Abstract
Finding appropriate systems with a large spin splitting of metallic surface-state band which can be fabricated on silicon using routine technique is an essential step in combining Rashba-effect based spintronics with silicon technology. We have found that originally poor structural and electronic properties of the  surface can be substantially improved by adsorbing small amounts of suitable species (e.g., Tl, In, Na, Cs). The resultant surfaces exhibit a highly-ordered atomic structure and spin-split metallic surface-state band with a momentum splitting of up to 0.052 Å−1 and an energy splitting of up to 190 meV at the Fermi level. The family of adsorbate-modified
surface can be substantially improved by adsorbing small amounts of suitable species (e.g., Tl, In, Na, Cs). The resultant surfaces exhibit a highly-ordered atomic structure and spin-split metallic surface-state band with a momentum splitting of up to 0.052 Å−1 and an energy splitting of up to 190 meV at the Fermi level. The family of adsorbate-modified  surfaces, on the one hand, is thought to be a fascinating playground for exploring spin-splitting effects in the metal monolayers on a semiconductor and, on the other hand, expands greatly the list of material systems prospective for spintronics applications.
surfaces, on the one hand, is thought to be a fascinating playground for exploring spin-splitting effects in the metal monolayers on a semiconductor and, on the other hand, expands greatly the list of material systems prospective for spintronics applications.
Similar content being viewed by others
Introduction
Generation of spin-polarized electrons on the basis of Rashba spin splitting in the two-dimensional electron-gas systems on semiconductors is considered to be an essential step in developing semiconductor spintronics applications1. To reach the goal, three requirements have to be satisfied as follows. First, spin splitting should be large enough to allow operations of the device at room temperature. Second, the surface-state band has to be metallic to allow significant spin transport. Third, the substrate should be a semiconductor as a large bulk current in metallic substrate would sweep off the surface spin signal. In addition, due to device application reasons it is highly desirable that the substrate would be a silicon, the most widely used semiconductor material. The last demand is that the structure could be easily fabricated using routine technique, e.g., molecular beam epitaxy.
The last decade has been marked by the step-by-step progress in this direction. The surface Rashba effect was first found on the metal surfaces like Au(111)2,3 and Bi(111)4,5 and a giant spin splitting was detected on Bi-covered Ag(111)6,7. The latter finding indicates that a large spin splitting is possible (and even enhances) when only a monolayer of a heavy element is placed on a surface of a light element. This discovery stimulated expanding the search area to semiconductor surfaces such as Si and Ge covered by heavy-metal monolayers. Large Rashba splitting has been found on Bi/Si(111)8,9,10, Bi/Ge(111)11, Tl/Si(111)12,13 and Pt/Si(110)14 surfaces but it occurs in the non-metallic surface-state bands. The first metal/semiconductor reconstruction with a spin splitting of metallic surface-state band found was the  15,16 followed by the
15,16 followed by the  17,18,19. As indicated in Ref. 15, the spin-splitting effect does not depend on any peculiar property of Ge, hence it seems possible to realize a similar electronic structure on a Si surface. In this respect, the
17,18,19. As indicated in Ref. 15, the spin-splitting effect does not depend on any peculiar property of Ge, hence it seems possible to realize a similar electronic structure on a Si surface. In this respect, the  reconstruction is thought to be a promising candidate as its atomic arrangement (described by the conjugated honeycomb chained-trimer (CHCT) model20,21 (see Fig. 1e)) is similar to that of the
reconstruction is thought to be a promising candidate as its atomic arrangement (described by the conjugated honeycomb chained-trimer (CHCT) model20,21 (see Fig. 1e)) is similar to that of the  22,23 and a strong Rashba-type spin-orbit splitting in it has already been predicted theoretically21. Substantial Rashba effect observed for self-assembled Au nanowires on vicinal Si surfaces24,25 also supports the suggested prospects of the Au/Si material systems. However, the structural and electronic properties of
22,23 and a strong Rashba-type spin-orbit splitting in it has already been predicted theoretically21. Substantial Rashba effect observed for self-assembled Au nanowires on vicinal Si surfaces24,25 also supports the suggested prospects of the Au/Si material systems. However, the structural and electronic properties of  surface are actually poor due to a presence of random domain walls. Fortunately, the breakthrough way was found to improve the surface, namely, adding small amount of In eliminates completely domain walls26 and enhances metallic surface band filling27. It has been recognized that after the transformation the basic CHCT structure of the original
surface are actually poor due to a presence of random domain walls. Fortunately, the breakthrough way was found to improve the surface, namely, adding small amount of In eliminates completely domain walls26 and enhances metallic surface band filling27. It has been recognized that after the transformation the basic CHCT structure of the original  surface is preserved, while the indium adsorbate forms a 2D gas of adatoms on it21,26,27.
surface is preserved, while the indium adsorbate forms a 2D gas of adatoms on it21,26,27.
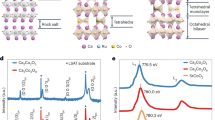
Adsorbate-induced transformations in structural and electronic properties of Au/Si(111).
STM images and ARPES spectra taken in the first  surface Brillouin zone (SBZ) along the
surface Brillouin zone (SBZ) along the  direction from (a) pristine
direction from (a) pristine  surface and the same surface after adsorption of 0.15 ± 0.05 ML of (b) Tl, (c) In and (d) Na. Scale of the STM images is 250 × 250 Å2, that of the insets is 50 × 50 Å2. Note that ARPES data are confined to the interior of SBZ as exact position of the
surface and the same surface after adsorption of 0.15 ± 0.05 ML of (b) Tl, (c) In and (d) Na. Scale of the STM images is 250 × 250 Å2, that of the insets is 50 × 50 Å2. Note that ARPES data are confined to the interior of SBZ as exact position of the  point is at 0.546 Å−1. (e) Atomic arrangement (CHCT model) of the
point is at 0.546 Å−1. (e) Atomic arrangement (CHCT model) of the  surface consisting of Au trimers (shown by orange circles) and Si trimers (shown by blue circles) residing on Si(111) bilayer (shown by black circles) and sketch of reciprocal space geometry with boundaries of the first
surface consisting of Au trimers (shown by orange circles) and Si trimers (shown by blue circles) residing on Si(111) bilayer (shown by black circles) and sketch of reciprocal space geometry with boundaries of the first  SBZ given by black lines (dashed hexagon depicts the 1 × 1 SBZ). The high symmetry points are marked by circles.
SBZ given by black lines (dashed hexagon depicts the 1 × 1 SBZ). The high symmetry points are marked by circles.
In the present study, we have revealed that the above effect is not a peculiar feature of only In, but is a common trait for a set of adsorbate species (e.g., Na, Cs and Tl). Scanning tunneling microscopy (STM) and low-energy electron diffraction (LEED) observations have shown that adsorption of each above mentioned species onto the  produces a homogeneous well-ordered surface. In addition, angle-resolved photoelectron spectroscopy (ARPES) and spin-resolved ARPES data as well as first-principles calculations clearly demonstrate occurrence of the well-defined metallic surface-state band with a large spin splitting. Thus, finding such a family of adsorbate-modified Au/Si(111) systems (having the same
produces a homogeneous well-ordered surface. In addition, angle-resolved photoelectron spectroscopy (ARPES) and spin-resolved ARPES data as well as first-principles calculations clearly demonstrate occurrence of the well-defined metallic surface-state band with a large spin splitting. Thus, finding such a family of adsorbate-modified Au/Si(111) systems (having the same  reconstruction) paves a way to combine Rashba-effect based spintronics with a silicon technology.
reconstruction) paves a way to combine Rashba-effect based spintronics with a silicon technology.
Results
Figure 1a illustrates structural and electronic properties of the pristine  surfaces. One can see that the main characteristic structural feature of the surface is a disordered meandering domain-wall network28. Such a surface displays the
surfaces. One can see that the main characteristic structural feature of the surface is a disordered meandering domain-wall network28. Such a surface displays the  LEED pattern with cloudlike diffraction streaks in between
LEED pattern with cloudlike diffraction streaks in between  spots27,29. As for its electronic properties, the surface is known to be metallic29, but its metallicity is not strongly expressed: the surface-state spectral features are smeared due to the domain walls and electron filling of the metallic S1 surface band is rather low, ~0.1 electrons per unit cell27. In the ARPES spectra, the S1 band is invisible in the first surface Brillouin zone (SBZ) (Fig. 1a) and its faint traces can be detected only in the higher SBZs27.
spots27,29. As for its electronic properties, the surface is known to be metallic29, but its metallicity is not strongly expressed: the surface-state spectral features are smeared due to the domain walls and electron filling of the metallic S1 surface band is rather low, ~0.1 electrons per unit cell27. In the ARPES spectra, the S1 band is invisible in the first surface Brillouin zone (SBZ) (Fig. 1a) and its faint traces can be detected only in the higher SBZs27.
Adding 0.15 ± 0.05 ML of Tl, In, Na or Cs atoms to this surface produces strong effect on its structure, namely, the domain walls disappear and homogeneous well-ordered surface forms (Fig. 1b–d, upper panel). Consequently, a sharp  LEED pattern without any other features develops (not shown). STM observations reveal that the adsorbed species are present at the surface as adatoms which are mobile at room temperature but can be frozen at fixed positions by cooling the sample down to ~100 K (except for Cs atoms which motion is frozen only at ~30 K).
LEED pattern without any other features develops (not shown). STM observations reveal that the adsorbed species are present at the surface as adatoms which are mobile at room temperature but can be frozen at fixed positions by cooling the sample down to ~100 K (except for Cs atoms which motion is frozen only at ~30 K).
ARPES observations demonstrate that upon adding adsorbates (Fig. 1b–d, lower panel) all spectral features of the initial  surface are preserved but become noticeably sharp due to removal of domain walls. All bands are shifted down towards the higher binding energy and the well-defined metallic S1 surface state band develops (being clearly seen even in the first SBZ). Electron filling of the S1 band increases three to seven times (up to ~0.3–0.7 electrons per unit cell). Next important feature is that the metallic S1 surface state is split. The splitting being modest along the
surface are preserved but become noticeably sharp due to removal of domain walls. All bands are shifted down towards the higher binding energy and the well-defined metallic S1 surface state band develops (being clearly seen even in the first SBZ). Electron filling of the S1 band increases three to seven times (up to ~0.3–0.7 electrons per unit cell). Next important feature is that the metallic S1 surface state is split. The splitting being modest along the  direction becomes substantial along the
direction becomes substantial along the  direction (Fig. 2a). To reveal a spin-split character of the metallic surface states, we have performed the spin-resolved ARPES measurement on Tl-adsorbed system. Figure 2b shows the spin-resolved energy distribution curves of
direction (Fig. 2a). To reveal a spin-split character of the metallic surface states, we have performed the spin-resolved ARPES measurement on Tl-adsorbed system. Figure 2b shows the spin-resolved energy distribution curves of  and
and  subbands at k|| = 0.30 Å−1 (here and below we refer inner and outer subbands as
subbands at k|| = 0.30 Å−1 (here and below we refer inner and outer subbands as  and
and  , respectively). This result clearly shows that the S1 state is spin-split and the spin orientations are opposite in A and B subbands.
, respectively). This result clearly shows that the S1 state is spin-split and the spin orientations are opposite in A and B subbands.
Splitting of dispersion curves measured by ARPES and spin-resolved ARPES.
(a) Band structure along the  of the Tl-adsorbed
of the Tl-adsorbed  surface determined with spin-unpolarized ARPES. Data are confined to the interior of SBZ as exact positions of the
surface determined with spin-unpolarized ARPES. Data are confined to the interior of SBZ as exact positions of the  and
and  points are at 0.546 Å−1 and 0.630 Å−1, respectively. (b) Spin-resolved ARPES spectra taken for the same surface at a fixed k|| = 0.30 Å−1 in the
points are at 0.546 Å−1 and 0.630 Å−1, respectively. (b) Spin-resolved ARPES spectra taken for the same surface at a fixed k|| = 0.30 Å−1 in the  direction.
direction.
Figure 3 summarizes the results for various adsorbate species. All spectra have a qualitatively similar appearance but the splitting value varies. Momentum splitting at the Fermi level Δk|| ranges from ~0.018 Å−1 obtained for Cs to that of ~0.052 Å−1 for Tl. Consequently, energy splitting ΔEF changes in the range from ~100 meV to ~190 meV. From the graph shown in Fig. 3b one can conclude that the splitting value is essentially controlled by position of the Fermi level, i.e., by the electron filling of the S1 band. The concept is illustrated in Fig. 3c where the experimental dots from Fig. 3b are superposed on the  and
and  dispersion curves of the calculated band structure. One can see that by choosing appropriate adsorbate species the Fermi level position can be tuned within the range of ~350 meV (shown by the pink shaded area in Fig. 3c). Position of Fermi level also varies slightly depending on concentration of a given adsorbate.
dispersion curves of the calculated band structure. One can see that by choosing appropriate adsorbate species the Fermi level position can be tuned within the range of ~350 meV (shown by the pink shaded area in Fig. 3c). Position of Fermi level also varies slightly depending on concentration of a given adsorbate.
Effect of adsorbate species.
(a) Fragments of the ARPES spectra taken in the  direction near the Fermi level of the Tl-, In-, Na- and Cs-adsorbed
direction near the Fermi level of the Tl-, In-, Na- and Cs-adsorbed  surfaces. (b) Momentum splitting at the Fermi level Δk|| plotted as a function of the Fermi wave vector kF for
surfaces. (b) Momentum splitting at the Fermi level Δk|| plotted as a function of the Fermi wave vector kF for  and
and  bands measured for various species (as indicated in the graph). (c) Calculated band structure along the
bands measured for various species (as indicated in the graph). (c) Calculated band structure along the  with the experimental dots from (b) superposed on the
with the experimental dots from (b) superposed on the  and
and  dispersion curves. Surface state bands are shown by filled yellow circles, shaded region indicates projected bulk bands. Range of the varied Fermi level position is indicated by the pink shaded area.
dispersion curves. Surface state bands are shown by filled yellow circles, shaded region indicates projected bulk bands. Range of the varied Fermi level position is indicated by the pink shaded area.
Figure 4a shows the ARPES Fermi surface of the Tl-adsorbed  . One can clearly see the two Fermi contours of which the outer (corresponding to the
. One can clearly see the two Fermi contours of which the outer (corresponding to the  band) has an almost circular shape while the inner (corresponding to the
band) has an almost circular shape while the inner (corresponding to the  band) has a shape of a smoothed hexagon. As the hexagon corners lies at the
band) has a shape of a smoothed hexagon. As the hexagon corners lies at the  directions, these are the directions of the minimal splitting, while the greatest splitting is at the hexagon sides (i.e., along the
directions, these are the directions of the minimal splitting, while the greatest splitting is at the hexagon sides (i.e., along the  directions). One can see that the calculated constant energy contours (Fig. 4b) properly reproduce all the principal features of the experimentally derived Fermi surface map. The only small deviation that can be noticed is the discrepancy between the calculated and experimental splitting along the
directions). One can see that the calculated constant energy contours (Fig. 4b) properly reproduce all the principal features of the experimentally derived Fermi surface map. The only small deviation that can be noticed is the discrepancy between the calculated and experimental splitting along the  direction where theory yields slightly underestimated value (Fig. 4c).
direction where theory yields slightly underestimated value (Fig. 4c).
Fermi surface and spin texture.
(a) Symmetrized Fermi surface of the Tl-adsorbed  surface determined with ARPES. The k-space area where the ARPES measurements were carried out is outlined by a dashed blue line. (b) Constant energy contours at energy marked by dashed blue line in Fig. 3c. Arrows along the contours and their length indicate the in-plane spin component. The out-of-plane spin component is indicated by the colour with red and blue corresponding to the upward and downward directions, respectively. White colour indicates fully in-plane spin alignment. (c) Azimuthal dependence of the Fermi wave vector kF for the
surface determined with ARPES. The k-space area where the ARPES measurements were carried out is outlined by a dashed blue line. (b) Constant energy contours at energy marked by dashed blue line in Fig. 3c. Arrows along the contours and their length indicate the in-plane spin component. The out-of-plane spin component is indicated by the colour with red and blue corresponding to the upward and downward directions, respectively. White colour indicates fully in-plane spin alignment. (c) Azimuthal dependence of the Fermi wave vector kF for the  and
and  bands. Experimental and calculated data are presented by dots and solid lines, respectively. (d) Azimuthal dependencies of the spin components, including in-plane components in the directions tangential and normal to the Fermi contour (upper panel) and out-of-plane component (lower panel).
bands. Experimental and calculated data are presented by dots and solid lines, respectively. (d) Azimuthal dependencies of the spin components, including in-plane components in the directions tangential and normal to the Fermi contour (upper panel) and out-of-plane component (lower panel).
Figure 4b also shows the clockwise and counterclockwise spin helicity for the inner and outer contours, respectively, with abrupt change of the sign for out-of-plane spin component at the  directions, which is intrinsic feature of the Rashba-split surface states at hexagonal surfaces12. The detailed spin texture is illustrated in Fig. 4d. Figure 4d (upper panel) shows the azimuthal dependencies of in-plane components in the tangential and normal directions to the
directions, which is intrinsic feature of the Rashba-split surface states at hexagonal surfaces12. The detailed spin texture is illustrated in Fig. 4d. Figure 4d (upper panel) shows the azimuthal dependencies of in-plane components in the tangential and normal directions to the  and
and  Fermi contours. One can see that tangential components for both bands demonstrate a very similar behavior with sharp maxima in the
Fermi contours. One can see that tangential components for both bands demonstrate a very similar behavior with sharp maxima in the  directions and wide minima around the
directions and wide minima around the  directions. The normal component (a signature of the Dresselhaus term) is small for the inner
directions. The normal component (a signature of the Dresselhaus term) is small for the inner  band but it becomes noticeable for the outer
band but it becomes noticeable for the outer  band and demonstrates an undulating behavior. Maximal deviation of the in-plane spin component from a purely tangential is ~3° and ~16° for the
band and demonstrates an undulating behavior. Maximal deviation of the in-plane spin component from a purely tangential is ~3° and ~16° for the  and
and  subbands, respectively. For both bands, the out-of-plane z component (Fig. 4d, lower panel) remains almost constant in the wide area near the
subbands, respectively. For both bands, the out-of-plane z component (Fig. 4d, lower panel) remains almost constant in the wide area near the  directions and abruptly changes its sign going through zero while crossing the
directions and abruptly changes its sign going through zero while crossing the  directions. As a result, spin has a fully in-plane alignment there. The calculations have also revealed that changing the Fermi level position affects the spin texture of the S1 band. Upon the downward shift of the Fermi level from its highest position, corresponding to Tl-adsorbed surface to its lowest position obtained on Cs-modified Au/Si(111) surface (see pink stripe in Fig. 3c) the maximal deviation of the in-plane spin component from a purely tangential increases up to ~24° for the
directions. As a result, spin has a fully in-plane alignment there. The calculations have also revealed that changing the Fermi level position affects the spin texture of the S1 band. Upon the downward shift of the Fermi level from its highest position, corresponding to Tl-adsorbed surface to its lowest position obtained on Cs-modified Au/Si(111) surface (see pink stripe in Fig. 3c) the maximal deviation of the in-plane spin component from a purely tangential increases up to ~24° for the  subband while that for the
subband while that for the  subband remains ~3°. At the same time this shift of the Fermi level leads to decreasing maximal out-of-plane spin tilt angle from ~ 68(63)° to ~ 64(53)° for the outer(inner) subband.
subband remains ~3°. At the same time this shift of the Fermi level leads to decreasing maximal out-of-plane spin tilt angle from ~ 68(63)° to ~ 64(53)° for the outer(inner) subband.
Discussion
Our results show that chemically very different species, alkali metals, Na and Cs and heavy group-III metals, In and Tl, when being adsorbed onto the  surface, affect its structural and electronic properties in a very similar way, namely, eliminate domain walls and donate electrons to the metallic S1 surface state band. DFT calculations for In-adsorbed surface26 showed that removal of domain walls is due to a stress-relieving effect produced by In adsorption. However, origin of this effect was not disclosed. Typically, surface lattice stress changes when foreign atoms become incorporated directly into the lattice and the greater the size difference between the host and foreign atoms the greater the stress. This typical scenario is apparently not held for the present case where foreign adatoms are not incorporated into the lattice and their atomic size does not play a decisive role. As an alternative, we suggest that adsorbates can affect the surface lattice stress by donating electrons to the substrate surface layer. As a result, the top layer possesses a non-compensated charge which means adding a Coulomb repulsion term into the interactions between surface atoms, hence changing the surface stress.
surface, affect its structural and electronic properties in a very similar way, namely, eliminate domain walls and donate electrons to the metallic S1 surface state band. DFT calculations for In-adsorbed surface26 showed that removal of domain walls is due to a stress-relieving effect produced by In adsorption. However, origin of this effect was not disclosed. Typically, surface lattice stress changes when foreign atoms become incorporated directly into the lattice and the greater the size difference between the host and foreign atoms the greater the stress. This typical scenario is apparently not held for the present case where foreign adatoms are not incorporated into the lattice and their atomic size does not play a decisive role. As an alternative, we suggest that adsorbates can affect the surface lattice stress by donating electrons to the substrate surface layer. As a result, the top layer possesses a non-compensated charge which means adding a Coulomb repulsion term into the interactions between surface atoms, hence changing the surface stress.
We have found a number of adsorbate species (Tl, In, Na and Cs) which make the  surface suitable for observing significant spin-orbit splitting. We believe that the list of such adsorbates can be extended (at least, at the expense of the left alkali metals). The main requirements for candidate species seem to be as follows. First, they are metals that could easily donate a sufficient amount of electrons to the surface state band. This could be attained with species like monovalent alkali metals with high electropositivity and/or species having several valent electrons, as Group-III metals. In this respect, it is worth noting the very recent finding that extra Au or Ge atoms produce a similar doping effect on
surface suitable for observing significant spin-orbit splitting. We believe that the list of such adsorbates can be extended (at least, at the expense of the left alkali metals). The main requirements for candidate species seem to be as follows. First, they are metals that could easily donate a sufficient amount of electrons to the surface state band. This could be attained with species like monovalent alkali metals with high electropositivity and/or species having several valent electrons, as Group-III metals. In this respect, it is worth noting the very recent finding that extra Au or Ge atoms produce a similar doping effect on  surface30, thus extending further the list of possible candidate species. Second, they have to preserve the original Au trimer structure (which is believed to be responsible for the spin-splitting effect18) or, in other words, they should not form 2D alloys with Au on Si(111) surface. For example, in contrast to In and Tl, the other group-III metals, Al and Ga, are not suitable, as they form binary reconstructions with Au,
surface30, thus extending further the list of possible candidate species. Second, they have to preserve the original Au trimer structure (which is believed to be responsible for the spin-splitting effect18) or, in other words, they should not form 2D alloys with Au on Si(111) surface. For example, in contrast to In and Tl, the other group-III metals, Al and Ga, are not suitable, as they form binary reconstructions with Au,  31, (Au,Al)/Si(111)2 × 231 and
31, (Au,Al)/Si(111)2 × 231 and  .
.
As the list is open, many species are expected to produce a similar effect on the structure and electronic properties of the  surface. However, similarity does not mean identity, that makes the family of adsorbate-modified
surface. However, similarity does not mean identity, that makes the family of adsorbate-modified  reconstructions to be a promising playground for exploring spin-splitting effects as a function of structural parameters tuned by adsorption of particular species. On the other hand, they represent a wide set of surface systems for the choice of the proper ones for spintronic device applications.
reconstructions to be a promising playground for exploring spin-splitting effects as a function of structural parameters tuned by adsorption of particular species. On the other hand, they represent a wide set of surface systems for the choice of the proper ones for spintronic device applications.
Another degree of freedom for tailoring electronic properties stems from ability to intermix Si and Ge into a desired SixGe1–x alloy layer. Though Si and Ge are akin elements and their  reconstructions have the same atomic arrangement, their electronic structures exhibit essential differences. While the
reconstructions have the same atomic arrangement, their electronic structures exhibit essential differences. While the  surface has a single metallic S1 band, the
surface has a single metallic S1 band, the  surface in addition to the electronic S1 band has the hole bands dispersing up to the Fermi level17,18. The spin textures of the S1 bands for these surfaces are similar but also not identical, namely, contribution of the Dresselhaus terms for the
surface in addition to the electronic S1 band has the hole bands dispersing up to the Fermi level17,18. The spin textures of the S1 bands for these surfaces are similar but also not identical, namely, contribution of the Dresselhaus terms for the  is substantially greater19. Thus, the variable
is substantially greater19. Thus, the variable  surfaces might be a new interesting object for exploring spin-splitting effects in metal monolayers on semiconductor.
surfaces might be a new interesting object for exploring spin-splitting effects in metal monolayers on semiconductor.
Methods
Sample preparation method
The STM and ARPES experiments were performed in an ultra-high-vacuum system with a base pressure of ~2.0 × 10−10 Torr. Atomically-clean Si(111)7 × 7 surfaces were prepared in situ by flashing to 1280°C after the samples were first outgassed at 600°C for several hours. Pristine  surfaces were formed by Au deposition onto Si(111)7 × 7 surface held at ~600°C. The adsorbate-modified Au/Si(111) surfaces were prepared by adsorbing 0.15 ± 0.05 ML of a given species, In, Na or Cs, onto the surface held at ~350°C. Due to significant desorption, deposition of Tl was performed at room temperature followed by annealing at ~350°C. Adsorbate deposition was terminated when STM shows domain-wall-free surface and LEED displays a sharp
surfaces were formed by Au deposition onto Si(111)7 × 7 surface held at ~600°C. The adsorbate-modified Au/Si(111) surfaces were prepared by adsorbing 0.15 ± 0.05 ML of a given species, In, Na or Cs, onto the surface held at ~350°C. Due to significant desorption, deposition of Tl was performed at room temperature followed by annealing at ~350°C. Adsorbate deposition was terminated when STM shows domain-wall-free surface and LEED displays a sharp  pattern without any other features.
pattern without any other features.
STM
STM images were acquired using Omicron variable-temperature STM-XA operating in a constant-current mode. Electrochemically-etched W tips and mechanically cut PtIr tips were used as STM probes after annealing in vacuum.
ARPES and spin-resolved ARPES
ARPES measurements were conducted in the ultrahigh vacuum chamber Omicron MULTIPROBE using VG Scienta R3000 electron analyzer and high-flux He discharge lamp (hν = 21.2 eV) with toroidal-grating monochromator as a light source. Spin-resolved ARPES and part of ARPES measurements were performed at the novel SR-ARPES facility of RGL at BESSY, U125-2_SGM beamline using VG Scienta R4000 analyzer with Mott detector operating at 25 keV.
First-principles calculations
Our calculations were based on DFT as implemented in the Vienna ab initio simulation package VASP32,33, using a planewave basis and the projector augmented-wave approach34 for describing the electron-ion interaction. The generalized gradient approximation (GGA) of Perdew, Burke and Ernzerhof (PBE)35 has been used for the exchange correlation (XC) potential. The Hamiltonian contains the scalar relativistic corrections and the spin-orbit interaction (SOI) was taken into account by the second variation method as has been implemented in VASP by Kresse and Lebacq36. To simulate the  -CHCT reconstruction we use a slab consisting of 12 bilayers. Hydrogen atoms were used to passivate the Si dangling bonds at the bottom of the slab. Both bulk Si lattice constant and the atomic positions within the three topmost bilayers of the slab were optimized including SOI self-consistently. The silicon atoms of deeper layers were kept fixed at the bulk crystalline positions.
-CHCT reconstruction we use a slab consisting of 12 bilayers. Hydrogen atoms were used to passivate the Si dangling bonds at the bottom of the slab. Both bulk Si lattice constant and the atomic positions within the three topmost bilayers of the slab were optimized including SOI self-consistently. The silicon atoms of deeper layers were kept fixed at the bulk crystalline positions.
References
Sinova, J. et al. Universal intrinsic spin Hall effect. Phys. Rev. Lett. 92, 126603 (2004).
LaShell, S., McDougall, B. A. & Jensen, E. Spin splitting of an Au(111) surface state band observed with angle resolved photoelectron spectroscopy. Phys. Rev. Lett. 77, 3419–3422 (1996).
Hoesch, M. et al. Spin structure of the Shockley surface state on Au(111). Phys. Rev. B 69, 241401 (2004).
Koroteev, Y. M. et al. Strong spin-orbit splitting on Bi surfaces. Phys. Rev. Lett. 93, 046403 (2004).
Kimura, A. et al. Strong Rashba-type spin polarization of the photocurrent from bulk continuum states: Experiment and theory for Bi(111). Phys. Rev. Lett. 105, 076804 (2010).
Ast, C. R. et al. Giant spin splitting through surface alloying. Phys. Rev. Lett. 98, 186807 (2007).
Bihlmayer, G., Blügel, S. & Chulkov, E. V. Enhanced Rashba spin-orbit splitting in Bi/Ag(111) and Pb/Ag(111) surface alloys from first principles. Phys. Rev. B 75, 195414 (2007).
Gierz, I. et al. Silicon surface with giant spin splitting. Phys. Rev. Lett. 103 (4), 046803–4 (2009).
Sakamoto, K. et al. Peculiar Rashba splitting originating from the two-dimensional symmetry of the surface. Phys. Rev. Lett. 103 (15), 156801–4 (2009).
Frantzeskakis, E., Pons, S. & Grioni, M. Band structure scenario for the giant spin-orbit splitting observed at the Bi/Si(111) interface. Phys. Rev. B 82 (8), 085440–11 (2010).
Ohtsubo, Y. et al. Spin-polarized semiconductor surface states localized in subsurface layers. Phys. Rev. B 82 (20), 201307–4 (2010).
Sakamoto, K. et al. Abrupt rotation of the Rashba spin to the direction perpendicular to the surface. Phys. Rev. Lett. 102 (9), 096805–4 (2009).
Ibañez-Azpiroz, J., Eiguren, A. & Bergara, A. Relativistic effects and fully spin-polarized Fermi surface at the Tl/Si(111) surface. Phys. Rev. B 84 (12), 125435–6 (2011).
Park, J. et al. Self-assembled nanowires with giant Rashba split band. Phys. Rev. Lett. 110 (3), 036801–5 (2013).
Yaji, K. et al. Large Rashba spin plitting of a metallic surface-state band on a semiconductor surface. Nature Commun. 1, 17–5 (2010).
Yaji, K., Hatta, S., Aruga, T. & Okuyama, H. Structural and electronic properties of the surface studied by PES and first-principles calculations. Phys. Rev. B 86 (23), 235317–6 (2012).
Höpfner, P. et al. Electronic band structure of the two-dimensional metallic electron system Au/Ge(111). Phys. Rev. B 83 (23), 235435–8 (2011).
Nakatsuji, K. et al. Anisotropic splitting and spin polarization of metallic bands due to spin-orbit interaction at the -Au surface. Phys. Rev. B 84 (3), 035436–4 (2011).
Höpfner, P. et al. Three-dimensional spin rotations at the Fermi surface of a strongly spin-orbit coupled surface system. Phys. Rev. Lett. 108 (18), 186801–5 (2012).
Ding, Y. G., Chan, C. T. & Ho, K. M. Theoretical investigation of the structure of the surface. Surf. Sci. 275 (3), L691–L696 (1992).
Hsu, C. H., Lin, W. H., Ozolins, V. & Chuang, F. C. Electronic structure of the indium-adsorbed surface: A first-principles study. Phys. Rev. B 85 (15), 155401–7 (2012).
Howes, P. B., Norris, C., Finney, M. S., Vlieg, E. & van Silfhout, R. G. Structure of determined by surface X-ray-diffraction. Phys. Rev. B 48 (3), 1632–1642 (1993).
Over, H., Wang, C. P. & Jona, F. Atomic bond configuration of : A low-energy electron-diffraction study. Phys. Rev. B 51 (7), 4231–4235 (1995).
Barke, I., Zheng, F., Rügheimer, T. K. & Himpsel, F. J. Experimental evidence for spin-split bands in a one-dimensional chain structure. Phys. Rev. Lett. 97 (22), 226405–4 (2006).
Okuda, T. et al. Large out-of-plane spin polarization in a spin-splitting one-dimensional metallic surface state on Si(557)-Au. Phys. Rev. B 82 (16), 161410–4 (2010).
Gruznev, D. V. et al. phase modified by In adsorption: Stabilization of a homogeneous surface by stress relief. Phys. Rev. B 73 (11), 115335–7 (2006).
Kim, J. K. et al. Two-dimensional electron gas formed on the indium-adsorbed surface. Phys. Rev. B 80 (7), 075312–7 (2009).
Nagao, T. et al. Structural phase transitions of : Phase transitions in domain-wall configurations. Phys. Rev. B 57 (16), 10100–10109 (1998).
Zhang, H. M., Balasubramanian, T. & Uhrberg, R. I. G. Surface electronic structure study of Au/Si(111) reconstruction: Observation of crystal-to-glass transition. Phys. Rev. B 66 (16), 165402–6 (2002).
Nakatsuji, K., Motomura, Y., Niikura, R. & Komori, F. Selective doping in a surface band and atomic structures of the Ge(111)( )R30°-Au surface. J. Phys.:Cond. Matt. 25 (4), 045007–9 (2013).
Khramtsova, E. A., Saranin, A. A., Chub, A. B. & Lifshits, V. G. Al and Au binary surface phases on the Si(111) surface. Surf. Sci. 331/333 (1), 594–599 (1995).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558 (1993).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Lebacq, O. The VASP manual. http://cms.mpi.univie.ac.at/vasp/vasp/vasp.html. Retrieved April 22, 2013.
Acknowledgements
Part of this work was supported by the Russian Foundation for Basic Research (Grant Nos. 11-02-98515r, 12-02-00416, 12-02-00430 and 12-02-31745), the Ministry of Education and Science of the RF (Grant Nos. 8022, 8581, 2.8575.2013 and 2.1004.2011), NSh-774.2012.2, the Basque Country Government, Departamento de Educación, Universidades e Investigación (Grant No. IT-366-07), the Spanish Ministerio de Ciencia e Innovación (Grant No. FIS2010-19609-C02-00), German-Russian Interdisciplinary Science Center (G-RISC) funded by the German Federal Foreign Office via the German Academic Exchange Service (DAAD) and Helmholtz Zentrum Berlin fur Materialien und Energie for support within a bilateral Program “Russian-German Laboratory” at BESSY-II. We thank S.S. Tsirkin for help with graphical presentation of results. D.U. and A.F. acknowledge support from SPbU grant.
Author information
Authors and Affiliations
Contributions
D.V.G. and L.V.B. carried out ARPES and STM under the support of A.A.Y. and A.Y.T. L.V.B., D.U., O.V. and A.F. carried out ARPES and spin-resolved ARPES at BESSY-II with guidance from D.V.V. S.V.E. and E.V.C. carried out the theoretical calculation. D.V.G., A.V.Z. and A.A.S. analyzed the data and wrote the manuscript with input from S.V.E. and E.V.C. and conceived and coordinated the project.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Bondarenko, L., Gruznev, D., Yakovlev, A. et al. Large spin splitting of metallic surface-state bands at adsorbate-modified gold/silicon surfaces. Sci Rep 3, 1826 (2013). https://doi.org/10.1038/srep01826
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01826
This article is cited by
-
Purely one-dimensional bands with a giant spin-orbit splitting: Pb nanoribbons on Si(553) surface
Scientific Reports (2017)
-
Synthesis of two-dimensional TlxBi1−x compounds and Archimedean encoding of their atomic structure
Scientific Reports (2016)
-
Spin-helical Dirac states in graphene induced by polar-substrate surfaces with giant spin-orbit interaction: a new platform for spintronics
Scientific Reports (2014)
-
A Strategy to Create Spin-Split Metallic Bands on Silicon Using a Dense Alloy Layer
Scientific Reports (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.