Abstract
Au nanoparticles (AuNPs), which easily aggregate in organic thin film, are observed to well-disperse in chicken albumen thin films. The incorporated AuNPs is distributed uniformly inside the thin film and is as dense as 650 (particles/μm3). In addition, enhanced ultraviolet (UV) fluorescence centered at 350 nm is observed from the AuNPs-containing chicken albumen thin film. The enhancement is proposed to be originated from the plasmon resonance energy transfer (PRET) from the d-band absorption of AuNPs to the chicken albumen protein. The enhanced fluorescence is further verified by the shorter fluorescence lifetime from the time-resolved fluorescence spectra. These results indicate that d-band transition of AuNPs can be used to interface with other UV-emitting biomolecules. Results in this study demonstrate that AuNPs exhibit future potentials in applications for both the organic thin-film technology and nano-biotechnology.
Similar content being viewed by others
Introduction
Localized surface plasmons are collective oscillation of conductive electrons propagating at the surface of noble metal nanoparticles1,2. The electric field is highly localized and very strong at the surface, which is ideal for electric field-related applications such as surface-enhanced Raman scattering (SERS)3. In addition, the light scattering induced by localized surface plasmon resonance (LSPR) of metal nanoparticles is very sensitive to the dielectric environment and is widely used for ultrasensitive chemical and biological sensing applications4,5,6,7. LSPR of nanoparticles are also affected by their sizes and shapes, which can be utilized to tune the LSPR for a specific wavelength or application8.
Surface plasmon resonance of a metal nanoparticle has been reported to enhance fluorescence from molecules9,10. The extinction crosssection from LSPR of a metal nanoparticle is usually at least 4 order of magnitude higher compared to organic fluorophores11, which indicates that more light is absorbed by the metal nanoparticles. The energy of the absorbed plasmon energy is able to transfer from the plasmonic nanoparticle to the attached molecules, resulting enhanced emission intensity from the molecules. This energy transfer process is referred as “Plasmon Resonance Energy Transfer; (PRET)”. PRET can occur between two plasmonic nanoparticles, such as an Au nanowire and another Ag nanowire12,13,14. Plasmon-exciton coupling between the fixed-frequency localized surface plasmons in Ag nanospheres and molecular J-aggregates was also reported15. The coupling between the LSPR of metal nanoparticles and excitons of molecule is very strong and is usually referred as “Plexiton” in some literatures16,17,18.
Au nanoparticles (AuNPs) are the most popular plasmonic nanoparticles. AuNPs with very small diameters (<10 nm) can also be used as fluorescence markers19. Larger AuNPs exhibit a tunable LSPR wavelength and have been widely used in recent nano-biotechnologies20. However, several problems still exist that have hindered the applications of AuNPs. First, AuNPs easily aggregates when the concentration becomes too high. The aggregation red-shifts its LSPR spectra and changes the optical and material properties. It is an ongoing research topic to find a method to avoid the aggregation while keeping the AuNPs concentration sufficiently high. The other problem is the spectra mismatch between the LSPR of AuNPs and the fluorescence spectra of most biomolecules. Most of the intrinsic light emission from biomolecules are excited with deep ultraviolet (UV) light and emit UV light, too. For examples, Tryptophan, which is an important aromatic amino acid in proteins, absorbs light around 280 nm and emits photons near 348 nm21. The fluorescence from Tryptophan is able to reveal the information about the protein structure and intermolecular interaction. Since the LSPR wavelength of AuNPs in water is longer than 530 nm, AuNPs is not suitable to study the plasmonic effect with these UV-emitting biomolecules. Although other types of nanoparticles, such as Ag and Al, exhibit a shorter LSPR wavelength, both of them easily oxidized and their LSPR properties easily change.
Biomaterials, such as DNA-hexadecyltrimethylammmonium chloride (DNA-CTMA)22,23,24 and silk25, have been reported to be used as fabrication materials for modern organic electronics. In addition, chicken albumen has been reported as the dielectric layer for an organic field-effect transistor26. These biomaterials are obtained from fossil-fuel free sources, which are potential materials for future green technologies. Among them, the chicken albumen approach is easily available and the chicken albumen exhibits several sulfide bonds within its structure, which should allow well-dispersion of AuNPs inside the albumen thin film.
In this research, dispersions of AuNPs in chicken albumen thin films are investigated. It is found that chicken albumen disperses AuNPs very well, which is confirmed from both transmission electron microscope (TEM) images and optical transmission spectra. The AuNPs concentration is estimated to be as high as 650 (particles/μm3). The well-dispersed AuNPs inside the albumen thin film will allow the use of AuNPs in modern thin-film based electronic devices. We also observed the coupling between the d-band absorption of AuNPs and the absorption band of the chicken albumen protein. The coupling enhances the UV emission intensity of the chicken albumen at 350 nm. The enhanced UV emission is verified by time-resolved fluorescence measurements, which reveals a shorter fluorescence lifetime for the AuNPs-containing chicken albumen thin film. These results indicate that the d-band transition for AuNPs, which is around 250 nm, is able to be used as the donors of plasmonic resonance energy transfer. This result will open the possibility for AuNPs to be used with other UV-emitting biomolecules and widen the use of AuNPs in recent nano-biotechnologies.
Results
Fig. 1 illustrates the TEM images of the AuNPs-containing thin films. Conventional polymer, such as polyvinyl alcohol (PVA), is not able to well disperse the AuNPs and the aggregation occurs when the concentration of AuNPs become sufficient high. The aggregation is easily observed in the TEM images of AuNPs-containing PVA thin film [Figs. 1(b1)–(b4)]. In contrast, chicken albumen liquid is able to disperse the AuNPs within the thin film and avoid the aggregation, as shown in Figs. 1(a1)–(a4). By comparing Figs. 1(a3) and 1(b3), it is easy to conclude that AuNPs indeed well-disperse inside the albumen thin film and are also very dense. It is believed that the sulfide bond within the albumen protein helps the dispersion of AuNPs due to the strong Au-sulfide. It is also possible that the electrostatic force between the negatively-charged AuNPs and the positively-charged protein helps the dispersion. The highest AuNPs concentration is estimated to be around 650 (particles/μm3) for the 10000 ppm albumen sample, which will be very useful for novel organic thin-film devices.
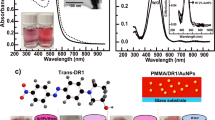
Fig. 2 illustrates the experimental transmission spectra of the obtained AuNPs-containing thin films. The transmission spectra for the AuNPs-only samples, shown in Fig. 2(a), reveal two extinction peaks located at 650 nm and 250 nm. The AuNPs-only samples are fabricated by spin-coating the AuNPs water solutions. The origins of these two extinction peaks are distinguished through results from electromagnetic simulation. The simulated transmission spectrum of a single Au nanoparticle with diameter of 20 nm is shown in Fig. 3. The Au nanoparticle is embedded in water. Two extinction peaks around 530 nm and 240 nm are observed. The extinction peak around 530 nm is identified as its LSPR dipole mode based on its average field-energy distribution shown in the inset of Fig. 3. The extinction peak at 240 nm also reveals the distribution of a dipole mode. Therefore, it is not originate from the LSPR quadruple or higher order modes. It is reasonable since the diameter for the Au nanoparticle is too small to sustain a quadruple LSPR mode. This extinction peak should correspond to the d-band transition by Au atoms. Excess electrons are created by absorption of the incident light and quickly dissipate its energy though heat, which is very similar to the behavior of the dipole mode.
The extinction peaks, for both the AuNPs-only and AuNPs-containing PVA samples, shown in Figs. 2(a) and 2(c), red-shift to longer wavelength, indicating AuNPs aggregation indeed happens in both samples. However, the extinction peak of the AuNPs-containing chicken-albumen, shown in Figs. 2(b), did not red-shift. These results confirm our observations while studying the TEM images that albumen thin film is able to well disperse AuNPs. The maximum AuNPs concentration can be as high as 650 (particles/μm3). In addition, chicken albumen thin films exhibit another extinction peaks located near 280 nm, which corresponds to the light absorption of proteins. Tryptophan and tyrosine are the two common aromatic amino acids in proteins that absorb 280 nm light. This peak is very close to or overlaps with the d-band extinction peak of the Au. It is very interesting to see if any coupling happens between these two extinction processes.
In order to verify if any coupling occurs between these two extinction processes, time-integrated fluorescence spectra, excited by a 280 nm UV light, were obtained from all the samples. As expected, AuNPs-only and AuNPs-containing PVA thin film did not exhibit any fluorescence responses, shown in Figs. 4(a) and (b), respectively. The neat chicken albumen reveals a broad emission centered at 350 nm, which corresponds to the emission from Tryptophan. To our surprise, the emission intensity of the chicken albumen thin film increases as AuNPs is incorporated. The intensity keeps increasing as the concentration increases from 500 to 5000 ppm. The enhancement reaches a maximum at the concentration of 10000 ppm and becomes less when even higher AuNPs concentrations are used. The AuNPs-concentration dependence is clearly observed in the inset of Fig. 4(c). It should be noted that the thickness of the neat chicken albumen film is measured to be 825 nm. The film thicknesses of 500, 5000 and 10000 ppm films are 819 nm, 830 nm and 821 nm, respectively. All of these thin films exhibit similar thickness. Therefore, the thickness effect is not what causes the observed fluorescence enhancement.
Experimental fluorescence spectra of (a) Au nanoparticle only, (b) Au-containing PVA, (c) Au-containing chicken albumen with various AuNPs concentrations.
Inset in (c) illustrates the integrated fluorescence intensities for albumen thin film with various AuNPs concentrations. Neat films in (b) and (c) correspond to the spectra for chicken albumen- and PVA- only films.
In order to explain the enhancement of emission intensity by AuNPs, a possible model is proposed and illustrated in Fig. 5. An Au nanoparticle is able to absorb 250 nm UV light through its d-band transition. The absorbed energy is transferred to the attached albumen protein and emits 350 nm UV light. It should be noted that the extinction corresponds to this d-band is higher than the extinction for the LSPR dipole mode, which indicates that the extinction crosssection for this d-band transition is also much higher than the absorption crosssection for protein molecules. Therefore, the resonance energy transfer will enhance the UV emission intensity. When too many AuNPs are incorporated, there will not be enough protein molecules to form functioning AuNPs-protein complexes. Most of the absorbed energy is dissipated non-radiatively into heat inside the AuNPs, results in a decreased UV emission intensity.
The proposed model for the enhancement of emission intensity.
The incident UV light is resonantly absorbed by the AuNPs and its energy is transferred to the attached chicken albumen molecule. The enhanced absorption caused by plasmonic effect enhances the fluorescence intensity by the albumen molecules.
If the UV emission intensity enhancement is originated from the metal-enhanced process, its radiative lifetime should be modified. This process can only be verified through time-resolved fluorescence measurements. The fluorescence decays for the AuNPs-containing chicken albumen thin films are illustrated in Fig. 6. Each decay profile can be fitted with a bi-exponential function:

The fitted parameters are summarized in Table 1. It is clearly observed that the decay time constant τ1 and τ2 becomes smaller as more AuNPs are incorporated. The time constants τ1 and τ2 becomes 35% and 20% smaller when 10000 ppm AuNPs are used. The smaller lifetime indicates faster radiative transitions, resulting higher emission intensity. The time-resolved fluorescence spectra provide strong evidences for the occurrences of metal-enhanced fluorescence inside the AuNPs-containing chicken albumen thin film.
Discussion
In this research, chicken albumen thin film is able to well-disperse AuNPs and prevents aggregations at high AuNPs concentrations. Both TEM and transmission spectra have confirmed this observation. The maximum AuNPs concentration can be as high as 650 (particles/μm3). By contrast, AuNPs easily aggregate inside conventional polymer, such as PVA, thin films. These AuNPs-containing albumen thin films can be potentially incorporated as a device layer in an optoelectronic device, which should leads to several interesting applications in the near future. In addition, AuNPs is found to be able to enhance the 350 nm emission intensity from a chicken albumen thin film. We propose that the origin of the enhancement is due to the plasmon energy transfer from the d-band Au absorption to the albumen protein emission, since both extinction processes occurs near 280 nm. The metal-enhanced process is verified by a shorter fluorescence lifetime measured by time-resolved fluorescence spectroscopy. This phenomenon is very different since most reported plasmon energy transfer processes are between the LSPR dipole modes of AuNPs to the attached molecules, which is limited to the wavelength range for 530 nm and larger. D-band transition is rarely reported to be used as the donor of plasmon energy transfer. This result should be very important since it allows the use of AuNPs with UV-emitting biomolecules, which is not possible by the LSPR dipole mode of AuNPs. This will make AuNPs more appearing for more biological applications. In addition to chicken albumen, duck albumen was also investigated and reveals a similar ability to well-disperse the AuNPs. However, we did not observe the enhanced fluorescence intensity at 350 nm in the AuNPs-containing duck albumen thin films. We are currently working to understand this phenomenon. In conclusion, results in this study reveals that AuNPs embedded in albumen thin films can be effectively used in modern optoelectronic device. In addition, we also found out that the AuNPs can interact with UV-emitting biomolecules through its d-band transition. These two findings surely will trigger several research topics both in bio-organic and nano-biophotonic research fields.
Methods
Thin film fabrication
Quartz or glass substrates that were cut into size of 15 × 15 mm2 were used in this study. The substrates were first cleaned with detergents and subsequently rinsed with deionized water. Acetone is used to clean the organic residue on the surface and subsequently blow-dried by nitrogen. The final substrate cleaning process was an UV-ozone treatment for 25 minutes. Albumen liquid was obtained from a chicken egg purchased at a supermarket using a stainless steel mesh spoon to separate the egg yolk, as shown in Fig. 7(a). Au nanoparticle solution was purchased from the company and the as-received concentration and nominal diameter are 1000 ppm and 20–50 nm, respectively. To increase the Au concentration, 1 ml of the as-received Au nanoparticle solution was centrifuged at 11000 rpm for 10 minutes [Fig. 7(b)]. To obtain a solution with 3-times Au nanoparticle concentration, 2/3 of the total clean solution was removed followed by ultrasonically re-suspension of the Au nanoparticles [Fig. 7(c)]. 3 of such Au nanoparticle solutions were mixed together to obtain 1 ml solution [Fig. 7(d)]. This Au nanoparticle solution was ultrasonically mixed 1 ml of the albumen liquid to obtain 2 ml of the Au-containing albumen liquid [Fig. 7(e)]. Finally, a thin film of the Au-containing albumen liquid was spin-coated on the cleaned substrates at 1000 rpm for 50 seconds [Fig. 7(f)]. The thin film is dried in air for at least 30 minutes. The final AuNPs concentrations in chicken albumen films chosen in this research are 500, 1500, 2500, 5000, 10000, 25000 ppm. The AuNPs-only samples are control samples that are fabricated directly using AuNPs water solution without mixing any albumen liquid or PVA solutions.
Schematically illustrations of the fabrication processes of the Au nanoparticle-chicken albumen complex thin film.
(a) Albumen liquid and the egg yolk were separated and (b)–(d) the Au concentration in solution was increased via the centrifugation, extraction of excess water and re-suspension. (e) The final Au solution is mixed with albumen liquid in a 1:1 ratio. (f) The final solution was spin-coated on top of the substrates.
Thin-film characterizations
The Au nanoparticle distributions insider the fabricated thin films were analyzed by a transmission electron microscope (TEM; Hitachi H-7500). Concentrations of AuNPs embedded in the chicken albumen thin films are estimated from the corresponding TEM images. The concentrations in PVA thin films are not estimated since AuNPs are not distributed uniformly inside the film. Therefore, we use the concentrations of the initial AuNPs solution as the sample names. Optical absorption spectra were obtained using a commercial UV-Visible-Near IR spectrophotometer (Hitachi U-4100). The time-integrated fluorescence spectra were performed by a commercial spectrometer (Perkin Elmer LS-55), whose excitation wavelength was 250 nm. Time-resolved fluorescence was excited with a frequency-tripled Ti:sapphire laser (266 nm, 10 mW, 80 MHz). The fluorescence signal was analyzed by a spectrometer equipped with a fast avalanche photodiode. The fluorescence-decay profiles were recorded and analyzed using a commercially available time-corrected single photon counting (TCSPC) module (Picoquant PicoHarp-300). The thickness of the thin film was measured using a step profiler (ET4000M, Kosaka, Japan).
Electromagnetic simulations
Electromagnetic simulations were performed with the three-dimensional finite-difference time-domain (3D-FDTD) method27, using a freely available software package28. The simulated structure in this research is an Au nanoparticle (D = 20 nm) embedded in water (ε = 1.77). The light source is a plane wave that is polarized in the x direction. A three-dimensional Cartesian coordinate system is used and all three dimensions of the grid cells are 1 nm. The surrounding boundaries in the z direction are perfectly matched layers. The x and y directions are set as periodic boundaries, where the periodicities are both 100 nm. The plasma frequency and damping constant of the Au are set at 1.2 × 1016 Hz and 0.8 × 1014 Hz, respectively.
References
Ritchie, R. H. Plasma Losses by Fast Electrons in Thin Films. Phys Rev 1, 874–881 (1957).
Halas, N. J., Lal, S. & Link, S. Nano-optics from sensing to waveguiding. Nat Photonics 1, 641–648 (2007).
Jeanmaire, D. L. & Vanduyne, R. P. Surface Raman Spectroelectrochemistry. 1. Heterocyclic, Aromatic and Aliphatic-Amines Adsorbed on Anodized Silver Electrode. J Electroanal Chem 84, 1–20 (1977).
Van Duyne, R. P., Bingham, J. M., Anker, J. N. & Kreno, L. E. Gas Sensing with High-Resolution Localized Surface Plasmon Resonance Spectroscopy. J Am Chem Soc 132, 17358–17359 (2010).
Kall, M., Chen, S. C. S., Svedendahl, M. & Van Duyne, R. P. Plasmon-Enhanced Colorimetric ELISA with Single Molecule Sensitivity. Nano Lett 11, 1826–1830 (2011).
Anker, J. N. et al. Biosensing with plasmonic nanosensors. Nat Mater 7, 442–453 (2008).
Hafner, J. H. & Mayer, K. M. Localized Surface Plasmon Resonance Sensors. Chem Rev 111, 3828–3857 (2011).
Haes, A. J. et al. Plasmonic materials for surface-enhanced sensing and spectroscopy. Mrs Bull 30, 368–375 (2005).
Tam, F., Goodrich, G. P., Johnson, B. R. & Halas, N. J. Plasmonic enhancement of molecular fluorescence. Nano Lett 7, 496–501 (2007).
Guzatov, D. V. et al. Plasmonic Enhancement of Molecular Fluorescence near Silver Nanoparticles: Theory, Modeling and Experiment. J Phys Chem C 116, 10723–10733 (2012).
Ming, T., Chen, H. J., Jiang, R. B., Li, Q. & Wang, J. F. Plasmon-Controlled Fluorescence: Beyond the Intensity Enhancement. J Phys Chem Lett 3, 191–202 (2012).
Wei, W. et al. Surface Plasmon-Mediated Energy Transfer in Heterogap Au-Ag Nanowires. Nano Lett 8, 3446–3449 (2008).
Jimenez, J. A., Lysenko, S. & Liu, H. Photoluminescence via plasmon resonance energy transfer in silver nanocomposite glasses. J Appl Phys 104 (2008).
Liu, G. L., Long, Y. T., Choi, Y., Kang, T. & Lee, L. P. Quantized plasmon quenching dips nanospectroscopy via plasmon resonance energy transfer. Nat Methods 4, 1015–1017 (2007).
Wiederrecht, G. P., Wurtz, G. A. & Hranisavljevic, J. Coherent coupling of molecular excitons to electronic polarizations of noble metal nanoparticles. Nano Lett 4, 2121–2125 (2004).
Fofang, N. T. et al. Plexcitonic Nanoparticles: Plasmon-Exciton Coupling in Nanoshell-J-Aggregate Complexes. Nano Lett 8, 3481–3487 (2008).
Fofang, N. T., Grady, N. K., Fan, Z. Y., Govorov, A. O. & Halas, N. J. Plexciton Dynamics: Exciton-Plasmon Coupling in a J-Aggregate-Au Nanoshell Complex Provides a Mechanism for Nonllinearity. Nano Lett 11, 1556–1560 (2011).
Manjavacas, A., de Abajo, F. J. G. & Nordlander, P. Quantum Plexcitonics: Strongly Interacting Plasmons and Excitons. Nano Lett 11, 2318–2323 (2011).
Lin, C. A. J. et al. Synthesis, Characterization and Bioconjugation of Fluorescent Gold Nanoclusters toward Biological Labeling Applications. Acs Nano 3, 395–401 (2009).
Saha, K., Agasti, S. S., Kim, C., Li, X. N. & Rotello, V. M. Gold Nanoparticles in Chemical and Biological Sensing. Chem Rev 112, 2739–2779 (2012).
Mine, Y. Egg bioscience and biotechnology. (Wiley-Interscience, 2007).
Singh, B., Sariciftci, N. S., Grote, J. G. & Hopkins, F. K. Bio-organic-semiconductor-field-effect-transistor based on deoxyribonucleic acid gate dielectric. J Appl Phys 100, 024514 (2006).
Yumusak, C., Singh, T. B., Sariciftci, N. S. & Grote, J. G. Bio-organic field effect transistors based on crosslinked deoxyribonucleic acid (DNA) gate dielectric. Appl Phys Lett 95, 263304 (2009).
Kim, Y. S. et al. High-mobility bio-organic field effect transistors with photoreactive DNAs as gate insulators. Appl Phys Lett 96, 103307 (2010).
Wang, C. H., Hsieh, C. Y. & Hwang, J. C. Flexible Organic Thin-Film Transistors with Silk Fibroin as the Gate Dielectric. Adv Mater 23, 1630–1634 (2011).
Chang, J. W. et al. Chicken Albumen Dielectrics in Organic Field-Effect Transistors. Adv Mater 23, 4077–4081 (2011).
Taflove, A. & Hagness, S. C. Computational electrodynamics : the finite-difference time-domain method. 3rd edn, (Artech House, 2005).
Oskooi, A. F. et al. MEEP: A flexible free-software package for electromagnetic simulations by the FDTD method. Comput Phys Commun 181, 687–702 (2010).
Acknowledgements
We thank the financial support from National Science Council, Taiwan under Grant Number (NSC 99-2112-M-006-015-MY2, NSC 99-2113-M-006-008-MY3, NSC 101-2112-M-006-013-MY2 and NSC 100-2627-B-006-014-). We also thank for the collaboration of Center of Micro/Nano Science and Technology, National Cheng Kung University, Tainan, Taiwan. The National Center for High-Performance Computing of Taiwan and the Computer and Network of National Cheng Kung University are also acknowledged for uses of high performance computing facilities. The valuable suggestions from Professors Yung-Chiang Lan and Shih-Hui Chang of Department of Photonics are also much appreciated.
Author information
Authors and Affiliations
Contributions
Y.C. Chang and T.F. Guo both conceived the design and supervised the whole projects. I.T. Chen, P.H. Chang fabricated the samples and performed various characterization. Y.C. Chang performed the electromagnetic simulations and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
-Tsung Chen, I., Chang, PH., Chang, YC. et al. Lighting Up Ultraviolet Fluorescence From Chicken Albumen Through Plasmon Resonance Energy Transfer of Gold Nanoparticles. Sci Rep 3, 1505 (2013). https://doi.org/10.1038/srep01505
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01505
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.