Abstract
We report a controllable wet method for effective decoration of 2-dimensional (2D) molybdenum disulfide (MoS2) layers with Au nanoparticles (NPs). Au NPs can be selectively formed on the edge sites or defective sites of MoS2 layers. The Au-MoS2 nano-composites are formed by non-covalent bond. The size distribution, morphology and density of the metal nanoparticles can be tuned by changing the defect density in MoS2 layers. Field effect transistors were directly fabricated by placing ion gel gate dielectrics on Au-decorated MoS2 layers without the need to transfer these MoS2 layers to SiO2/Si substrates for bottom gate devices. The ion gel method allows probing the intrinsic electrical properties of the as-grown and Au-decorated MoS2 layers. This study shows that Au NPs impose remarkable p-doping effects to the MoS2 transistors without degrading their electrical characteristics.
Similar content being viewed by others
Introduction
Due to their unique properties, atomically thin two-dimensional (2D) materials with a layered structure such as graphene1,2,3, hexagonal boron nitride (h-BN)4,5 and transition-metal dichalcogenide (LTMDs)6,7,8 have been attracting increasing attention. Molybdenum disulfide (MoS2), belongs to the layered 2D nanomaterial family, is traditionally used as a solid state lubricant and a catalyst for hydrodesulfurization (HDS) and hydrogen evolution reaction (HER)9,10,11. In recent years, it has been demonstrated that ultra-thin MoS2 crystals with a typical thickness of ~0.65 nm can be obtained by either physical6,12 or chemical exfoliation methods13,14, MoS2 layers with uniform thickness can also be synthesized in large scale by chemical vapor deposition15,16,17,18,19. The monolayer MoS2 exhibits excellent electrical6,7,8 and optical performance20,21 compared to its bulky counterpart.
MoS2 can be seen as an inorganic graphene analogue, similar to graphene and h-BN. The structure of MoS2 is based on a hexagonal crystal22, where Mo atom is six-fold coordinated and hexagonally packed between two trigonally coordinated sulphur atoms. One S-Mo-S quintuple-layer is weakly bonded to another S-Mo-S layer by van der Waals forces. It has been reported that the basal plane of MoS2 is catalytically inactive and the activity in the HDS and HER is associated with the edge termination of MoS2 clusters9,10,11,23. Especially for the MoS2 nanocrystals form, it has been investigated as an inexpensive alternative to platinum or other noble metals for the electrochemical or photochemical generation of hydrogen from water10,24,25,26,27, which could be a promising clean energy source. The better understanding of catalytical active edge sits of MoS2 is critical for the advanced catalysts design and developing its practical applications28. However, the precise molecular structure modulation and engineering, especially for a well-defined edge site, is fundamentally challenging and rarely reported. On the other hand, noble metal nanoparticles such as Au, Pt and Ag are a class of materials with unique chemical and physical properties which find great applications in biosensing29,30, photonics31,32 and catalysis33,34 It can be expected that the noble metal nanoparticles (NPs) anchored on the MoS2 sheet could potentially extend its functionalities as novel catalytic, magnetic and optoelectronic nanomaterials28,35. Furthermore, compared with graphene, MoS2 is a semiconducting material with a suitable band gap around 1.2 to 1.9 eV, depending on its thickness6,20,21. It is possible to form p-n junctions with other semiconducting materials as new type of electronic devices. While substantial research efforts have been paid, the Fermi level engineering of MoS2 layers by chemical doping methods are still lack of investigation. It was reported metal NPs can be selectively decorated on carbon nanomatierals by controlling the surface wetting properties36 and it was also suggested the edges of 2D nanotmateirals may favor the anchoring of metal NPs37. However, location dependence of metal NPs decoration on MoS2 layers was rarely reported. In this contribution, we study the controllable decoration of isolated MoS2 single crystal prepared by chemical vapor deposition method (CVD-MoS2) by Au NPs. The CVD-MoS2 layers are in well-defined triangular shapes, providing a perfect template for the nanoparticle decoration. It was found that AuCl4− ions can be reduced to neutral gold atoms selectively on the edge part, or defective sites of MoS2 layers by a simple solution dip casting method. The quality of MoS2-Au NPs composite was evaluated by atomic force microscopy (AFM), transmission electron microscopy (TEM), selected area electron diffraction (SAED), X-ray energy dispersive spectroscopy (EDS), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS) and electrical characterizations.
Results
With the recently developed techniques for MoS2 growth by CVD method, single layer and crystalline MoS2 can be produced in a large scale on single crystal substrates16,17,18 or even an atomically thin graphene layer15. These highly crystalline CVD-MoS2 layers serve as good model 2D material systems for us to investigate the growth mechanism of Au NPs on 2D materials. Fig. 1 (A) shows the AFM images of the MoS2 sheets obtained on the sapphire substrates. Smooth surface morphology of MoS2 sheet is observed under AFM, which suggests that a layer structure of MoS2 is formed. These continuous MoS2 layers are formed by small triangular MoS2 single crystals with a typical thickness of 0.71 nm (See supporting information Figure S1). According to the previous report38, the triangular shape is a unique feature of the single-layer MoS2 clusters, since the S edge terminations is considerably more stable. The inset in Fig. 1 (A) displays the non-continuous MoS2 single crystals in a triangular shape, where the well-defined feature allows us to further study the reactivity of MoS2 edges and basal planes. Fig. 1(B) provides AFM images of the MoS2 nanosheets with Au NPs grown on them. The AFM images clearly show that small NPs with a diameter around 5 nm were formed after AuCl4− doping and there is no particles found on the surface of substrate which suggests the selectively reduction reaction of AuCl4− on MoS2 nanosheets. Interestingly, the Au NPs tend to form on the edge sites of MoS2 with only few particles on the basal plane of MoS2 layers. Previously we report the Au NPs tend to nucleate on the graphene wrinkles due to the morphology change which could trap the precursor and act as the initial nucleation centers for Au particle growth39. Different from the chemical vapor deposited graphene (CVD-G), there is no obvious wrinkle formation on CVD-grown MoS2 nanosheets. The atomically flat surface morphology excludes the influence of different surface energy induced by surface smoothness. In this case, the preferential formation of Au NPs on the edge sites of MoS2 indicates that the edge site is more reactive than the basal plane region. The edge area could attract the AuCl4− precursor at the initial stage and act as the first nucleation center for Au growth. The intensity of photoluminescence (PL) peak and the energy separation between the Raman A1g and E2g peaks have been found to relate to the number of MoS2 layers20,21. Fig. 1 (C) shows the Raman and PL spectra for the MoS2 layer, where the excitation light source is a continuous laser with a wavelength of 473 nm. The Raman peaks at 405 and 385.6 cm−1 are identified as the A1g and E2g vibration modes, which are characteristics for MoS2. The PL spectrum displays a distinct main emission peak at ~663 nm, which is in good agreement with the MoS2 thin layer obtained from exfoliation method. The PL and Raman characterizations suggest the good crystallinity of the CVD-MoS2.
MoS2 nano-flakes on c-face sapphire substrate substrates.
(A) Typical AFM height images of MoS2 flakes grown on c-face sapphire substrates. Inset shows a non-continuous MoS2 layers formed in triangular feature; (B) AFM image of the corresponding regions after Au nano particles decoration. For these samples, the Au precursor adsorption time was 20 seconds and rinsed with DI water after dip casting; (C) photoluminescence spectra for the obtained CVD-MoS2 layers, inset shows the Raman spectra of the CVD-MoS2. Both Raman and PL experiments were performed using a confocal spectrometer with 476 nm excitation laser.
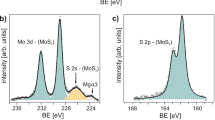
XPS was applied to reveal the chemical composition of the nano-sized particles. Fig. 2 shows the XPS spectra of the Au doped MoS2 nanosheets on Sapphire substrates. As shown in Fig. 2 (A) and (B), the Mo 3d shows two peaks at 229.2 eV and 232.3 eV, which can be attributed to the doublet of Mo 3d5/2 and Mo 3d3/2. The binding energy for S 2p3/2 and S 2p1/2 are 162.0 eV and 163.3 eV, shown in Fig. 2 (B). The Mo and S binding energies are in good agreement with reported value40, indicating that the doping of Au does not severely change the crystallinity of MoS2 and the MoS2 monolayer is chemically stable in HAuCl4 aqueous solutions. The XPS scans for the MoS2 samples after AuCl4− doping confirm the chemical bonding states of the MoS2 layers and Au as displayed in Fig. 2 (C). No detectable Cl signal within the bonding energy ranging from 196 eV to 204 eV has been observed in Fig. 2 (D). This suggested that there is no physical adsorption of AuCl4− on MoS2 surface and AuCl4− has been fully reduced to Au. The calculated atomic concentration of Mo, S and Au from XPS are 33.9%, 64.6% and 1.5% with a ratio of 1 : 1.90 : 0.044.
To explore the location dependency of Au NPs growth on MoS2 layers and understand the tendency of the epitaxial stacking manner of Au NPs on MoS2 nanosheets, the Au NPs/CVD-MoS2 was investigated by analyzing the high resolution TEM (HRTEM) and the corresponding fast Fourier transform (FFT) images. The results were also compared with the Au doped chemical exfoliated MoS2 (Au NPs/CE-MoS2) which has a higher defect density.
Fig. 3 (A) and (B) are the TEM images of Au NPs/CVD-MoS2, triangular nanosheets with well-proportioned NPs along the edge and sporadic ones on the surface region which is in good consistence with the AFM results. As observed in Fig. 3 (B), NPs appeared to be darker in contrast when compared to that of the MoS2 nanosheets. We noticed that due to the low content of gold in the CVD-MoS2 nanosheets, no Au element can be obviously detected in Au NPs/CVD-MoS2 according to EDS (see supporting information Figure S2). Fig. 3 (C) displays a HRTEM image taken from the edge part of Au NPs/CVD-MoS2. The inset shows the SAED pattern from the surface of CVD-MoS2, which can be attributed to the 2H-MoS2 single crystal15,16,18,19. For the SAED analysis, in order to avoid cover more than one piece of triangular MoS2 flake, the aperture in TEM analysis was set to be 10 μm (with the electron beam size of 150 nm), which is a bit smaller than that of the Au decorated MoS2 flakes. Fig. 3 (D) depicts the HRTEM image of an Au NP which clearly reveals a single crystal nature of Au nano-domain. The measured d spacing is around 0.23 nm which is in good consistence with the Au (111) spacing41. We also noticed although it is hard to indicate the grain boundaries of continuous CVD-MoS2 layer, after Au decoration and rinsing process, part of the MoS2 film could break and left mechanically formed MoS2 edges (See supporting information Figure S3). The Au NPs tend to form on the exposed MoS2 edges rather than on the basal plane region of MoS2. These observations align well and suggest that the MoS2 defects, especially for the edge area, may be more reactive than the basal surface.
TEM images of the Au NPs decorated CVD-MoS2 nano-flakes.
(A) Low magnification TEM image of isolated single crystal MoS2 flakes in triangular shape. (B) A zoomed-in TEM image of MoS2 flakes with nano-size particles mostly adsorbed on the edge parts. (C) A typical HRTEM image taken from the edge of a MoS2 flake. Inset shows the SAED pattern from the MoS2 surface. (D) shows an enlarged HRTEM image of Au NPs on MoS2 edge, the measured d spacing distance is around 0.23 nm.
In order to further examine the assumption regarding the relationship between Au NPs and the defects in MoS2, MoS2 films were also prepared by chemical exfoliation (CE-MoS2) method and characterized by TEM. The chemical exfoliation method involves lithium ion intercalation between MoS2 layers and ultra-sonication was applied to assist the further exfoliation of MoS2 layers and forming few layer or monolayer MoS2 nanosheets, therefore CE-MoS2 was expected to possess more defects than the CVD-MoS2. The HRTEM taken for CE-MoS2 suggest that the CE-MoS2 films exhibit poor crystallinity compared to the CVD-MoS2 as expected. (See supporting materials Figure S4). Fig. 4 (A) and (B) displays the TEM image of the chemically exfoliated MoS2 film after Au doping. Dramatically different from the CVD-MoS2, both large and small Au NPs can be found on CE-MoS2 as shown in Fig. 4 (A) and 4 (B). The particle density is also much higher than that observed from CVD-MoS2. The inset images in Fig. 4 (B) shows the corresponding SAED images. The SAED image of a typical Au NPs decorated CE-MoS2 film exhibits three distinguished rings which can be assigned to the MoS2 {100}, {110} and Au {111} planes with lattice spacing of 0.27, 0.16 and 0.23 nm respectively. The characteristic electron diffraction ring assigned to the {111} planes of the face-centred-cubic (fcc) lattice of Au is much sharper and stronger in comparison with the other planes, revealing the predominant orientation of the Au {111} planes. The increase of Au NPs density on CE-MoS2 strongly suggests that the forming of Au NPs is associated with the defects of MoS2. The edge parts or defects in MoS2 layers could interact with the AuCl4− precursor at the initial stage and act as the first nucleation center for Au NPs growth, therefore the results suggest the Au NPs could effectively work as a defect marker for MoS2 layers. HRTEM images were taken to further investigate the orientation relationship between MoS2 film and Au particles Fig. 4 (C) and (D) show the HRTEM images of typical Au NPs on CE-MoS2 with different particle sizes ranging from ~2 nm to ~10 nm. The HRTEM image in Fig. (D) displays an in plane lattice spacing of 0.23 nm which is in good agreement with the number of Au (111). Although it is not easy to determine the accurate lattice of CE-MoS2 due to its defective polycrystalline nature, the SEAD and HRTEM results suggest that the defective sites in MoS2 layers could effectively attract Au NPs precursor and serve as nucleation sites and the surface plane of MoS2 serves as a growth temple for Au NPs along its {111} plane. It was previously reported single layer MoS2 nano-islands can form on crystalline Au (111) surfaces with their (0001) basal plane oriented parallel to the Au (111) substrate surface38. In this study, we propose at the very beginning, the AuCl4− ions adsorb onto the defective sites in MoS2 layers and reduced to Au atoms which subsequently form Au nanoclusters, therefore the density of Au NPs depends on the defect density of MoS2 layer. Meanwhile, the Au {111} has the lowest surface energy42,43 and small lattice mismatch with MoS2 (0001), which promotes the preferential orientation of Au (111) crystal planes on MoS2 surface.
TEM images of the Au NPs/CE-MoS2 film.
(A) Low magnification TEM image of Au NPs/CE-MoS2 film. (B) Enlarged TEM image of Au NPs/CE-MoS2 film with various Au NPs size. The inset shows the SAED pattern from the film. ) (C) A typical HRTEM image of small particle (~2 nm) located on the CE-MoS2 films; (D) HRTEM image of large Au particle (~10 nm) located on the CE-MoS2.
Discussion
Various research methods have been developed aiming at tuning the electronic properties of 2D materials. According to our previous report39,44, the work function of graphene layer can be effectively controlled by immersing graphene film in AuCl4− solution, due to the spontaneous reduction of Au3+ to Au0. Raman spectroscopy is a powerful nondestructive characterization tool to reveal the level of doping in single-layer MoS2 due to the strong electron-phonon interaction45,46. It has been reported that n-doping results in softening of the A1g phonon which results in decrease of relative intensity and peak frequency difference between A1g and E2g modes46 while p-doping in MoS2 layers should cause relative shifting of the Raman-active modes in opposite direction. Figure 5 (A) shows typical Raman spectra for a single layer MoS2 sheet before and after Au NPs decoration. The MoS2 layer exhibits two Raman characteristic bands at ~404 and 386 cm−1, corresponding to the A1g and E2g modes respectively. It was reported oxygen in the ambient may attack Mo-S-Mo bands and cause the shift of Raman vibrations modes45. To reduce the influence from the ambient oxygen, the Raman spectra were taken right after the CVD synthesis and/or Au NPs decoration. It was also noticed that the Raman spectra shows some variation across the sample surface, therefore to reveal the doping effect of Au NPs a set of data points were taken and statistical analysis were carried out as shown in Figure 5 (B) and (C). Figure 5(B) and (C) compare the relative peak intensity and frequency difference shift of A1g and E2g Raman modes, respectively. The results showing a detectable upshift of A1g frequency and an increase of A1g/E2g peak intensity ratio, which suggest distinct p-doping caused by Au NPs decoration45,46. To further evaluate doping effect of Au NPs on CVD-MoS2 electrically, top-gate transistors were fabricated by evaporating Au/Ti electrodes directly on top of the MoS2 layers on sapphire substrate. The MoS2 electric double-layer transistors (EDLTs)47,48 were formed with an ionic gel film which works as dielectric layer for the transistor. Details of device fabrication can be found in the previous report48. It is worthy to mention, since the devices are directly fabricated on the growth substrates and no transfer process is involved, the intrinsic transport properties of CVD-MoS2 layers and the effect of Au NPs on the transistor performance can be directly accessed. Fig. 5 (D) shows the transfer curve of CVD-MoS2 EDLTs before and after Au NPs decoration. Supporting information Figure S5 shows the I-Vg curves plotted in log scale. The pristine CVD-MoS2 EDLTs show typical transfer curves of n-typed behavior which is consistent with the other reports. Au/Ti (80 nm and 5 nm, respectively) were used as the source/drain electrodes. The work function of Ti is closer to the conduction band edge of MoS2 and results an expected n-typed behaviors. After Au NPs decoration, the threshold gate voltage changes from 0.47 V to 1.29 V and the transfer curve shifts to the right hand side which indicates that a p-doing effect has been introduced by the Au NPs. The electrical results are consistent with the Raman characterizations confirming that the AuCl4− ions in solution can strongly withdraw electrons from MoS2 layers and reduces to Au NPs, therefore, it is anticipated for a down shift of Fermi level in MoS2 layers. It is found that the on/off ratio for the device after Au NPs doing is around 3.54 × 103, which is around 50 times larger than that of the pristine MoS2 electric double-layer transistors (EDLT). The measured maximum current for the doped and pristine MoS2 EDLT is 13.7 mA and 22.5 mA, respectively. The mobility of the MoS2 EDLT can be derived from the slope of the transfer characteristics using the standard equation Id = (μWVDCi/L)(VG-Vth) for the linear region48. Where Id is the drain current, μ is the field-effect mobility, W is the channel width, VD is the drain voltage, Ci is the specific capacitance of the dielectric, L is the channel length, VG is the gate voltage and Vth is the threshold voltage. From the calculation, the mobility for Au NPs doped MoS2 is around 2.44 cm2/(Vs), which is 2 times smaller than the pristine MoS2 EDLT.
(A) Typical Raman spectra of MoS2 sheets before and after Au NPs decoration; (B) and (C) show the statistical analysis of the relative intensity and frequency change in E2g and A1g vibration mode induced by the Au NPs decoration; (D) Thin-film MoS2 EDLT constructed with an ion gel dielectric layer.
Black and red lines correspond to the transfer characteristics of the MoS2 EDLT before and after Au NPs decoration. The inset schematically illustrates the device structure, the source and drain electrodes were electrically isolated from the ion gel by a thin PDMS layer.
In conclusion, we have proposed a simple method to decorate the CVD-MoS2 and CE-MoS2 thin layers with Au NPs. AFM, TEM, XPS characterization methods were carried out to investigate the growth mechanism and epitaxial stacking manner of Au NPs and MoS2. The Au NPs exhibited a remarkable p-doping effect to the MoS2 transistors. Hence, the proposed Au NPs decoration method can provide promising nanomaterials hybrids for the application of future optoelectronics devices.
Methods
CVD-MoS2 synthesis
High-crystal-quality MoS2 were grown on a sapphire substrates by chemical vapor deposition method inside a horizontal tube furnace. The detailed fabrication procedure can be found elsewhere17,18. To be brief, the MoS2 films were synthesized on c-face sapphire substrates in a hot-wall furnace. High purity MoO3 (99%, Aldrich) and S powder (99.5, Alfa) were placed in two separated Al2O3 crucibles and the substrates were placed on the downstream side of the Ar carrying gas. The MoS2 samples were obtained by annealing at 650°C for 15 min with a heating rate of 15°C/min and Ar flow rate was kept at 1 sccm.
Chemical exfoliated MoS2
The MoS2 nanoflakes were obtained by lithium intercalation method follow the procedure reported before20. 0.5 g of natural MoS2 crystals from Sigma-Aldrich were dispersed in 5 mL of 1.6 M butyllithium solution in hexane (Sigma-Aldrich) for 48 hours in a flask filled with argon gas. The LixMoS2 was retrieved by filtration and washed with hexane (60 mL) to remove excess lithium and organic residues. Exfoliation was achieved by ultrasonicating the obtained LixMoS2 slurry in DI-water for 1 h. The mixture was centrifuged and re-dispersed in DI water for at least 5 times to remove excess lithium in the form of LiOH and unexfoliated material. Thin films were prepared by filtering through a mixed cellulose ester membrane with 0.025 μm pores (Millipore). The film was delaminated on water surface for subsequent transfer onto substrates or TEM Grids.
Au NPs decoration on CVD-MoS2 and CE-MoS2
AuCl3 dissolved in water was used for the growth of Au nano particles (Au NPs) on MoS2. To prepare Au NPs/CVD-MoS2, the as prepared CVD-MoS2 on sapphire substrates were directly immersed in 5 mM AuCl3 for 20 sec, followed by gently rinsing with DI water. To prepare Au NPs/CE MoS2 samples, the CE MoS2 film was firstly delaminated on water surface by the previously described method20. Several drops of 5 mM AuCl3 water solution were added into the water subsequently. Finally, the Au NPs/CE-MoS2 floating on water surface can be picked up by TEM grids or arbitrary substrates.
Transfer of the as-grown Au NPs/MoS2 hybrid to arbitrary substrates
The CVD-MoS2 and Au NPs decorated MoS2 films was transferred by coating the film with a thin layer (~100 nm) of Poly[methylmethacrylate] (PMMA). After etching the underlying sapphire substrates with KOH aquariums (with a concentration of 2 M) at 80°C, the PMMA/MoS2 film was transferred to DI water and was suspended on the surface of water to remove the etchant residue. Subsequently, the film can be transferred to any substrate or TEM grids for analysis and characterization. Finally, the top layer of PMMA can be removed by acetone or by directly annealing the samples in an Ar and H2 atmosphere at 400°C for 2 hours.
Characterizations
Surface morphology of the samples was examined with commercial atomic force microscope (AFM, Veeco Icon). Raman spectra were collected in a NT-MDT confocal Raman microscopic system with exciting laser wavelength of 473 nm and the laser spot-size is around 0.5 μm. For the Raman characterization, the Si peak at 520 cm−1 was used as reference for wavenumber calibration. Field-emission transmission electron microscope (JEOL JEM-2010F, operated at 200 keV), equipped with an energy dispersive spectrometer (EDS) was used to obtain the information of the microstructures and the chemical compositions. Chemical composition was determined by X-ray photoelectron spectroscope (XPS, Phi V5000). XPS measurements were performed with an Al Kα X-ray source. The energy calibrations were made against the C 1s peak to eliminate the charging of the sample during analysis.
References
Geim, A. K. & Novoselov, K. S. The rise of graphene. Nat Mater 6, 183–191 (2007).
Novoselov, K. S. et al. Two-dimensional gas of massless Dirac fermions in graphene. Nature 438, 197–200 (2005).
Novoselov, K. S. et al. Electric Field Effect in Atomically Thin Carbon Films. Science 306, 666–669 (2004).
Kubota, Y., Watanabe, K., Tsuda, O. & Taniguchi, T. Deep Ultraviolet Light-Emitting Hexagonal Boron Nitride Synthesized at Atmospheric Pressure. Science 317, 932–934 (2007).
Dean, C. R. et al. Boron nitride substrates for high-quality graphene electronics. Nat Nano 5, 722–726 (2010).
Radisavljevic, B., Radenovic, A., Brivio, J., Giacometti, V. & Kis, A. Single-layer MoS2 transistors. Nat Nano 6, 147–150 (2011).
Lembke, D. & Kis, A. Breakdown of High-Performance Monolayer MoS2 Transistors. ACS Nano 6, 10070–10075 (2012).
Wang, H. et al. Integrated Circuits Based on Bilayer MoS2 Transistors. Nano Lett 12, 4674–4680 (2012).
Chang, Y.-H. et al. Highly Efficient Electrocatalytic Hydrogen Production by MoSx Grown on Graphene-Protected 3D Ni Foams. Adv Mater 25, 756–760 (2013).
Li, Y. et al. MoS2 Nanoparticles Grown on Graphene: An Advanced Catalyst for the Hydrogen Evolution Reaction. J Am Chem Soc 133, 7296–7299 (2011).
Kong, D. et al. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Lett 13, 1341–1347 (2013).
Mak, K. F., Lee, C., Hone, J., Shan, J. & Heinz, T. F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Physical Review Letters 105, 136805 (2010).
Coleman, J. N. et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 331, 568–571 (2011).
Gordon, R. A., Yang, D., Crozier, E. D., Jiang, D. T. & Frindt, R. F. Structures of exfoliated single layers of WS2, MoS2 and MoSe2 in aqueous suspension. Physical Review B 65, 125407 (2002).
Shi, Y. et al. van der Waals Epitaxy of MoS2 Layers Using Graphene As Growth Templates. Nano Lett 12, 2784–2791 (2012).
Liu, K.-K. et al. Growth of Large-Area and Highly Crystalline MoS2 Thin Layers on Insulating Substrates. Nano Lett 12, 1538–1544 (2012).
Lin, Y.-C. et al. Wafer-scale MoS2 thin layers prepared by MoO3 sulfurization. Nanoscale 4, 6637-6641 (2012).
Lee, Y.-H. et al. Synthesis of Large-Area MoS2 Atomic Layers with Chemical Vapor Deposition. Adv Mater 24, 2320–2325 (2012).
Zhan, Y., Liu, Z., Najmaei, S., Ajayan, P. M. & Lou, J. Large-Area Vapor-Phase Growth and Characterization of MoS2 Atomic Layers on a SiO2 Substrate. Small 8, 966–971 (2012).
Eda, G. et al. Photoluminescence from Chemically Exfoliated MoS2 . Nano Lett 11, 5111–5116 (2011).
Splendiani, A. et al. Emerging Photoluminescence in Monolayer MoS2 . Nano Lett 10, 1271–1275 (2010).
Brivio, J., Alexander, D. T. L. & Kis, A. Ripples and Layers in Ultrathin MoS2 Membranes. Nano Lett 11, 5148–5153 (2011).
Laursen, A. B., Kegnaes, S., Dahl, S. & Chorkendorff, I. Molybdenum sulfides-efficient and viable materials for electro - and photoelectrocatalytic hydrogen evolution. Energy & Environmental Science 5, 5577–5591 (2012).
Chen, Z. et al. Core–shell MoO3–MoS2 Nanowires for Hydrogen Evolution: A Functional Design for Electrocatalytic Materials. Nano Lett 11, 4168–4175 (2011).
Zong, X. et al. Photocatalytic H2 Evolution on MoS2/CdS Catalysts under Visible Light Irradiation. The Journal of Physical Chemistry C 114, 1963–1968 (2010).
Bonde, J., Moses, P. G., Jaramillo, T. F., Norskov, J. K. & Chorkendorff, I. Hydrogen evolution on nano-particulate transition metal sulfides. Faraday Discuss 140, 219–231 (2009).
Zhou, W. et al. Synthesis of Few-Layer MoS2 Nanosheet-Coated TiO2 Nanobelt Heterostructures for Enhanced Photocatalytic Activities. Small 9, 140–147 (2013).
Jaramillo, T. F. et al. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 317, 100–102 (2007).
Dong, X., Shi, Y., Huang, W., Chen, P. & Li, L.-J. Electrical Detection of DNA Hybridization with Single-Base Specificity Using Transistors Based on CVD-Grown Graphene Sheets. Adv Mater 22, 1649–1653 (2010).
He, S. et al. Graphene-Based High-Efficiency Surface-Enhanced Raman Scattering-Active Platform for Sensitive and Multiplex DNA Detection. Anal Chem 84, 4622–4627 (2012).
Qian, H., Zhu, Y. & Jin, R. Atomically precise gold nanocrystal molecules with surface plasmon resonance. Proceedings of the National Academy of Sciences. DOI 10.1073/pnas.1115307109 (2012).
Huang, F. & Baumberg, J. J. Actively Tuned Plasmons on Elastomerically Driven Au Nanoparticle Dimers. Nano Lett 10, 1787–1792 (2010).
Lu, Y.-C. et al. Platinum−Gold Nanoparticles: A Highly Active Bifunctional Electrocatalyst for Rechargeable Lithium−Air Batteries. J Am Chem Soc 132, 12170–12171 (2010).
Murdoch, M. et al. The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles. Nat Chem 3, 489–492 (2011).
Huang, X. et al. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Nat Commun 4, 1444 (2013).
Yick, S., Han, Z. J. & Ostrikov, K. Atmospheric microplasma-functionalized 3D microfluidic strips within dense carbon nanotube arrays confine Au nanodots for SERS sensing. Chem Commun 49, 2861–2863 (2013).
Rider, A. E., Kumar, S., Furman, S. A. & Ostrikov, K. Self-organized Au nanoarrays on vertical graphenes: an advanced three-dimensional sensing platform. Chem Commun 48, 2659–2661 (2012).
Helveg, S. et al. Atomic-Scale Structure of Single-Layer MoS2 Nanoclusters. Phys Rev Lett 84, 951–954 (2000).
Kim, K. K. et al. Enhancing the conductivity of transparent graphene films via doping. Nanotechnology 21, 285205 (2010).
Baker, M. A., Gilmore, R., Lenardi, C. & Gissler, W. XPS investigation of preferential sputtering of S from MoS2 and determination of MoSx stoichiometry from Mo and S peak positions. Appl Surf Sci 150, 255–262 (1999).
Huang, X. et al. Synthesis of hexagonal close-packed gold nanostructures. Nat Commun 2, 292 (2011).
Chen, M., Wu, B., Yang, J. & Zheng, N. Small Adsorbate-Assisted Shape Control of Pd and Pt Nanocrystals. Adv Mater 24, 862–879 (2012).
Wang, A.-J. et al. Melamine assisted one-pot synthesis of Au nanoflowers and their catalytic activity towards p-nitrophenol. New J Chem 36, 2286–2291 (2012).
Shi, Y. et al. Work Function Engineering of Graphene Electrode via Chemical Doping. Acs Nano 4, 2689–2694 (2010).
Li, H. et al. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv Funct Mater 22, 1385–1390 (2012).
Chakraborty, B. et al. Symmetry-dependent phonon renormalization in monolayer MoS2 transistor. Phys Rev B 85, 161403 (2012).
Braga, D., Gutiérrez Lezama, I., Berger, H. & Morpurgo, A. F. Quantitative Determination of the Band Gap of WS2 with Ambipolar Ionic Liquid-Gated Transistors. Nano Lett 12, 5218–5223 (2012).
Pu, J. et al. Highly Flexible MoS2 Thin-Film Transistors with Ion Gel Dielectrics. Nano Lett 12, 4013–4017 (2012).
Acknowledgements
This work is supported by SUTD-MIT international design center fund to Dr Yang Hui Ying. L.J. Li thanks the support from Academia Sinica (IAMS and Nano program) and National Science Council Taiwan (NSC-99-2112-M-001-021-MY3). S.F. Yu and L.M. Jin thank PolyU grant (Grant No. G-YJ73).
Author information
Authors and Affiliations
Contributions
Y. Shi and H.Y. Yang conceived and designed the study. Y. Shi and H.Y. Yang wrote the manuscript. Y. Shi, J. Huang and L. Jin performed the experiments. Y. Hsu helped to do the experiments. L.J. Li and S.F. Yu gave scientific advice. All the authors contributed to discussion and reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
supporting information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Shi, Y., Huang, JK., Jin, L. et al. Selective Decoration of Au Nanoparticles on Monolayer MoS2 Single Crystals. Sci Rep 3, 1839 (2013). https://doi.org/10.1038/srep01839
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01839
This article is cited by
-
High temperature anomalous Raman and photoluminescence response of molybdenum disulfide with sulfur vacancies
Scientific Reports (2023)
-
Two-dimensional MoS2/diamond based heterojunctions for excellent optoelectronic devices: current situation and new perspectives
Rare Metals (2023)
-
High-κ perovskite membranes as insulators for two-dimensional transistors
Nature (2022)
-
Fabrication of p-MoS2/n-Si heterojunction by AuCl3 treatment for IR detection
Applied Physics A (2022)
-
Theory of nonvolatile resistive switching in monolayer molybdenum disulfide with passive electrodes
npj 2D Materials and Applications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.