Abstract
New hydrophilic 2D graphene oxide (GO) nanosheets with various oxygen functional groups were employed to maintain high sensitivity in highly unfavorable environments (extremely high humidity, strong acidic or basic). Novel one-headed polymer optical fiber sensor arrays using hydrophilic GO and hydrophobic reduced graphene oxide (rGO) were carefully designed, leading to the selective sensing of volatile organic gases for the first time. The two physically different surfaces of GO and rGO could provide the sensing ability to distinguish between tetrahydrofuran (THF) and dichloromethane (MC), respectively, which is the most challenging issue in the area of gas sensors. The eco-friendly physical properties of GO allowed for faster sensing and higher sensitivity when compared to previous results for rGO even under extreme environments of over 90% humidity, making it the best choice for an environmentally friendly gas sensor.
Similar content being viewed by others
Introduction
Gas sensors based on micro and nano technology have allowed for the detection of an important set of gases in several applications. For optimal gas sensor performance, several issues such as sensitivity, selectivity, stability and time of response should be simultaneously addressed. One of the most challenging issues for the realization of an effective gas sensor is to achieve selectivity with high sensitivity and maintain high sensitivity under high humidity conditions. Selectivity allows a gas sensor to detect the presence of particular gases in media, including other gases and can be very hard to achieve under normal atmospheric conditions. There are two general approaches to enhance the selective properties in sensors. The first method is aimed at preparing a material that is specifically sensitive to one compound and has almost zero cross-sensitivity to other compounds that may be present in the working atmosphere. Specific sensitivity to one compound is regularly achieved either by modulation of the sensor temperature1,2 or through the use of sensor arrays3,4 due to difficulties in distinguishing the specific sensitivity to one compound when only one sensor signal is employed. The second approach is based on the preparation of materials that can discriminate among several analytes in a mixture5. Such discrimination is possible because of the different adsorption and reactivity properties of the analytes to the materials. Many studies have been focused on the development of optical sensor systems6,7; these types of devices offer interesting advantages compared to electronic ones such as their light weight, remote measuring capability and electromagnetic immunity. Therefore, no electric signal is necessary, which can eliminate any kinds of risks from explosion in the detection of specific volatile organic compounds (VOCs).
For highly sensitive gas detection, 1-dimensional (1D) semiconducting metal oxide nano wires have been extensively investigated because of their high sensitivities toward different gaseous species8,9. In recent years, 2-dimensional (2D) graphene nano sheets consisting of a monolayer of hexagonally arrayed sp2-bonded carbon atoms10 have been demonstrated as a promising sensing material11,12 because of their large surface area and excellent mechanical13, thermal14 and electrical10,15 properties. Several recent studies have reported that the incorporation of nanocrystals/nanoparticles in graphene-based gas sensors could improve sensor performance in terms of the sensitivity/detection limit, response time, or recovery time16,17,18. In addition, several other groups have demonstrated that reduced graphene oxide (rGO) can act as a gas/vapour sensor with promising results11,12,19,20,21,22. However, at present there have been no reports demonstrating selectivity using graphene and rGO in gas sensors. Furthermore, no studies have been published regarding the use of graphene oxide (GO) as a gas sensor for VOCs. It is highly expected that the use of both hydrophobic rGO and hydrophilic GO in a gas sensor array can provide high selectivity toward VOC gases. In addition, the utilization of hydrophilic 2D GO nanosheets with various oxygen functional groups is expected to provide the best solution to the most challenging issue in gas sensors: maintaining high sensitivity under extremely humidity conditions and in highly acidic or basic environments.
Herein, we report on a polymer optical fiber (POF) sensor array using GO and rGO to selectively sense VOC gases. To demonstrate the selective sensing properties of GO and rGO, we designed POF sensors with one-headed GO and rGO tip patterned with sunlight. In addition, two-headed tip POF sensor array systems were fabricated in which, as control samples, one optical fiber tip was coated with GO and another with rGO (called a GO/rGO array), or both fiber tips were coated with either GO or rGO (GO/GO and rGO/rGO arrays, respectively). To the best of our knowledge, the changes in the optical properties of GO and rGO upon their interaction with VOCs have not previously been reported. In addition, we carefully introduce a GO sensor that has various oxygen functional groups and a high surface area, providing much better gas/vapour sensing capabilities than that of previously reported rGO due to the strong adsorption of the VOC on the high surface area of the sensor23,24,25. For a highly sensitive eco-friendly VOC sensor, we also evaluated the sensing ability of GO when compared to rGO under extremely humid conditions and in highly acidic or basic environments for very low concentrations (500 ppb) of eight different VOCs (hydrazine, ethanol, methanol, dichloromethane (MC), acetone, tetrahydrofuran (THF), nitromethane and diethylamine). This is the first report on the attainment of high selectivity with a simple GO-rGO sensor array system and high sensitivity in extreme environments, including 90% humidity and strongly acidic or basic conditions.
Results
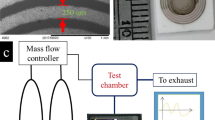
A schematic illustration of the experimental setup used to evaluate the sensor performance for VOCs is shown in Fig. 1a, while schematic representations of a POF reflectance probe with GO, rGO and GO-rGO as the selectively sensing layers are shown in Fig. 1b–d. For the POF sensors with one-headed GO and rGO tips, the rGO pattern on the GO was formed simply by exposing the GO layer to sunlight (Fig. 1e)26. For the preparation of the two-headed tip GO/GO, GO/rGO and rGO/rGO POF sensor array systems, also previously reported solar radiation was applied to prepare rGO layers26. This method was employed to prepare a uniform graphene layer on the POF end face for the sensing studies. X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) and thermogravimetric analysis (TGA) were employed for characterization of the GO and rGO. Based on the XPS analyses, the as-prepared GO had a very high oxygen atomic percentage (C/O = 2.2). In contrast, the C/O ratio of rGO was 11.6. Based on the XPS data, we concluded that the rGO fabricated from this process contained far less oxygen, which confirmed the high quality of the rGO (Fig. 2a, b). The XRD spectra of the synthesized GO and rGO are shown in Fig. 2c. TGA was used to assess the quality of the rGO when compared to GO. TGA plots of GO (black) and rGO (red) are shown in Fig. 2d. In the case of GO, the majority of the weight was lost between 100 and 200°C, indicating that CO and CO2 were released from the most labile functional groups during pyrolysis. At temperatures below 700°C, the total weight loss of GO was about 72%, while that of rGO was 18.6%.
Schematic illustration of the experimental setup used to evaluate the performance of the GO and rGO polymer optical fiber sensors.
(a) Schematic representation for fabrication of one-headed POF sensor with (b) GO, (c) rGO and (d) GO-rGO. (e) Schematic representation for fabrication processes of GO-rGO POF sensor by converting GO into rGO with sunlight.
Characterization and sensing ability of GO and rGO.
High-resolution C 1s XPS spectra for (a), GO and (b), rGO. (c), XRD patterns of GO (black) and rGO (red). (d), TGA plots of GO (black) and rGO (red). Plots of the adsorption and desorption responses of (e), only GO and (f), only rGO POF sensors; the concentration of acetone vapour was varied from 500 ppb to 500 ppm.
The principle of operation of sensor transduction relies on the dependence of the reflectance on the optical and geometric properties of the sensing layers when vapour molecules are adsorbed on the GO and rGO layers, as depicted in Fig. 1. This means that any change in the features of the GO and rGO layers due to chemical or physical adsorption of a target analyte would induce a consequent change in the layer's reflectance. According to our previous report, a variation in the refractive index of the GO and rGO layers as well as the external media (all VOCs) will lead to changes in the reflectance at the fiber-coated interfacial layer and thus, induce changes in the sensor output signal27. As we know that GO is hydrophilic and rGO is hydrophobic, these surface characteristic difference will affect the sensing capability of GO and rGO. The oxygen containing functional groups of GO are playing the key roles for GO's gas sensing activity. The interaction of the oxygen containing groups of GO with VOCs will be different in comparison to that of rGO. The oxygen containing groups can strongly interact with those polar VOCs that are NO2, NH2 and other oxygen containing functional groups due to the formation of intermolecular polar interactions28,29. So, the wettability of hydrophilic GO and hydrophobic rGO will definitely make a difference of gas sensibility. The responses of the GO and rGO POF sensor arrays to VOCs are shown in Fig. 2e, f and 3. The GO and rGO POF sensor array could selectively detect different VOCs, including hydrazine, ethanol, methanol, dichloromethane (MC), acetone, tetrahydrofuran (THF), nitromethane and diethylamine. Fig. 2e, f showed the recording of the changes in the reflected optical power during successive injections of acetone concentrations ranging from 500 ppm to 500 ppb for the only GO and only rGO POF sensor, respectively. The GO and rGO sensor exhibited a negative variation in the reflectance for a given VOC. According to the experimental analysis, a larger dilution of the vapour will lead to smaller variations in the reflected optical power. The detection results for acetone vapours with and without the GO and rGO layers on the POF end face are displayed in Fig. S1. The results show that the GO and rGO layers were sensitive to the vapours and played a key role in the detection of the VOCs. Shown in Fig. 3 are the comparison results for changes in the detector output for all vapours used in the study (i.e., hydrazine, ethanol, methanol, dichloromethane, acetone, tetrahydrofuran, nitromethane and diethylamine) at a 500 ppb concentration, which is quite low according to previous reports30 for both POF sensors. It is clearly seen that the only GO and only rGO POF sensors showed different sensitivities toward the various vapours (Fig. 3a, b). The intensity of the reflected optical response for the only GO and only rGO POF sensor was highest for diethylamine and nitromethane vapours at the same concentration, respectively and was lowest for methanol and dichloromethane vapours, respectively. The use of thinner GO or rGO layers as the active element in the sensor array facilitated an increase in vapour detection when compared to the use of thicker layers30. Furthermore, according to the experimental results, the sensitivity of GO to VOCs (mainly nitro and amine containing compounds) is much higher than that of rGO due to the presence of numerous polar functional groups (Fig. 3b). This experimental finding is good confirmation of our hypothesis. The measured responses of the only GO and only rGO POF sensors suggested that GO and rGO showed selectivity toward sensing VOCs (Fig. 3a, b).
Comparative plots of the sensing responses to eight different vapours at a 500 ppb concentration level.
(a) Only GO POF. (b), Only GO and only rGO POF. (c) A plot of selectively sensing responses of GO and rGO POF to THF, dichloromethane and ethanol with a two-headed POF sensor. The two-headed GO/GO, rGO/rGO and GO/rGO POF sensors were prepared with either GO or rGO, or one head was coated with GO and another head with rGO, respectively. (d) A plot of the selectivity of one-headed GO-rGO POF to THF, dichloromethane and ethanol.
Selective gas sensing by using two different hydrophilic GO and hydrophobic rGO surfaces
It was found that GO exhibited no response to dichloromethane and rGO displayed no response to THF. The selectivity properties of GO and rGO were confirmed through additional experiments. We carried out selectivity experiments with GO/rGO, GO/GO and rGO/rGO POF sensor arrays to detect MC, THF and ethanol vapours. For the GO/rGO POF sensor arrays, one optical fiber tip was coated with rGO while the other tip was coated with GO (GO/rGO array) (Fig. S2). For control samples, both fiber tips were coated with either GO or rGO (GO/GO and rGO/rGO arrays). The developed sensors clearly showed selectivity. Specifically, the new type of sensor array allowed for selectivity by detecting the differential responses for MC and THF vapours. Ethanol was used as a control vapour because it could be detected by both GO and rGO POF sensor arrays. In the case of MC, the reflected optical response of the GO and rGO coated optical fiber sample (GO/rGO array) was half that of the sample where both fibers were coated with only rGO (rGO/rGO array), as shown in Fig. 3c, S3. Similarly, in the case of THF, the reflected optical response of the GO/rGO array was half that of the sample where both fibers were coated with only GO (GO/GO array) (Fig. S4). In the case of ethanol, a reflected optical response was observed due to the sensing response of both GO and rGO in the GO/rGO array (Fig. S5). According to this experience, we prepared our newly designed GO-rGO coated POF sensor in one-headed tip (Fig. 1d, e). We have carried out the sensing ability test of the newly designed one-headed GO-rGO coated POF sensor for MC, THF and ethanol vapours respectively. Fig. 3d showed that in case of MC, the reflected optical response of the one-headed GO-rGO coated optical fiber sample was half of that of the one-headed only rGO POF sensor (Fig. S7). Similarly in case of THF, the reflected optical response of the one-headed GO-rGO coated optical fiber sample was half of that of the one-headed only GO POF sensor. In case of ethanol, the reflected optical response was observed lower than that of the one-headed only GO POF sensor and higher than that of the one-headed only rGO POF sensor, which is reasonable, due to sensitivity difference of GO and rGO for ethanol. We can use this concept to distinguish ratios of the gas mixture. Firstly we passed separately only MC, only THF and their gas mixture with 50:50 ratio to each of the 3 different sensors such as only GO (Fig. 1b), only rGO (Fig. 1c) and GO-rGO (Fig. 1d). For each case, we got sensitivity response data for each sensor (Fig. 3d). We found that the sensitivity intensity of only rGO to MC and only GO to THF is ~1:2 ratio with same concentration and that maintain even in the presence of MC and THF 50:50 mixtures (Fig. 3d, S7 and Table S3). Total sensitivity intensity of only GO and only rGO sensor for gas or gases mixture, is two-times of than that of GO-rGO sensor (Table S3). These sensitivity intensity responses will be our standard data that can be allowed to calculate any new concentration ratio of MC and THF. To verify this concept, we applied different gas ratios of MC and THF to 3 different sensors separately. Based on experimental results, the calculated gas ratio was ~30:70 and ~70:30 respectively (Table S3). Actually by this novel way, we are able to distinguish how much amount of MC or THF are present in the unknown gas mixtures. Now we can design our new one-headed only GO, only rGO and GO-rGO POF sensor array system to detect the amount of MC and THF in their gas mixtures at a time (Fig. S8). Also we can design nanolithography of rGO on GO surface for VOC sensor by the use of conductive atomic force microscopy (C-AFM) as reported in our previous paper31.
Furthermore, based on previously published studies, signal recovery was normally slow when the sensor was removed from the chamber due to the strong chemisorption of different vapours upon the surface of the material30,32. The observed signal recovery upon exposure to UV irradiation was consistent with the photodesorption of adsorbed gases, as previously noted for carbon nanotube films30,33. According to earlier reports, our rGO and GO layers behaved in a similar manner, i.e., when exposed to different vapours, the signal could return to the baseline through the use of UV irradiation and the response value is coming to steady state within ~8 mins (Fig. S9). However, in the case of only GO layers based on Fig. 2e,f, the time to return to the baseline is faster than that observed for only rGO, which implies that the recovery of the GO POF sensor is superior to than that previously reported rGO sensor. To prove our hypothesis that the functional groups of GO play a key role in the sensing ability and working under extremely strong acidic and basic conditions, we carried out a sensing ability test with ethanol, nitromethane and diethylamine vapours for GO suspensions with different pH values.
Gas sensing ability of GO under extreme pH conditions
We know that GO contains many polar functional groups (e.g., carboxyl, hydroxyl and epoxy groups) that can easily trapped H+ so as to obtain a positive charge under highly acidic conditions (pH = 1). Consequently, positively charged GO layers are formed. The amount of charges would increase with increasing [H+] ion concentration. Conversely, [OH−] ions at high pH (11) would lead to reactions of the epoxy and carboxyl groups with OH− and thus, deprotonate the carboxylic acid to form negatively charged GO layers. However, GO under the neutral condition (pH = 7) does not contain any charges34. Our as-prepared GO also contains positive charge, as the pH of the suspension is 5. Changing the pH of the GO suspension clearly had an effect on VOC sensing, which agrees with our assumption that is highest sensitivity at very strong acidic and basic conditions (Fig. 4a). At pH values of 1 and 11, the GO contained a high amount of positive and negative charges and thus, showed much higher sensitivity when compared to GO at pH 7, which is also higher than that of our as-prepared GO (pH = 5). Thus, GO is stable at extremely high acid and basic conditions to sense VOCs with high response. Surprisingly, the GO sheets with charges are allowed to form a relatively porous layer due to repulsive forces, whereas a closely packed layer at the neutral condition was formed. The most possible mechanism for a highest sensitivity of GO layers under strong acidic and basic environments is that the VOC molecules can be easily intercalated into the relatively porous GO layers, leading to a swelling of the relatively porous GO layers and finally an increase in the light absorption of the GO layers. On the other hand, with a closely packed GO layer, the VOC molecules could be adsorbed only on the surface of the GO layer to form a VOC liquid layer, which is helpful to reflect light. Hence, the light intensity of the GO layers generated at high and low pH decreased. In contrast, the light intensity was increased for GO layers at pH 7, thereby inducing a positive variation of the reflectance for a given VOC (Fig. 4a). To prove this assumption, we prepared 4 GO papers at pH values of 1, 5, 7 and 11 with a thickness of approximately 30 to 35 μm. We then used nitromethane as a VOC that could be exposed on the GO paper for 20 h. Optical microscopy was ultimately employed to measure the thickness of the GO papers before and after exposure of the VOC. We found that the thickness of all GO papers increased after exposure of the VOC. In the case of GO papers prepared at pH 1 and 11, the thickness increased up to ~70 and ~77%, whereas for papers prepared at pH 5 and 7, the thickness increased by ~17 and ~10%, respectively (Fig. S10–13, Table S1). The XRD data for the GO papers also confirmed our assumption with the interlayer distance of each layer before and after VOC exposure. We found that in the case of GO paper prepared at pH 1 and 11, the interlayer distance was higher when compared to GO paper fabricated at pH 5 and 7 due to the presence of repulsive forces with a high amount of similar charge. In contrast, the GO paper prepared at pH 5 contained a low amount of similar charges, while the paper fabricated at pH 7 did not contain any charges (Table S2). In the case of GO paper prepared at pH 1 and 11, the interlayers increased by ~6% after VOC exposure, whereas the interlayer increase for GO papers fabricated at pH 5 and 7 was ~2% and ~1%, respectively (Fig. S14, Table S2). The experimental data clearly supported our assumption. It can be clearly demonstrated that the GO papers that have ionic charges at pH 1, 5 and 11 would form the relatively porous layer due to repulsive forces, whereas the neutral condition at pH 7 will form a closely packed layer. Therefore, the VOC molecules can be easily adsorbed into the relatively porous GO layer and induce a swelling of the GO papers. To understand the relatively porous morphology, scanning electron microscopy (SEM) measurements were performed of different GO papers (Fig. S15).
Sensing properties of GO and rGO POF sensors with different pH and humidity at a 500 ppb concentration level of VOCs.
(a), A plot of the sensitivity of the GO suspension at pH 1, 5, 7 and 11 for 3 VOCs (ethanol, nitromethane and diethylamine). (b), A comparative plot for sensing eight different VOCs with the only GO and only rGO POF sensor under a maximum amount of humidity.
Gas sensing ability of GO under extremely high humidity
Humidity is a notorious interferent for gas sensing. Until now, the hydrophobic carbon nanotube (CNT) sensor has shown a negligible humidity interfering effect and high sensor stability due to the hydrophobicity and chemical stability of the material, respectively. Cantalini and co-workers were one of the first groups to report on almost negligible interfering effects at 80% relative humidity (RH) with CNTs, but the results were only achieved at an elevated temperature of 165°C35. Minami et al. also reported that graphitic SWNTs could achieve a negligible humidity interfering effect up to 50% RH at room temperature36. It was previously shown that hydrophobic rGO sheets were insusceptible to humidity interfering36. However, the negligible humidity interfering effect of hydrophobic carbon materials beyond 80% RH at room temperature has not yet been reported. To overcome the current limitation of the negligible humidity interfering effect for hydrophobic carbon materials at room temperature, we introduced a novel concept of GO nanosheets with a hydrophilic rather than hydrophobic surface. It was expected that the hydrophilic GO sheets, when compared with hydrophobic rGO, could effectively interact with adsorbed molecules in a higher humidity environment. To prove that the GO-coated POF sensor was atmosphere friendly, we evaluated the sensing ability of GO with respect to rGO under very high humidity conditions (up to RH 90%). All other experiments in this work were conducted at 20% RH so as to simulate the conditions of daily life at room temperature. As shown in Fig. 4b, the GO could sense VOCs (500 ppb concentration) at a higher humidity condition with high sensitivity when compared to rGO. It was confirmed that GO had a higher sensitivity than rGO, even under harsh conditions. We also observed that, as the RH was gradually increased, the sensitivity decreased in both cases at a 500 ppb VOC concentration, but VOCs could still be sensed especially with the GO sensor (Fig. S16,17). We found that our as-prepared GO at pH = 5 showed high sensitivity toward ethanol (RH 90%), nitromethane (RH 50%), acetone (RH 80%), methanol (RH 90%), THF (RH 90%), diethylamine (RH 60%) and hydrazine (RH 60%) at a 500 ppb concentration (Fig. 4b, S18). Sensing response of pure humidity of GO and rGO sensors was reported (Fig. S19). GO sensors with different pH values could sense VOCs in the presence of similar high humidity limits (Fig. S20). Here, the negligible interfering effect of humidity even at room temperature made GO attractive as a material for gas sensing because humidity is a notorious interferent. In contrast, metal oxide sensors, which are widely used for gas detection, usually require higher operating temperatures above 100°C to prevent humidity interference37. In general, the problems of sensor drift and stability are common in chemical sensors and are not ignorable factors during long-term continuous use38. Such factors can change the sensor sensitivity due to the degradation of sensitive layers. However, in our study, we observed that the stability of the GO layer was excellent over the sensor evaluation period (∼1 h) because of its chemical inertness, oxidation resistance and sensor fabrication technique (Fig. S21), which are comparable to those reported for the rGO system30. There is no noticeable damage to the GO and rGO layers, thereby suggesting that there is no covalent bond formation between the vapour and the materials. XPS data obtained for the GO and rGO layers before and after prolonged exposure to diethylmethane and dichloromethane vapour, respectively, showed no additional peaks from nitrogen or chlorine, which suggested that molecules did not chemically react with the various active sites present in the GO and rGO layers (Fig. S22, 23). The water contact angles of the GO and as-obtained rGO were 48.8 and 79.3°, respectively (Fig. S24)39. The experimental evidence demonstrates that, like rGO, GO could act as a POF sensor for VOCs, as the efficiency of the GO sensor was better than that of the rGO sensor.
Discussion
Therefore, according to our experimental result we could conclude that, due to the higher sensitivity of GO when compared to the best known rGO even under highly humid conditions and strongly acidic or basic conditions, GO is the best choice for use as a POF sensor for VOCs. The oxygen functional groups played a key role in the improvement of the GO sensing properties, confirmed with the results of selectivity and pH dependence experiments. Newly designed GO and rGO array provided the ability to distinguish between THF and MC, respectively to GO and rGO gas sensing, even at very low concentrations. In addition, the advantages of GO over rGO in terms of physical properties, such as easy dispersibility in water, simple formation of the films, the presence of different functional groups and no need for reduction to rGO (which requires more reaction steps and induces low dispersibility), can make GO a versatile sensor in the near future.
In summary, new one-headed POF sensor arrays with hydrophilic GO and hydrophobic rGO were carefully designed and successfully fabricated, resulting in the selective sensing of VOC gases for the first time. It was clearly shown that the GO and rGO sensor array had a high sensing ability to distinguish between THF and MC, respectively. The selective VOC detection of the GO-rGO POF sensor array can provide a good prospect for use with low cost and ease of fabrication. In addition, for the first time, the hydrophilic GO sensor with various oxygen functional groups was employed to overcome the previously unsolved issue of low sensitivity in a high humidity environment and under strong acidic and basic conditions. Due to the swelling effect of GO layers in the presence of VOCs, which was confirmed with thickness measurements obtained by optical microscopy and the interlayer distance acquired by XRD, the sensitivity of the hydrophilic GO sensor was higher than that of hydrophobic rGO in air at room temperature, even under highly humid conditions and in strongly acidic (pH 1) and basic (pH 11) environments. Thus, the eco-friendly physical properties of GO, combined with its fast and high sensitivity, would make it an ideal choice for an environmentally friendly VOC sensor. The physically and chemically stable properties of 2D GO, which allow the notorious humidity issues inherent in gas sensors to be overcome, are expected to facilitate the commercialization of GO and GO-rGO array based gas sensors.
Methods
Preparation of graphene oxide (GO)
GO was prepared from natural graphite powder by the modified Hummers and Offenman's method using sulfuric acid, potassium permanganate and sodium nitrate40.
POF reflectance probe fabrication
The polymer optical fiber (POF) reflectance probe was coated with graphene oxide (GO) (Fig. 1 b) or reduced graphene oxide (rGO) (Fig. 1c) as the sensitive layer. A POF with a 1 × 2 fiber coupler (50:50, Industrial Fiber Optics Inc., IF-562) was used for the detection of the reflected signal by providing the necessary connections between the light sources and the sensing interface. A 3 μL 1 mg/mL GO solution was dropped on the end of the optical fiber and the fiber was continuously dried at 60°C for 20 h to form a GO layer. An rGO layer was prepared in a similar manner followed by sunlight reduction26. Before deposition of the GO or rGO, the fiber end was cleaved with a precision cleaver and polished to obtain a uniform and planar cross section. The fiber with GO coating was fixed on the tip and half of the coating had been covered by printing paper. Solar radiation using converging lens was focused on remaining half of GO coating which was not covered by printing paper. The paper were removed and the half GO and half rGO combined structure was achieved (Fig. 1e). Similar demonstration was done on GO paper in as given video.
Experimental setup
A schematic view of the experimental setup used to evaluate the sensor performance for VOCs is shown in Fig. 1a. The POF end face with a GO or rGO coating was located in the test chamber (volume = 350 mL) and known quantities of VOC were injected to achieve various VOC concentrations. The sensor performance was evaluated using a blue light source (spectral range of 450–495 nm and optical power of 2 mW). The reflected light intensity was measured using a Si photodiode detector (PDA36A, Thorlab), which has a spectral range of 350–1100 nm and connects with a computer interfaced digital multimeter (2700, Keithley). The microstructure was observed by field emission scanning electron microscopy (SEM; JSM-6701F/INCA Energy, JEOL). To help the signal recover, UV irradiation (254 nm, VL-4.LC) was used as needed during the experiment. All experiments were carried out in a dark room at room temperature.
Chemicals and characterization
Natural graphite (Bay Carbon, SP-1 graphite), sulfuric acid (95–97%), hydrogen peroxide (30 wt%), potassium permanganate and sodium nitrate were obtained from commercial sources and used as received. All X-ray photoemission spectroscopy (XPS) measurements were acquired with a SIGMA PROBE (ThermoVG, U.K.) with a monochromatic Al-Kα X-ray source at 100 W. The XRD patterns were obtained using a D8 Advance instrument (Germany) with Cu-Kα radiation. The thermal properties of the rGO were characterized by TGA under N2 gas atmosphere (Polymer Laboratories, TGA 1000 plus).
References
Chakraborty, S., Sen, A. & Maiti, H. S. Selective detection of methane and butane by temperature modulation in iron doped tin oxide sensors. Sens. Actuators, B 115, 610–615 (2006).
Nakata, S., Okunishi, H. & Nakashima, Y. Distinction of gases with a semiconductor sensor depending on the scanning profile of a cyclic temperature. Analyst 131, 148–154 (2006).
Sysoev, V. V., Goschnick, J., Schneider, T., Strelcov, H. & Kolmakov, A. A gradient microarray electronic nose based on percolating SnO2 nanowire sensing elements. Nano Lett. 7, 3182–3188 (2007).
Röck, F., Barsan, N. & Weimar, U. Electronic nose: current status and future trends. Chem. Rev. 108, 705–725 (2008).
Umar, A. & Hahn, Y. B. Metal Oxide Nanostructure and Their Applications 3, 31–52 (2010).
Bariáin, C., Matías, I. R., Romeo, I., Garrido, J. & Laguna, M. Detection of volatile organic compound vapors by using a vapochromic material on a tapered optical fiber. J. Appl. Phys. Lett. 77, 2274–2276 (2000).
Shi, C. et al. Molecular fiber sensors based on surface enhanced raman scattering (SERS). J. Nanosci. Nanotechnol. 9, 2234–2246 (2009).
Franke, M. E., Koplin, T. J. & Simon, U. Metal and metal oxide nanoparticles in chemiresistors: does the nanoscale matter? Small 2, 36–50 (2006).
Eranna, G., Joshi, B. C., Runthala, D. P. & Gupta, R. P. Oxide materials for development of integrated gas sensors-A comprehensive review. Crit. Rev. Solid State Mater. Sci. 29, 111–188 (2004).
Geim, A. K. & Novoselov, K. S. The rise of graphene. Nat. Mater. 6, 183–191 (2007).
Schedin, F. et al. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 6, 652–655 (2007).
Robinson, J. T., Perkins, F. K., Snow, E. S., Wei, Z. & Sheehan, P. E. Reduced graphene oxide molecular sensors. Nano Lett. 8, 3137–3140 (2008).
Frank, I. W., Tanenbaum, D. M., Van der Zande, A. M. & McEuen, P. L. Mechanical properties of suspended graphene sheets. J. Vac. Sci. Technol. B 25, 2558–2561 (2007).
Balandin, A. A. et al. Superior thermal conductivity of single-layer graphene. Nano Lett. 8, 902–907 (2008).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Kaniyoor, A., Jafri, R. I., Arockiadoss, T. & Ramaprabhu, S. Nanostructured Pt decorated graphene and multi walled carbon nanotube based room temperature hydrogen gas sensor. Nanoscale 1, 382–386 (2009).
Vedala, H., Sorescu, D. C., Kotchey, G. P. & Star, A. Chemical sensitivity of graphene edges decorated with metal nanoparticles. Nano Lett. 11, 2342–2347 (2011).
Yi, J., Lee, J. M. & Park, W. Il Vertically aligned ZnO nanorods and graphene hybrid architectures for high-sensitive fiexible gas sensors. Sens. Actuators, B 155, 264–269 (2011).
Lu, G. et al. Toward practical gas sensing with highly reduced graphene oxide: a new signal processing method to circumvent run-to-run and device-to-device variations. ACS Nano 5, 1154–1164 (2011).
Lu, G., Yu, K., Ocola, L. E. & Chen, J. Ultrafast room temperature NH3 sensing with positively gated reduced graphene oxide field-effect transistors. Chem. Commun. 47, 7761–7763 (2011).
Ji, Q. et al. Layer-by-layer films of graphene and ionic liquids for highly selective gas sensing. Angew. Chem. Int. Ed. 49, 9737–9739 (2010).
Lu, G., Ocola, L. E. & Chen, J. Reduced graphene oxide for room-temperature gas Sensors. Nanotechnology 20, 445502–445511 (2009).
Rao, C. N. R., Sood, A. K., Subrahmanyam, K. S. & Govindaraj, A. Graphene: the new two-dimensional nanomaterial. Angew. Chem., Int. Ed. 48, 7752–7777 (2009).
Mao, S. et al. Tuning gas-sensing properties of reduced graphene oxide using tin oxide Nanocrystals. J. Mater. Chem. 22, 11009–11013 (2012).
Ponzoni, A., Comini, E. & Sberveglieri, G. Ultrasensitive and highly selective gas sensors using three-dimensional tungsten oxide nanowire networks. App. Phys. Lett. 88, 203101–203103 (2006).
Eswaraiah, V., Aravind, S. S. J. & Ramaprabhu, S. Top down method for synthesis of highly conducting graphene by exfoliation of graphite oxide using focused solar radiation. J. Mater. Chem. 21, 6800–6803 (2011).
Zhang, H. et al. Detection of acetone vapor using graphene on polymer optical fiber. J. Nanosci. Nanotechnol. 11, 5939–5943 (2011).
Compton, O. C. et al. Tuning the mechanical properties of graphene oxide paper and its associated polymer nanocomposites by controlling cooperative intersheet hydrogen bonding. ACS Nano 6, 2008–2019 (2012).
Paredes, J. I., Villar-Rodil, S., Martínez-Alonso, A. & Tascón, J. M. D. Graphene oxide dispersions in organic solvents. Langmuir 24, 10560–10564 (2008).
Dua, V. et al. All-organic vapor sensor using inkjet-printed reduced graphene oxide. Angew. Chem. Int. Ed. 49, 2154–2157 (2010).
Seo, S. et al. Electric field-induced nanopatterning of reduced graphene oxide on Si and a p–n diode junction. J. Mater. Chem. 21, 5805–5811 (2011).
Lu, G., Ocola, L. E. & Chen, J. Gas detection using low-temperature reduced graphene oxide sheets. Appl. Phys. Lett. 94, 083111–083113 (2009).
Chen, R. et al. Molecular photodesorption from single-walled carbon nanotubes. Appl. Phys. Lett. 79, 2258–2260 (2001).
Que, R. et al. Flexible nanogenerators based on graphene oxide films for acoustic energy harvesting. Angew. Chem. Int. Ed. 51, 5418–5422 (2012).
Cantalini, C. et al. Sensitivity to NO2 and cross-sensitivity analysis to NH3, ethanol and humidity of carbon nanotubes thin film prepared by PECVD. Sens. Actuators, B 95, 195–202 (2003).
Sasaki, I., Minami, N., Karthigeyan, A. & Iakoubovskii, K. Optimization and evaluation of networked single-wall carbon nanotubes as a NO2 gas sensing material. Analyst 134, 325–330 (2009).
Hsu, L.–C. et al. Evaluation of commercial metal-oxide based NO2 sensors. Sensor Review 27, 121–131 (2007).
Natale, C. D., Davide, F. A. M. & D'Amico, A. A self-organizing system for pattern classification: time varying statistics and sensor drift effects. Sens. Actuators, B 27, 237–241 (1995).
Cote, L. J., Cruz-Silva, R. & Huang, J. Flash reduction and patterning of graphite oxide and its polymer composite. J. Am. Chem. Soc. 131, 11027–11032 (2009).
Moon, I. K., Lee, J., Ruoff, R. S. & Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 1, 73–79 (2010).
Acknowledgements
This work was supported by the Creative Research Initiatives (project title: Smart Molecular Memory) of MEST/NRF. The authors declare no competing financial interests.
Author information
Authors and Affiliations
Contributions
S.S. and Y.X. contributed equally to this paper. S.S. and H.L. designed the research, S.S. characterized the GO and rGO, Y.X. carried out VOC experiments, Y.K. and Y.Y. synthesized the GO and GO papers and S.S., Y.X., Y.K., Y.Y., H.Q., A.K., T.K. and H.L. analyzed and interpreted the data. S.S. and H.L. co-wrote the manuscript and supporting text.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Supplementary Information
Video, GO to rGO (Lee).
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Some, S., Xu, Y., Kim, Y. et al. Highly Sensitive and Selective Gas Sensor Using Hydrophilic and Hydrophobic Graphenes. Sci Rep 3, 1868 (2013). https://doi.org/10.1038/srep01868
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01868
This article is cited by
-
Optical fiber sensors coated with GO/PANI nanocomposite integrating a microcontroller-based acquisition and processing system
Journal of Optics (2024)
-
Thermal reduced graphene oxide-based gas sensor for rapid detection of ammonia at room temperature
Journal of Materials Science (2023)
-
Synergistic antibacterial performance of rosemarinic acid - graphene oxide in electrospun polylactic acid membranes for air filtration
Journal of Polymer Research (2023)
-
Advanced Strategies to Improve Performances of Molybdenum-Based Gas Sensors
Nano-Micro Letters (2021)
-
Anti-biofouling NH3 gas sensor based on reentrant thorny ZnO/graphene hybrid nanowalls
Microsystems & Nanoengineering (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.