Abstract
Increased connectivity with the mainland has led to the arrival of many invasive species to the Galápagos Islands, including novel pathogens, threatening the archipelago's unique fauna. Here we consider the potential role of the mosquito Aedes taeniorhynchus in maintaining the flavivirus West Nile virus [WNV] should it reach the islands. We report on three components of vectorial capacity - vector competency, distributional abundance and host-feeding. In contrast to USA strains, Galápagos A. taeniorhynchus is a competent and efficient WNV vector, capable of transmission at 5 days post-exposure. Based on 25 blood-meals, mammalian feeding suggests a potential bridge vector role should contact with key amplification taxa occur. Vector population abundance is driven primarily by climatic factors, peaking between January and March. As a ubiquitous competent vector, A. taeniorhynchus may facilitate future WNV establishment, therefore it is vital to ensure the biosecurity of Galápagos to prevent introductions of pathogens such as WNV.
Similar content being viewed by others
Introduction
The Galápagos Islands hold immense conservation value. The archipelago, famous for its unique range of endemic fauna and flora which has evolved in isolation over millennia, is recognised as a United Nations Education Scientific and Cultural Organisation [UNESCO] World Heritage site1 and generates considerable economic income for Ecuador through ecotourism. However, as increasing connectivity with the continental Americas, primarily driven by a rapidly expanding tourism industry and growing human population, diminish geographic barriers, the Galápagos ecosystem is threatened by invasive species and novel pathogens2,3,4. Here we quantify epidemiological factors key to the establishment and transmission of West Nile virus [WNV] should this mosquito-borne virus be introduced to Galápagos, focusing on Aedes taeniorhynchus, a native species and the most abundant and widely-distributed mosquito on the islands5.
West Nile virus (Flaviviridae) is maintained in an avian host - mosquito vector enzootic cycle, but affects a broad range of hosts6. After emergence in the USA in 1999, WNV showed unprecedented severity and range expansion6,7. It was associated with high rates of mortality with subsequent declines of several US bird species, leading to concern over potential impacts in the rest of the Americas8. Although WNV has yet to be detected in continental Ecuador, the virus is known to have reached South America by 20049,10. While this region has not experienced the same impact on people and wildlife from WNV as seen in the USA, possibly due to a degree of cross-protection from related viruses already in circulation, the impact of WNV incursion to Galápagos could be grave. Critically, the endemic fauna of Galápagos have evolved in the absence of any native flaviviruses and therefore lack previous exposure to the genus (authors' unpublished data11). As such, the endemic fauna are immunologically naïve and consequently may show heightened susceptibility to WNV infection and disease12.
Predicting the likely infection dynamics of WNV in Galápagos is useful to both conservation and public health practitioners. Understanding the relative importance of candidate vector species could guide interventions, such as mosquito control, to limit the threat of WNV. Currently, little is known of the ecology of Galápagos mosquitoes, or their potential role in WNV transmission.
The introduced mosquitoes, Aedes aegypti and Culex quinquefasciatus have been present on Galápagos since the 1980s. Aedes aegypti feeds almost exclusively on humans and therefore is not a concern for wildlife disease. Previously, we discussed the WNV vector competency of the invasive Culex quinquefasciatus, a notorious vector of wildlife disease elsewhere and showed it to be moderately competent3. However, its distribution is limited to habitats heavily modified by humans and it is far less abundant than A. taeniorhynchus3,13. Here, we focus on the latter species since, if a competent vector, it is likely to be the most important species for WNV epidemiology in Galápagos.
Aedes taeniorhynchus is a salt marsh-associated coastal species that can swarm in large numbers14. The Galápagos strain colonised the Islands naturally around 200,000 years ago and is believed to occur throughout the archipelago in both human modified and natural habitats, including the highland interiors5,15. Although A. taeniorhynchus is not considered to be an important WNV vector elsewhere16, the Galápagos strain shows deep genetic divergence from populations in the rest of the Americas to the extent that it may constitute a distinct, locally adapted species5 with diverged vector ecology.
Vectorial capacity provides a quantitative summary of the basic ecological attributes of a vector population in relation to parasite transmission17,18. It has been used to describe the relative importance of ticks and mosquitoes in the transmission of diseases such as malaria, filariasis and dengue19,20,21. We present three key components of WNV vectorial capacity of Galápagos A. taeniorhynchus. Firstly, we look at mosquito-virus interaction. Elsewhere A. taeniorhynchus is a known vector for zoonotic viral pathogens, including Venezuelan equine encephalitis virus22, however non-Culex species are infrequently considered important for WNV transmission due to poor vector competency (or inappropriate feeding behaviour)16,23. In the USA, despite being found in WNV-positive surveillance pools since 2002, experimental infection of A. taeniorhynchus showed infection rates of no greater than 12% and zero transmission23,24. There can however, be extensive geographical variation in WNV vector competency for the same mosquito species25. Secondly, we look at the distribution of A. taeniorhynchus populations in Galápagos and test ecological and environmental correlates of temporal and spatial variation in their abundance. Thirdly, we examine feeding patterns, comparing blood-meal fractions against the host community composition. Previous research suggests that Galápagos A. taeniorhynchus utilises both reptile and mammal blood5; we hypothesise that avian species would also be included in its diet and that A. taeniorhynchus could act as an enzootic bridge vector for WNV transmission in Galápagos.
Kilpatrick et al (2006) previously evaluated the introduction risk of WNV posed by natural and human mediated transport routes to Galápagos, based on an assumption that WNV would invade and persist in Galápagos (i.e. R0 would be > 1)26. Data were not then available to make an informed assessment of the likelihood of establishment or spread of WNV on the islands. Here, through the evaluation of vector competence, distribution, abundance and host-feeding behaviour of the predominant mosquito species on the Islands, we provide local data on parameters essential to inform impact risk assessments and mitigation measures for WNV reaching the Galápagos archipelago.
Results
Vector competency
Galápagos A. taeniorhynchus demonstrated evidence of both midgut infection (52%) and ability to transmit (11%) WNV at 5 days EIP. At 10 days, transmission rates were over 30%. Detection of infection (χ2 = 8.1, df = 2, P = 0.015) and dissemination (χ2 = 25.0, P = 0.000004) differed significantly across the three EIP time-points, however transmission rates were not significantly different (χ2 = 4.1, df = 2, P = 0.13) (figure 1). Galápagos A. taeniorhynchus showed a high efficiency for WNV infection to disseminate beyond the midgut (43%, 95% and 100% of infected mosquitoes, at 5, 10 and 14 days post-exposure respectively). Moderate transmission efficiency was demonstrated; 21.4%, 35% and 40.9% of mosquitoes with infections and 50%, 36.8% and 40.9% of those with disseminated infections, could transmit at days 5, 10 and 14 respectively.
Abundance and distribution
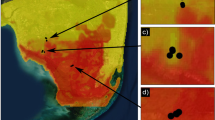
A total of 26,683 mosquitoes were captured on Santa Cruz Island in the five-year period, January 2006 to February 2011, in a total of 1,073 individual overnight collections. Aedes taeniorhynchus represented 88% of all mosquitoes captured (Culex quinquefasciatus was the only other mosquito species caught) and trap-counts ranged from 0 to over 3,000 individuals per night. Large numbers of A. taeniorhynchus were frequently observed, often with aggressive biting behaviour, at coastal sites outside urban areas. The species was encountered at all of the 131 sample sites across Galápagos at least once during the course of monitoring, including the five inhabited islands (Santa Cruz, Baltra, San Cristobal, Floreana and Isabela) plus the islands of Santiago, Española and Santa Fe. A map of the sampled distribution of A. taeniorhynchus across the archipelago is shown in figure 2.
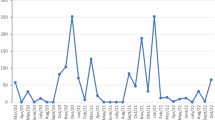
Mosquito abundance generated non-normal count data (Shapiro-Wilks test; W = 0.23, P < 0.001). In constructing a predictive model of abundance, the backward selection procedure yielded a zero-inflated GLM which was found preferable to a standard GLM (Vuong test V = 2.698, P = 0.003). The significant influences on A. taeniorhynchus abundance (model summarized in table 1) were identified as ‘vegetation zone’, ‘lunar cycle’, concurrent ‘maximum tide height’, ‘mean temperature’, ‘prior average rainfall’ and ‘distance to urbanisation (logged)’. Simultaneously, lower ‘mean temperature’ and lower ‘prior average rainfall’ controlled zero-counts, i.e. when absence of A. taeniorhynchus was expected. Temporal evolution of the meteorological parameters can be seen against predicted vector population in Supplementary figure S1 online. Although certain vegetation habitats were predicted to have relatively higher abundance (agricultural, urban and mangrove), minimal spatial dependence existed, verified visually by exponential variograms of model residuals and site latitude-longitude and by a geostatistical model fitted to an object including latitude and longitude (range = 0.14, partial sill = 0.21, nugget = 1.64). The model performed adequately when tested at novel sites; a paired t-test showed no significant difference (t = −1.46, df = 329, P = 0.146) when both model-predicted abundance and field-observed counts were transformed to the ‘relative abundance risk’ score. The relative abundance of A. taeniorhynchus varied in a bimodal distribution across months of the year (Wilcoxon signed rank test on means; V = 78, P = 0.0005). Peak abundance occurred between February and March, with a secondary increase around September (figure 3).
Feeding behaviour
Nearly half of blood-meal samples came from Santa Cruz Island, in the vicinity of Vivienda 10 in the Galápagos National Park (a coastal site around 15 metres above sea level) or Loyola lodge (a highland site in the transitional/agricultural zone). Additional collections were made elsewhere on Santa Cruz, on San Cristóbal (near Puerto Baquerizo), Baltra (FAE airbase) and Isabela Islands (Puerto Villamil and one highland site), figure 4. From a total of 121 engorged or gravid mosquitoes collected from the field, 25 vertebrate DNA amplifications were successfully sequenced to provide host data for Galápagos A. taeniorhynchus. The fraction of mosquito blood-meals from each host species according to major habitat is shown in figure 5.
The majority of blood-meals identified were derived from mammals (84%, n = 21). One reptilian blood-meal (marine iguana, Amblyrhynchus cristatus) was detected and three from avian species – all poultry (two chicken, Gallus gallus and one turkey, Meleagris gallopavo) in the highlands. Human beings and Bos taurus (domestic or wild cow) were the most commonly bitten hosts, each providing 24% (n = 6) of the blood-meals. No mixed species blood-meals were detected.
Forage indices (Fi) indicate a significant avian feeding aversion by A. taeniorhynchus. Finch species (Geospiza spp), flycatchers and mockingbirds each constituted at least 8% of the vertebrate community, yet no blood-meals were derived from them (F < 1; P < 0.01). In contrast, mammals were preferentially fed on in fractions significantly greater than their abundance, for example in the highlands A. taeniorhynchus had a forage ratio of 3.8 (P < 0.01) for pigs (Sus scrofa) and of 7.5 (P < 0.001) for cows (Bos taurus). On the coast, the domestic dog (Canis familiaris) was a more predominant host (F = 6.8; P < 0.01).
Discussion
A fundamental component of investigating emerging pathogens in a new ecosystem is to identify factors which influence their establishment and infection dynamics. Such knowledge assists in public health policies and the design of control and mitigation measures that can be implemented before disease impacts occur. In this study we aimed to quantify the WNV vector competence, relative abundance, distribution and local feeding behaviour of Aedes taeniorhynchus, the most widespread mosquito in Galápagos. Although further components of vectorial capacity exist, useful epidemiological predictions can still be made by considering dominant entomological variables44 and in combination, these components define the epidemiological role of A. taeniorhynchus for WNV in Galápagos, should this pathogen reach these islands. Whilst West Nile virus has not yet emerged in Galápagos, it is important to assess the threat of novel pathogens reaching this region of high conservation and evolutionary significance1,3. Although further evidence of interaction with competent vertebrate hosts is required, our results indicate that, in the event of WNV introduction to Galápagos, A. taeniorhynchus has several characteristics favourable to supporting cycling of the virus, including the capacity to act as a ‘bridge-vector’ such as broad feeding habits, high species distribution and abundance and an ability to transmit the pathogen3,45.
Firstly, we found that Galápagos A. taeniorhynchus is a highly competent vector; 30% could transmit WNV within 10 days of exposure to a biologically relevant dose. In contrast, USA strains of A. taeniorhynchus have been demonstrated to be inefficient WNV vectors, with no transmission and low infection rates 12-15 days after exposure to 7.2 log10 PFU/mL22. Furthermore, whilst USA A. taeniorhynchus appears to have a midgut escape barrier to WNV infection (3% dissemination rates; although 93% transmission when inoculated intrathoracically), Galápagos A. taeniorhynchus shows no indication of this barrier (dissemination efficiency of up to 100%) and thus is a more efficient vector. This disparity in vector competence (although we used a more recent clade of WNV dominating the Americas, WN02 and not NY99) could be a phenotypic expression of the strong genetic divergence between Galápagos and continental A. taeniorhynchus5. A further consideration is the absence of Wolbachia symbionts in Galápagos A. taeniorhynchus29, a bacterium which elsewhere has been demonstrated to reduce the flavivirus transmission ability of vectors46. Importantly, Galápagos A. taeniorhynchus can transmit WNV as early as 5 days post-exposure. Considered alongside gonotrophic length for this mosquito (approximately 5 days between blood-meals; Eastwood, unpublished data), this finding has implications for infection dynamics. Potentially, early transmission could increase the number of hosts infected, perpetuate epidemics and inflate the pathogen reproductive rate (R0). Rapid WNV development has been reported in USA Culex pipiens, with transmission ability being a product of time and temperature; mosquitoes held at 32°C showed transmission at 12, 36 and 60 hours47.
Secondly, along with the results of the vector competency experiments, the widespread and abundant nature of Galápagos A. taeniorhynchus suggests that sustained WNV transmission on Galápagos will be feasible. Particularly when the vector to host ratio is low, the foci and prevalence of infection with a vector-borne pathogen is strongly dependent upon changes in vector density48. By identifying the spatio-temporal drivers of vector populations, predictions of the occurrence of vector-borne disease can therefore be improved. This knowledge can assist vector control measures to intervene in disease outbreaks. Since an active mosquito presence was detected throughout the year, one presumes that blood-feeding continues and that there is potential for year-round WNV transmission; however with a likely higher risk between January and April (‘rainy season’) when A. taeniorhynchus was found with the greatest abundance across the archipelago. Swarming, i.e. very high localised abundance and aggressive biting behaviour was observed for A. taeniorhynchus, which could increase the rate of infection transmission to new vertebrate hosts and mosquitoes.
We showed an influence of tide on A. taeniorhynchus abundance; this likely is a trigger for egg eclosion. Tide was indicated by Bataille et al (2010) to be a determinant of coastal abundance of this species, however the inland abundance could also be affected by tide as this species has a strong flying ability and can disperse widely15,49. Nevertheless, in Galápagos, ecological differences in habitat have been suggested to drive a genetic differentiation of the inland and coastal A. taeniorhynchus populations5 and during this study we identified larvae in water bodies of highland interiors supporting the notion of independent populations15. Agricultural zones (habitat located in the highlands) of Galápagos are particularly associated with A. taeniorhynchus, possibly related to breeding site availability from water provision for livestock or from the heavier rainfall that occurs in the Galápagos highlands33. Darker lunar phase has been shown elsewhere to augment light-trap catches of mosquitoes50. Although A. taeniorhynchus is the predominant mosquito across the Galápagos Islands, 12% of mosquitoes captured during longitudinal monitoring were Culex quinquefasciatus, which we have already shown to be a moderately competent laboratory vector of WNV3 and in southern USA states this species is a renowned WNV vector. Since feeding patterns of Cx. quinquefasciatus in Galápagos do include passerine birds (Eastwood et al., unpublished data), i.e. supporting interaction with probable virus amplification hosts, it is possible that, despite its lower abundance and more-restricted distribution, this mosquito species also could represent a risk of WNV transmission in Galápagos.
Finally, our blood-feeding analyses showed Galápagos A. taeniorhynchus to feed on mammals, reptiles and birds. Broad feeding habits would provide a mechanism for WNV to extend beyond a bird-mosquito cycle to infect a wide range of hosts, including human beings. As in its continental range, this mosquito is primarily mammophillic, but feeding on reptiles is corroborated5. Bataille et al (2009) showed Galápagos A. taeniorhynchus to take blood-meals from tortoises and marine iguanas with clear support for reptile feeding5. Galápagos has over 22 species of endemic reptile, many of which are classified by the IUCN as threatened51. Although not having a widely recognized role in the amplification of WNV infection, reptiles are not immune to WNV infection or disease52,53. Assessing the susceptibility and/or host competency of Galápagos reptiles would help to elucidate their potential role in the epidemiology of WNV on the archipelago. Birds however are the typical reservoir for WNV. In the current study, the only avian blood-hosts we detected for A. taeniorhynchus were domestic poultry. These are not competent hosts for WNV as they are only capable of maintaining viremic levels great enough to infect mosquitoes when very young, although they can serve as WNV sentinels54,55.
The limited proportion of avian blood-meals detected here questions the role of A. taeniorhynchus in WNV epidemiology. Nonetheless, Bataille et al (2012) report a single blood-meal from a flightless cormorant (Phalacrocorax harrisii)29 and there are anecdotal accounts of Galápagos seabirds abandoning their nests due to molestation by A. taeniorhynchus56. Furthermore, a variety of wild bird species in Galápagos are infected with pathogens such as hemoproteus and filarial pathogens that are probably vectored by mosquitoes2,57,58. Therefore it seems likely that A. taeniorhynchus has more contact with avian species than can be demonstrated so far. Our sample size of A. taeniorhynchus blood-meals was low with a poor recovery rate (21%) of host DNA. This could have been due to the blood-meal DNA being degraded (e.g. effect of time post-ingestion59), or due to primers being sub-optimal for detecting the DNA of endemic Galápagos avifauna arising from primer site mismatches.
While the current results suggest that there is a strong likelihood for the establishment and spread of WNV in Galápagos, several issues could be assessed to evaluate this further: i) Importantly, evidence of feeding on WNV-competent hosts is needed; either by further examination of engorged specimens or by testing the host-competency of established blood-hosts such as marine iguana. ii) Whether vertical transmission in A. taeniorhynchus could provide a mechanism for WNV persistence in Galápagos. iii) How the WNV competency or feeding patterns of A. taeniorhynchus responds to seasonal changes in Galápagos (climate, host-availability). In the USA for example, the vector Cx. pipiens was found to host-switch in response to bird migration, resulting in temporal variation of WNV transmission to human beings60. Temperature and viral dose can influence WNV development within mosquito vectors47,61 and may have significance for the infection dynamics of WNV in Galápagos.
Our results indicate that A. taeniorhynchus could act as a competent WNV vector in Galápagos, emphasizing the need for ongoing and improved biosecurity in Galápagos to prevent the introduction of WNV and other pathogens. In particular, there is a continuing need to focus on managing the risk from human-mediated transport, as recommended by Kilpatrick et al (2006)26. Over 1300 invasive species have already arrived to Galápagos, including regular introductions of arthropods of medical importance62,63. The expanded knowledge of A. taeniorhynchus ecology presented here aids scientific understanding of a disease vector, but importantly highlights the need to avoid complacency in ensuring that disease prevention measures are in place.
Methods
Vector competency
Mosquitoes
Aedes taeniorhynchus were collected in Galápagos during December 2010. Eggs were obtained using oviposition traps lined with seed paper (Fisher, UK), lured with mango leaf infusion. Also first-instar larvae were collected from local pools. Eggs and larvae were transported under USDA (no.47279) and CDC (no.2010-05-090) permits to the Wadsworth Center Arbovirus Laboratory (New York State Department of Health, USA). Mosquitoes were reared to adult stage in a BioSafety Level 2 quarantine insectary maintained at 26 ± 1°C with 12 hour[h]:12 h (light:dark) [L:D] photoperiod and 85% relative humidity [RH]. Emerged adults were held in 0.47 L mesh-topped cartons and fed 10% sucrose ad lib. Experimental infection took place under BioSafety Level 3.
Infection
Mosquitoes were infected with WNV as described by Eastwood et al (2011)3. Briefly, mosquitoes were presented with a rabbit blood-meal preparation containing WNV strain WN02-1956, initially isolated from an American Crow kidney in New York27. The WNV titer was 7.84 – 7.89 log10 PFU/mL. Fed female mosquitoes were separated from unfed or male mosquitoes under CO2 and were held at 26°C for an extrinsic incubation period [EIP] of 5, 10 or 14 days; the shortest EIP was designed to test for evidence of rapid transmissibility. At each time-point, approximately 25 mosquitoes were immobilised using triethylamine (Sigma, CA) and mosquito body, legs and salivary secretions were harvested, as described by Eastwood et al (2011)3.
Assay
A plaque assay on Vero cell culture was used to screen harvested mosquito samples for West Nile virus, as described by Eastwood et al (2011) based on Payne et al. (2006)3,28. Observed rates of infection, dissemination and transmission were compared across EIPs using a Pearson's chi-squared test (5% significance level) to determine any differences over time.
Mosquito abundance and distribution
Population monitoring
A mosquito sampling program was implemented on Santa Cruz Island between January 2008 and February 2011. Findings were supplemented by data previously collected in 2006 and 200715,29. CO2−baited CDC light-traps (JW Hock, FL) were employed overnight (approximately from 6 pm to 6 am). Trap contents were sorted at the Galápagos Genetics, Epidemiology and Pathology Laboratory [GGEPL]. Aedes taeniorhynchus were identified morphologically and the number of female individuals recorded. To assess the distribution of A. taeniorhynchus, additional sampling was conducted over a wider geographical area using CDC gravid traps (JW Hock) and human catch landings. The lure used within gravid traps was mango leaf-infused water. Any engorged or gravid mosquitoes were retained for blood-meal analysis (see below).
Study sites
Thirty-eight sites (listed in supplementary table S1 online) were monitored on Santa Cruz on at least five occasions for abundance modelling. A further 93 sites across the archipelago were visited less intensively to determine mosquito distribution by recording presence-absence. Environmental and climatic data associated with the site and timing of each collection was acquired from the following sources:
-
a
Site characteristics: Vegetation zone (one of seven habitat types) was noted and the spatial easting, northing and elevation measured using a GPS handset. From a digital map of the Islands (Galápagos National Park), distance to the nearest urban area and distance to the sea, were calculated (shortest direct line) in a geographic information system [GIS] (ArcGIS 9.2, ESRI), adjusting for slope using Pythagoras theorem.
-
b
Environmental data: Maximum, minimum and mean temperature, mean RH and precipitation were recorded using Hobo data loggers (Onset Corporation, MA) with supplementary data from the Charles Darwin Foundation (www.darwinfoundation.org) climate stations in Bellavista and Puerto Ayora. High tides were obtained from Ecuadorian Naval Oceanography [INOCAR] or from www.mobilegeographics.com30. Moon phase was parameterized using a cosine function which assumed the value of 0 at new moon31.
Distribution
The extent of A. taeniorhynchus distribution (i.e. where the species was detected during the five-year monitoring period) was projected in a GIS to create a basic species distribution map for A. taeniorhynchus in Galápagos. Mean abundance per sample site was calculated and geo-referenced.
Abundance modelling
The spatio-temporal abundance of A. taeniorhynchus was modelled against site and climatic variables using R software32. Eight time-lagged variables were created, under the hypothesis that prior events (such as rainfall triggering egg eclosion) would be influential upon subsequently observed adult abundance; based on an egg - adult development duration of 7 – 14 days (Eastwood, unpublished data). Where possible, time variables such as ‘month’ were replaced by climatic variables such as temperature (due to inter-annual variation in Galápagos monthly climate and sporadic phenomena such as El Niño33). Spatial coordinates were considered mutually within models and were afterwards included to form a geostatistical model, along with variograms, to check for spatial dependency in the final model. Pearson's product-moment tests and plots identified any collinearity in available variables. A zero-inflated generalized linear model [GLM] with negative binomial error structure was constructed after eliminating collinearity (which also reduced the number of explanatory variables). This type of regression is flexible for overdispersed data and incorporates an element to explain excess zeros (e.g. lack of rain)34. A Vuong test compared model performance against standard negative binomial regression using Kullback-Liebler criterion35. A backward selection process (based on p-values) was then applied to determine which factors significantly explained patterns of abundance. Multi-way interaction effects were examined but were not adopted due to increased log-likelihood and/or reduced interpretability.
Validation of the model was performed using a set of sampling data from novel sites in Galápagos (n = 330 records). The predicted abundance of A. taeniorhynchus at these sites was compared to abundance observed in the field, using a paired t-test (95% two-tailed). A relative abundance risk index, constructed arbitrarily based on the count of mosquitoes, was applied to both observed and model-predicted abundance. Absolute abundance counts of zero received a risk score of 0 ( = minimal risk), counts between 0.1 and 5 scored 1 ( = low risk), from 5.01 to 15.0 scored 2 ( = medium risk) and those over 15 scored 3 ( = high risk). We present predicted seasonal abundance of these potential vectors, compared to observed patterns.
Feeding behaviour
Sample collection
Blood-engorged A. taeniorhynchus were collected in June 2009, February 2010 and November 2010, at 13 locations. Engorged mosquitoes were aspirated either from resting traps (high-sided pots placed on their sides overnight) or opportunistically, or were captured in light traps during the abundance monitoring. Specimens identified as A. taeniorhynchus were stored in punctured vial tubes within sealed plastic bags containing silica desiccant (Silicagelpackets.co.uk) prior to analysis.
Blood-meal analysis
DNA was extracted from the excised abdomen of each engorged mosquito using Chelex 100 (Bio-Rad, CA) in a protocol adapted from Walsh et al (1991)36. Briefly, each sample was ground in a 300 μL 10% Chelex solution, held in a 95°C heat block for 10 min, then pulse-vortexed for 10 s before centrifugation at 8000 rpm for 1 min. Polymerase chain reaction [PCR] assays targeting the cytochrome b (cytb) gene were performed using primer sets described by Cupp et al (2004)37. Cycling conditions were 95°C for 2 minutes, followed by 40 cycles of 94°C for 20 s, 50°C for 30 s and 72°C for 45 s, ending with 7 minutes at 72°C. To improve success rate, a second PCR was performed using product from the first amplification as template. Products were sequenced at a concentration of 25 nM. Edited nucleotide sequences were compared against sequences available on Genbank to identify vertebrate hosts on which A. taeniorhynchus had fed38,39.
Host-foraging index
To indicate whether Galápagos A. taeniorhynchus feeding was representative of the background vertebrate community, we estimated the relative abundance of fauna in the vicinity of traps using three unlimited distance point-transects at five Galápagos regions (repeated on four occasions). Surveys took place at approximately 6 am or 6 pm (dawn and dusk peak feeding times for A. taeniorhynchus), for a 10-minute period during which each vertebrate detected by sight or ear was identified to species level and an estimate of their radial distance from the monitoring point recorded (in metres). An initial 1-minute settling-down period allowed for any disturbance created when approaching the point40. We used the program Distance to estimate the density of key Galápagos vertebrate species at each location41, which acknowledges differences in species-detectability thus reducing bias40. We pooled both blood-meal and host data according to Highland or Coastal criteria, analysing point-survey data within the multiple covariate sampling engine41. An index of relative abundance was formed from the species density estimates. To meet normality assumptions, all relative abundance values were log-transformed. Forage ratios, equation (1), were calculated to indicate if A. taeniorhynchus displayed any preference or avoidance in feeding behaviour42:

If host species i was fed on opportunistically by mosquitoes in proportion to their abundance, the forage index, Fi, would be 1. Multinomial simulations (of the number of blood-meals uij from a host species i at site j) and ratio tests compared the observed distribution of blood-meals with those drawn under the null hypothesis of Fi = 1. If no blood-meals were detected of species i present at site j (due to avoidance or lack of samples), uij = 0.5 was assumed and for observation probabilities less than 0.05, a forage index Fi = 0.5 was reported as a conservative estimate of minimum avoidance43.
References
UNESCO (available at http://whc.unesco.org/en/list/1/). Galápagos Islands. World Heritage List. United Nations Educational Scientific and Cultural Organization. (2010).
Padilla, L. R., Santiago-Alarcon, D., Merkel, J., Miller, R. E. & Parker, P. G. Survey for Haemoproteus spp., Trichomonas gallinae, Chlamydophila psittaci, and Salmonella spp. in Galápagos Islands Columbiformes. Journal of Zoo and Wildlife Medicine 35, 60–64 (2004).
Eastwood, G., Kramer, L. D., Goodman, S. J. & Cunningham, A. A. West Nile virus vector competency of Culex quinquefasciatus mosquitoes in the Galápagos Islands. American Journal of Tropical Medicine and Hygiene 85, 426–433 (2011).
Galápagos National Park (Victor Carrion). Online report - Invasive species in Galápagos. in Conservation and sustainable development 2 (Galápagos National Park, 2010).
Bataille, A. et al. Natural colonization and adaptation of a mosquito species in Galápagos and its implications for disease threats to endemic wildlife. Proceedings of the National Academy of Sciences of the United States of America 106, 10230–10235 (2009).
Kramer, L. D., Styer, L. M. & Ebel, G. D. A global perspective on the epidemiology of West Nile virus. Annual Review of Entomology 53, 61–81 (2008).
CDC. West Nile virus activity in the United States - Centers for Disease Control & Prevention (CDC) Division of Vector-Borne Infectious Diseases. in West Nile Virus - Statistics, Surveillance and Control Archive (2010).
LaDeau, S. L., Kilpatrick, A. M. & Marra, P. P. West Nile virus emergence and large-scale declines of North American bird populations. Nature 447, 710–713 (2007).
Mattar, S. et al. West Nile Virus antibodies in Colombian horses. Emerging Infectious Diseases 11, 1497–1498 (2005).
Bosch, I. et al. West Nile virus, Venezuela. Emerging Infectious Diseases 13, 651–653 (2007).
Travis, E. K. et al. Hematology, serum chemistry and serology of Galápagos penguins (Sphenicus mendiculus) in the Galápagos Islands, Ecuador. Journal of Wildlife Diseases 42, 625–632 (2006)
Wikelski, M., Foufopoulos, J., Vargas, H. & Snell, H. Galápagos birds and diseases: Invasive pathogens as threats for island species. Ecology and Society 9, e5 (2004).
Keyghobadi, N., LaPointe, D., Fleischer, R. C. & Fonseca, D. M. Fine-scale population genetic structure of a wildlife disease vector: the southern house mosquito on the island of Hawaii. Molecular Ecology 15, 3919–3930 (2006).
Apperson, C. The black salt marsh mosquito, Aedes taeniorhynchus. in Wing Beats 9 ( Florida Mosquito Control Association, 1991).
Bataille, A., Cunningham, A. A., Cruz, M., Cedeno, V. & Goodman, S. J. Seasonal effects and fine-scale population dynamics of Aedes taeniorhynchus, a major disease vector in the Galápagos Islands. Molecular Ecology 19, 4491–4504 (2010).
Turell, M. J., Sardelis, M. R., Dohm, D. J. & O'Guinn, M. L. Potential North American vectors of West Nile virus. West Nile Virus: Detection, Surveillance and Control 951, 317–324 (2001).
Reisen, W. K. Epidemiology of vector-borne diseases. in Medical and Veterinary Entomology (ed. G. Mullen, Durden,L.) (Academic Press, San Diego, 2002).
Fine, P. E. M. Epidemiological principles of vector-mediated transmission. in Vectors of disease agents (ed. J. J. McKelvey, B. F. Eldridge & K. Maramorosch) 77–91 (Praeger Scientific, New York, 1981).
Spielman, A., Levine, J. F. & Wilson, M. L. Vectorial capacity of North American Ixodes ticks. The Yale Journal of Biology and Medicine 57, 507–513 (1984).
Anderson, J. T. & Rico-Hesse, R. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. American Journal of Tropical Medicine and Hygiene 75, 886–892 (2006).
Eldridge, B. F. & Edman, J. D. Medical entomology: A textbook on public health and veterinary problems (Springfield, 1994).
Ortiz, D. & Weaver, S. Susceptibility of Ochlerotatus taeniorhynchus (Diptera: Culicidae) to infection with epizootic (Subtype IC) and enzootic (Subtype ID) Venezuelan Equine Encephalitis viruses: Evidence for epizootic strain adaptation. Journal of Medical Entomology 41, 987–993 (2004).
Turell, M. J., O'Guinn, M. L., Dohm, D. J., Jones, J. W. Vector competence of North American mosquitoes (Diptera : Culicidae) for West Nile virus. Journal of Medical Entomology 38, 130–134 (2001).
CDC. Entomology - Mosquito species. in West Nile Virus Activity - Division of Vector Borne Infectious Diseases (Center for Disease Control and Prevention, 2009).
Vaidyanathan, R. & Scott, T. W. Geographic variation in vector competence for West Nile virus in the Culex pipiens (Diptera: Culicidae) complex in California. Vector Borne and Zoonotic Diseases 7, 193–198 (2007).
Kilpatrick, A. M. et al. Predicting pathogen introduction: West Nile virus spread to Galápagos. Conservation Biology 20, 1224–1231 (2006).
Ebel, G. D., Carricaburu, J., Young, D., Bernard, K. A. & Kramer, L. D. Genetic and phenotypic variation of West Nile virus in New York, 2000 – 2003. The American Journal of Tropical Medicine and Hygiene 71, 493–500 (2004).
Payne, A. F., Binduga-Gajewska, I., Kauffman, E. B. & Kramer, L. D. Quantitation of flaviviruses by fluorescent focus assay. Journal of Virological Methods 134, 183–189 (2006).
Bataille, A. Host selection and parasite infection in Aedes taeniorhynchus, endemic disease vector in the Galápagos Islands. Infection, Genetics and Evolution 12, 1831–1841 (2012)
INOCAR - Instituto Oceanografico de la Armada de Ecuador. Online database - Tabla de Mareas. (2006–2011).
Röösli, M. et al. Sleepless night, the moon is bright: longitudinal study of lunar phase and sleep. Journal of Sleep Research 15, 149–153 (2006).
R. The R foundation for statistical computing - R Version 2.13.0. (2011).
Charles Darwin Foundation. Datazone: Climate Database. (2011).
Bruin, J. Zero-inflated negative binomial regression. (Statistical Consulting Group. UCLA: Academic Technology Services, 2006).
Vuong, Q. H. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica 57, 307–333 (1989).
Walsh, S. P., Metzger, D. A. & Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10, 506–513 (1991).
Cupp, E. W. et al. Identification of reptilian and amphibian blood meals from mosquitoes in an Eastern Equine encephalomyelitis virus focus in central Alabama. American Journal of Tropical Medicine and Hygiene 71, 272–276 (2004).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. in Nucleic Acids Research 3389–3402 (1997).
Hall, T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT in Nucleic Acids Symposium Series No. 41, 95–98 (1999).
Buckland, S. T., Anderson, D. R., Burnham, K. P. & Laake, J. L. DISTANCE sampling: Estimating abundance of biological populations. (Chapman & Hall, 1993).
Thomas, L. et al. Distance 6.0. Release 2. (ed. Research Unit for Wildlife Population Assessment) (University of St. Andrews, UK, 2009).
Hess, A. D., Hayes, R. O. & Tempelis, C. H. The use of the forage ratio technique in mosquito host preference studies. Mosquito News 28, 386–389 (1968).
Kilpatrick, A. M., Daszak, P., Jones, M. J., Marra, P. P. & Kramer, L. Host heterogeneity dominates West Nile virus transmission. Proceedings of the Royal Society B-Biological Sciences 273, 2327–2333 (2006).
Dye, C. Vectorial capacity: must we measure all its components? Parasitology Today 2, 203–209 (1986).
Kilpatrick, A. M. et al. West Nile virus risk assessment and the bridge vector paradigm. Emerging Infectious Diseases 11, 425–429 (2005).
Bian, G., Xu, Y., Lu, P., Xie, Y. & Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathogens 6, e1000833 (2010).
Kilpatrick, A. M., Meola, M. A., Moudy, R. M. & Kramer, L. D. Temperature, viral genetics and the transmission of West Nile virus by Culex pipiens mosquitoes. Plos Pathogens 4, e1000092 (2008).
Grenfell B. T., Dobson A. & Eds. Ecology of infectious diseases in natural populations (Cambridge Universitiy Press, 1995).
Tetens-Nielsen, E. & Tetens-Nielson, A. Field observations on the habits of Aedes taeniorhynchus. Ecology 34, 141–156 (1953).
Provost, M. W. The influence of moonlight on light-trap catches of mosquitoes. Annals of the Entomological Society of America 52, 261–271 (1959).
Jiménez-Uzcátegui, G., Márquez, C. & Snell, H. L. CDF checklist of Galápagos reptiles - FCD lista de especies de reptiles de Galápagos in Lista de Especies de Galápagos de la Fundación Charles Darwin (ed. F. Bungartz, et al.) (Charles Darwin Foundation, Puerto Ayora, Galápagos: 2011).
Miller, D. L. et al. West Nile virus in farmed alligators. Emerging Infectious Diseases 9, 794-799 (2003).
Steinman, A. et al. West Nile virus infection in crocodiles Emerging Infectious Diseases. 9, 887–889 (2003).
Kradel, D. C. West Nile encephalitis and poultry. (ed. College of Agricultural Sciences) (The Pennsylvania State University, 2002).
Langevin, S. A., Bunning, M., Davis, B. & Komar, N. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerging Infectious Diseases 7, 726–729 (2001).
Anderson, J. D. & Fortner, S. Waved Albatross egg neglect and associated mosquito ectoparasitism. Condor 90, 727–729 (1988).
Parker, P. G. et al. 110 Years of Avipoxvirus in the Galápagos Islands. PLoS ONE 6, e15989 (2011).
Gottdenker, N. L. et al. Causes of mortality of wild birds submitted to the Charles Darwin Research Station, Santa Cruz, Galápagos, Ecuador from 2002–2004. Journal of Wildlife Diseases 44, 1024–1031 (2008).
Oshaghi, M. A. et al. Effects of post ingestion and physical conditions on PCR amplification of host blood meal DNA in mosquitoes. Iranian Journal of Public Health 34, 12–19 (2005).
Kramer, L. D. & Kilpatrick, A. M. Unraveling a complex transmission cycle: Implications for control. in Vector Biology, Ecology and Control (ed. P. W. Atkinson) 191–202 (Springer, New York, 2010).
Richards, S. L., Mores, C. N., Lord, C. C. & Tabachnick, W. J. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector Borne and Zoonotic Diseases 7, 629–636 (2007).
Bataille, A. et al. Evidence for regular ongoing introductions of mosquito disease vectors into the Galápagos Islands. Proceedings of the Royal Society - Biological Sciences 276, 3769–3775 (2009).
Causton, C. et al. Alien insects: Threats and implications for conservation of Galápagos Islands. Annals of the Entomological Society of America 99, 121–143 (2006).
Acknowledgements
We are grateful to Marilyn Cruz, Alberto Vélez, Virna Cedeño and Grace Loyola in Galápagos. We thank Arnaud Bataille for the kind provision of supplementary sampling data. Alex Ciota and Ryan Peters for laboratory support whilst at Wadsworth Arbovirus Laboratory and for reviewing an early manuscript draft. The cooperation of the Galápagos National Park is also appreciated, in support of the overall project (PNG09-21) and for granting access for sample collection.
Author information
Authors and Affiliations
Contributions
G.E. performed the field sampling, analysis, laboratory experiments and wrote the main manuscript text. G.E., S.J.G., A.A.C. and L.D.K. designed the study and analyses. S.J.G., A.A.C. and L.D.K. critically reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Eastwood, G., Goodman, S., Cunningham, A. et al. Aedes Taeniorhynchus Vectorial Capacity Informs A Pre-Emptive Assessment Of West Nile Virus Establishment In Galápagos. Sci Rep 3, 1519 (2013). https://doi.org/10.1038/srep01519
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01519
This article is cited by
-
Culex quinquefasciatus: status as a threat to island avifauna and options for genetic control
CABI Agriculture and Bioscience (2021)
-
Zika virus detection, isolation and genome sequencing through Culicidae sampling during the epidemic in Vitória, Espírito Santo, Brazil
Parasites & Vectors (2019)
-
Using Avian Surveillance in Ecuador to Assess the Imminence of West Nile Virus Incursion to Galápagos
EcoHealth (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.