measured soil CO2 fluxes are still positive but could potentially be underestimated at night.
The underestimation of night time flux rates could have further implications for calculations of ecosystem respiration because it is the regression between respiration and temperature at night that is used to extrapolated ecosystem respiration during the daytime6,37,38. In ecosystems with saline/alkaline soils, underestimation of night time flux would significantly underestimate the daytime C efflux and thus result in an overestimation of the net primary productivity.
In summary, the recognition of an inorganic component in the soil C flux has widespread consequences for the study of the global C cycle. We suggest an inorganic module, such as the model presented in the current study, should be added for the saline/alkaline half of the global land. Otherwise, C cycling analysis could be inaccurate in both mechanism and quantification.
Methods
Site description
Field soil CO2 flux measurements were conducted at the Fukang Station of Desert Ecology (44°17′N, 87°56′E and 475 m a.s.l.), Chinese Academy of Sciences. The climate of the region is arid temperate with annual mean temperature of 5–7°C and mean annual precipitation of 160 mm. The main soil types were classified as Solonchaks in the FAO/UNESCO soil classification system12. The soil is silty clay-loam with high salinity/alkalinity [electrical conductivity (EC) > 4 dS m−1, pH > 8.2 for soil solution of soil/water ratio of 1 : 5], with the soil solution mainly consisting of sulfate and chloride salts. Total nitrogen and organic C contents of the soil are 0.068 and 1.064%, respectively7. The plant community is dominated by Tamarix spp., a deep-rooted halophyte shrub39, with canopy coverage of approximately 17% of the land surface.
In situ measurement of soil CO2 flux
Soil CO2 flux was measured during the growing season (from May 5 to Oct 10) in 2009, with an LI−8150 Automated Soil CO2 Flux System, equipped with six long-term monitoring chambers. Soil collars were arranged along a projecting line from the center of a shrub with average crown diameter to interplant space; distances to the center were 0.5, 1, 2, 3, 4 and 5 m, respectively. More details about field measurement can be found in Ma et al.7 In this study, we only compared soil CO2 flux under the canopy with that in inter-plant spaces (distance to center is 5 m) to capture spatial variation derived from the effect of the plant canopy40.
Control experiment
To determine the potential contribution of inorganic flux to total soil CO2 flux, we compared the fluxes between sterilized and unsterilized soils. An autoclaving method was found to be most suitable35 and was used in this study. In total, 14 types of soil samples were collected from different places to represent pH and EC gradients in nature. Soil samples were ground, air-dried and sieved (16-mesh) to remove gravel and roots. The samples were then well mixed to make them homogeneous. Soil pH and soil EC were determined by a Sartorius PP–20 Professional Meter (Sartorius, Germany) with soil solution centrifuged from water-saturated soil.
Controlled experiments were carried out on air-dried soils (with residue moisture at approximately 2.5%) and soils with moisture content of 10% (v/v). These levels of soil moistures were chosen because soils in arid zone are generally dry due to high evaporation and low precipitation. For the 10% treatment, distilled water was first added to both unsterilized and sterilized soils to attain a 10% moisture level. Then soils were put into six metal drums (three for sterilization and another three for natural conditions), whose bottom were sealed. The heights of the drums were 40 cm, because the diurnal variation of soil temperature below 40 cm was < 1.5°C in the field and was assumed to be negligible in the current study. For sterilized soils, the tops of the metal drums were sealed by layers of filter and brown paper to minimize water infiltrating into the soil and were sterilized in a medical autoclave for 24 h at 120°C. After sterilization, soil drums were placed in a UV-sterilized room to allow soil cores to equilibrate with the surrounding conditions. To increase the comparability of the results between sterilized and unsterilized soil, the drums filled with control (i.e. unsterilized) soils were also covered with filter papers at the top and balanced with their surroundings synchronously. The metal drums were then reburied in the field to maintain natural fluctuations of soil temperature. The soil surface in the drums was as at an equivalent height to the surrounding soil. CO2 flux was measured every 30 min for 2 d for each set of soil samples. For the air-dried soil, the soil was put into drums directly and sterilization and measurements processes were same as the soils with 10% moisture.
To confirm the accuracy of the LI–COR 8150 measurements, we compared the surface CO2 flux from artificial matrices, with three different treatments: quartz sand, quartz sand with distilled water and quartz sand with sterilized soil solution. The purpose of using analytically pure quartz sand was to check whether the instruments would measure zero flux when it was expected to be zero. By contrast, the difference between treatments of quartz with soil solution and quartz sand with distilled water was to determine whether “anomalous fluxes” occurred under these controlled and sterilized conditions. Before measurements, distilled water and sterile soil solution were added to corresponding drums to attain a 10% moisture level and reburied in the field after equilibrium. Measurement processes were the same as that of soil CO2 flux.
Soil temperature was measured at 5, 10, 15 and 20 cm below the soil surface in a soil profile close to the chambers, using a thermocouple connected to LI–8150 long-term chambers. The soil moisture content was determined by conventional oven-drying method after each set of measurement.
Model description
CO2 flux in or out of the soil surface is considered to be the combined result of two major processes: the production or absorption of CO2 and gas transport process41,42. Based on the one-dimensional (vertical) soil CO2 transport model described by Nickerson & Risk43 and Phillips et al.5, we add a new function of CO2 concentration change adjusted by effusion or dissolution equilibrium between CO2 and soil solution26,29,30,44, which was regulated by temperature. The vertical CO2 flux can be expressed by the following mass balance equation41,42:

Fdiff and Fadv describe the CO2 fluxes caused by diffusion in the gas phase and by advection of soil air, respectively; PRs represents the total CO2 production by the respiration source and in our study PRs = 0 for we assume all the biological processes or microorganism's activities were excluded by the pretreatments of root removal and sterilization; t denotes time (s) and z the depth below the soil surface.
The modeled environment assumes a well-mixed atmospheric boundary layer (Supplementary Fig. S2). To simplify the model, the total soil column was considered homogenous and total soil porosity (Φ) was considered to be constant with depth and any air/water-filled pore space during the simulation keep constant over the length of the entire soil column. As changes in soil water volume are always matched by changes in gas volume in the opposite direction.
Submodel of the effusion-dissolution equilibrium of CO2 with soil solution
In the absence of biotic processes, the chemical equilibria in the soil-water-air continuum can be described by the chemical reactions26,29,30,44.




where pCO2 is the partial pressure of CO2 in the soil air (atm); KH is Henry's Law constant; and K0, K1 and K2 are equilibrium constants of dissolution and first and second order dissociation reaction constants for carbonic acid, respectively (temperature dependence of the constants for saline and alkaline soils are listed in Supplementary Table S1). So, the total amount of DIC was calculated as the sum of all carbonaceous species resulting from CO2 dissolution31, which can be obtained from equations (2)– (5).
Submodel of CO2 transport component
Under the assumption of horizontal homogeneity, where the horizontal loss or gain of CO2 and the dispersion of CO2 in the gas phase caused by vertical gas movement are negligible, one-dimensional CO2 transport in each layer of soils exchanges gases along soil CO2 concentration gradients with its nearest two neighboring soil layers, governed by Fick's First Law5,41,42:

where  is the difference of CO2 concentration between two neighboring layers; Dga and Dgs are the effective diffusion coefficient of CO2 in the atmosphere and soil respectively; and ξg is the tortuosity factor of gas diffusion through the soil as a function of air-filled porosity45,46,47,48.
is the difference of CO2 concentration between two neighboring layers; Dga and Dgs are the effective diffusion coefficient of CO2 in the atmosphere and soil respectively; and ξg is the tortuosity factor of gas diffusion through the soil as a function of air-filled porosity45,46,47,48.
Up to now there has been no effective method to quantify the tortuosity factor of CO2 diffusion in the liquid phase. However, the diffusion of CO2 in the liquid phase is about 10000 times lower than that in the gas phase, thus the contribution of the liquid phase to the total effective diffusion is far less than that of gas phase diffusion, except when soil is close to saturation41. So ignoring liquid phase diffusion will not cause a serious error in the modeling and the new CO2 concentration in each layer at each model time-step (e.g. 1 s) was calculated as43,49:

where Fdiff (z + 1, t + 1) is the CO2 flux from the layer (z) below, Fdiff (z, t + 1) is the CO2 flux leaving the layer z; L is the total depth of the soil column and N is the total number of soil layers. CO2change(z, t + 1) is the change of CO2 concentration at layer z at time (t + 1), which can be expressed by:

For the numerical solution of equation (1), the soil was divided into 40 horizontal layers with thickness of 1 cm. Soils in metal drums were assumed to exhibit no respiratory CO2 production after sterilization treatment. Soil porosity, pH and CO2 concentration for each soil were set constant with time and soil depth. The model was programmed and run in Matlab 7.7. To minimize model run time, the model was initialized for a steady state and the initial CO2 concentration in the atmospheric layer and at each soil depth was typically assigned to a constant CO2 concentration, 380 ppm. The time steps used in the iterative solving of the model equations was 1 s and model output to file was 1 min.
Simulated inorganic fluxes from the soil surface were validated by the half-hourly flux data measured after soil sterilization treatment with soil moisture content of 10%.
References
Chapin, F. S. et al. Reconciling carbon−cycle concepts, terminology and methods. Ecosystems 9, 1041–1050 (2006).
Valentini, R. et al. Respiration as the main determinant of carbon balance in European forests. Nature 404, 861–865 (2000).
Baggs, E. M. Partitioning the components of soil respiration: a research challenge. Plant Soil 284, 1–5 (2006).
Baldocchi, D. D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: past, present and future. Global Change Biol 9, 479–492 (2003).
Phillips, C. L., Nickerson, N., Risk, D. & Bond, B. J. Interpreting diel hysteresis between soil respiration and temperature. Global Change Biol 17, 515–527 (2011).
Cable, J. M. et al. The temperature responses of soil respiration in deserts: a seven desert synthesis. Biogeochemistry 103, 71–90 (2011).
Ma, J., Zheng, X. J. & Li, Y. The response of CO2 flux to rain pulseds at a saline desert. Hydrological Process 26, 4029–4037 (2012).
Yuste, J. C., Janssens, I. A., Carrara, A., Meiresonne, L. & Ceulemans, R. Interactive effects of temperature and precipitation on soil respiration in a temperate maritime pine forest. Tree Physiol 23, 1263–1270 (2003).
Raich, J. W. & Schlesinger, W. H. The global carbon−dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B 44, 81–99 (1992).
Stone, R. Ecosystems − Have desert researchers discovered a hidden loop in the carbon cycle? Science 320, 1409–1410 (2008).
Wohlfahrt, G., Fenstermaker, L. F. & Arnone, J. A. Large annual net ecosystem CO2 uptake of a Mojave Desert ecosystem. Global Change Biol 14, 1475–1487 (2008).
Xie, J. X., Li, Y., Zhai, C. X., Li, C. H. & Lan, Z. D. CO2 absorption by alkaline soils and its implication to the global carbon cycle. Environ Geol 56, 953–961 (2009).
Hastings, S. J., Oechel, W. C. & Muhlia-Melo, A. Diurnal, seasonal and annual variation in the net ecosystem CO2 exchange of a desert shrub community (Sarcocaulescent) in Baja California, Mexico. Global Change Biol 11, 927–939 (2005).
Kindler, R. et al. Dissolved carbon leaching from soil is a crucial component of the net ecosystem carbon balance. Glob Change Biol 17, 1167–1185 (2011).
Battin, T. J. et al. The boundless carbon cycle. Nat Geosci 2, 598–600 (2009).
Brandt, L. A., Bohnet, C. & King, J. Y. Photochemically induced carbon dioxide production as a mechanism for carbon loss from plant litter in arid ecosystems. J Geophys Res 114, G02004 (2009).
Rutledge, S., Campbell, D. I., Baldocchi, D. & Schipper, L. A. Photodegradation leads to increased carbon dioxide losses from terrestrial organic matter. Global Change Biol 16, 3065–3074 (2010).
Austin, A. T. & Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442, 555–558 (2006).
Emmerich, W. E. Carbon dioxide fluxes in a semiarid environment with high carbonate soils. Agr Forest Meteorol 116, 91–102 (2003).
Serrano-Ortiz, P. et al. Hidden, abiotic CO2 flows and gaseous reservoirs in the terrestrial carbon cycle: Review and perspectives. Agr Forest Meteorol 150, 321–329 (2010).
Schlesinger, W. H. The Formation of caliche in soils of the Mojave-Desert, California. Geochim Cosmochim Ac 49, 57–66 (1985).
Mielnick, P., Dugas, W. A., Mitchell, K. & Havstad, K. Long−term measurements of CO2 flux and evapotranspiration in a Chihuahuan desert grassland. J Arid Environ 60, 423–436 (2005).
Ball, B. A., Virginia, R. A., Barrett, J. E., Parsons, A. N. & Wall, D. H. Interactions between physical and biotic factors influence CO2 flux in Antarctic dry valley soils. Soil Biol Biochem 41, 1510–1517 (2009).
Schlesinger, W. H., Belnap, J. & Marion, G. On carbon sequestration in desert ecosystems. Global Change Biol 15, 1488–1490 (2009).
Ghassemi, R., Jakeman, A. J. & Nix, H. A. Salinisation of Land and Water Resources: Human Causes, Extert, Management and Case Studies. (University of New South Wales Press, Sydney; 1995).
Lindsay, W. L. Chemical Equilibria in Soils. (John Wiley and Sons Ltd, New York; 1979).
Jasoni, R. L., Smith, S. D. & Arnone, J. A. Net ecosystem CO2 exchange in Mojave Desert shrublands during the eighth year of exposure to elevated CO2 . Global Change Biol 11, 749–756 (2005).
Luo, H. Y. et al. Mature semiarid chaparral ecosystems can be a significant sink for atmospheric carbon dioxide. Global Change Biol 13, 386–396 (2007).
Gamnitzer, U., Moyes, A. B., Bowling, D. R. & Schnyder, H. Measuring and modelling the isotopic composition of soil respiration: insights from a grassland tracer experiment. Biogeosciences 8, 1333–1350 (2011).
Plummer, L. N. & Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO2−H2O solutions between 0°C and 90°C and an evaluation of the aqueous model for the system CaCO3−CO2−H2O. Geochim Cosmochim Ac 46, 1011–1040 (1982).
Karberg, N. J., Pregitzer, K. S., King, J. S., Friend, A. L. & Wood, J. R. Soil carbon dioxide partial pressure and dissolved inorganic carbonate chemistry under elevated carbon dioxide and ozone. Oecologia 142, 296–306 (2005).
Jackson, R. B. et al. A global analysis of root distributions for terrestrial biomes. Oecologia 108, 389–411 (1996).
Amundson, R. G. & Davidson, E. A. Carbon−dioxide and nitrogenous gases in the soil atmosphere. J Geochem Explor 38, 13–41 (1990).
Kendall, C. & McDonell, J. J. Isotope tracers in catchment Hydrology. (Elsevier Science Publishers, New York; 1998).
Stevenson, B. A. & Verburg, P. S. J. Effluxed CO2−13C from sterilized and unsterilized treatments of a calcareous soil. Soil Biol Biochem 38, 1727–1733 (2006).
Shanhun, F. L., Almond, P. C., Clough, T. J. & Smith, C. M. S. Abiotic processes dominate CO2 fluxes in Antarctic soils. Soil Biol Biochem 53, 99–111 (2012).
Lloyd, J. & Taylor, J. A. On the Temperature−dependence of soil respiration. Funct Ecol 8, 315–323 (1994).
Fang, C. & Moncrieff, J. B. The dependence of soil CO2 efflux on temperature. Soil Biol Biochem 33, 155–165 (2001).
Xu, H., Li, Y., Xu, G. Q. & Zou, T. Ecophysiological response and morphological adjustment of two Central Asian desert shrubs towards variation in summer precipitation. Plant Cell Environ 30, 399–409 (2007).
Li, J., Zhao, C., Zhu, H., Li, Y. & Wang, F. Effect of plant species on shrub fertile island at an oasis−desert ecotone in the South Junggar Basin, China. J Arid Environ 71, 350–361 (2007).
Simunek, J. & Suarez, D. L. Modeling of carbon-dioxide transport and production in soil .1. Model Development. Water Resour Res 29, 487–497 (1993).
Fang, C. & Moncrieff, J. B. A model for soil CO2 production and transport 1: Model development. Agr Forest Meteorol 95, 225–236 (1999).
Nickerson, N. & Risk, D. Physical controls on the isotopic composition of soil−respired CO2 . J Geophys Res-Biogeo 114, G01013 (2009).
Schulz, K. G., Riebesell, U., Rost, B., Thoms, S. & Zeebe, R. E. Determination of the rate constants for the carbon dioxide to bicarbonate inter−conversion in pH-buffered seawater systems. Mar Chem 100, 53–65 (2006).
Currie, J. A. Gaseous diffusion in porous media. Part 3 − Wet granular materials. Br. J. Appl. Phys. 12, 275–281 (1961).
Moldrup, P., Olesen, T., Schjonning, P., Yamaguchi, T. & Rolston, D. E. Predicting the gas diffusion coefficient in undisturbed soil from soil water characteristics. Soil Sci Soc Am J 64, 94–100 (2000).
Penman, H. L. Gas and vapour movements in the soil I. The diffusion of vapours through porous solids. J Agr Sci 30, 437–462 (1940).
Penman, H. L. Gas and vapour movements in the soil II. The diffusion of carbon dioxide through porous solids. J Agr Sci 30, 570–581 (1940).
Pumpanen, J., Ilvesniemi, H. & Hari, P. A process−based model for predicting soil carbon dioxide efflux and concentration. Soil Sci Soc Am J 67, 402–413 (2003).
Acknowledgements
The authors thank all staff at the Fukang Station of Desert Ecology for technical support and theoretical discussions. This research was funded by the National Basic Research Program of China (Grant no. 2009CB825102) and “Strategic Priority Research Program" of China (No.XDA05030500).
Author information
Authors and Affiliations
Contributions
All authors commented on manuscript at all stages. Y.L. developed the concept of the paper and oversaw the study; J.M., W.Z.Y. and Z.X.J. did the in situ measurements of soil CO2 flux and the sterilization experiment; J.M. and Y.L. analyzed all the data and wrote the paper; B.A.S worked on every version of the paper and had significant contribution in structuring and presenting the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Ma, J., Wang, ZY., Stevenson, B. et al. An inorganic CO2 diffusion and dissolution process explains negative CO2 fluxes in saline/alkaline soils. Sci Rep 3, 2025 (2013). https://doi.org/10.1038/srep02025
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep02025
This article is cited by
-
Effect of phosphogypsum application on aluminum speciation in acid pasture soils
Journal of Soils and Sediments (2022)
-
Precipitation Pattern Regulates Soil Carbon Flux Responses to Nitrogen Addition in a Temperate Forest
Ecosystems (2021)
-
Patterns and drivers of multi-annual CO2 emissions within a temperate suburban neighborhood
Biogeochemistry (2021)
-
Biotic and Abiotic Contribution to Diurnal Soil CO2 Fluxes from Saline/Alkaline Soils
Scientific Reports (2020)
-
Global CO2 emissions from dry inland waters share common drivers across ecosystems
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.