Abstract
Ultra-definition, large-area displays with three-dimensional visual effects represent megatrend in the current/future display industry. On the hardware level, such a “dream” display requires faster pixel switching and higher driving current, which in turn necessitate thin-film transistors (TFTs) with high mobility. Amorphous oxide semiconductors (AOS) such as In-Ga-Zn-O are poised to enable such TFTs, but the trade-off between device performance and stability under illumination critically limits their usability, which is related to the hampered electron-hole recombination caused by the oxygen vacancies. Here we have improved the illumination stability by substituting oxygen with nitrogen in ZnO, which may deactivate oxygen vacancies by raising valence bands above the defect levels. Indeed, the stability under illumination and electrical bias is superior to that of previous AOS-based TFTs. By achieving both mobility and stability, it is highly expected that the present ZnON TFTs will be extensively deployed in next-generation flat-panel displays.
Similar content being viewed by others
Introduction
The introduction of flat-panel, thin-film transistor liquid-crystal display (TFT-LCD) enormously expanded the horizon of display application by enabling production of large-screen TV sets and various mobile devices. Nevertheless, the demand for more realistic visual experiences is driving academic as well as industrial research efforts towards next-generation displays incorporating “ultra” definition (4000 × 2000 pixels), large-area (> 70 inch) and three-dimensional effects. The feasibility of such high-end devices, whether employing liquid crystal or organic light-emitting diode (OLED), hinges on high performance TFT components that can switch on or off each pixel at unprecedented rates. Technically, a transistor that exhibits field-effect mobility values higher than 20 cm2V−1s−1 is required1,2.
Most of present LCD devices employ hydrogenated amorphous silicon (a-Si:H) as the channel layer in TFTs owing to low fabrication cost and excellent cell-to-cell uniformity. However, the directional sp3 bonding intrinsically limits the field-effect mobility of amorphous silicon below 1 cm2V−1s−1, which is far lower than the above requisite for next-generation displays. Nomura et al. demonstrated that the intrinsic drawback of the amorphous network can be circumvented by employing materials with non-directional s character in the conduction band such as In-Ga-Zn-O (a-IGZO), so called amorphous oxide semiconductors (AOS)3,4. Several prototypical displays incorporating AOS-TFTs have been demonstrated with promising results2,5.
However, it is suggested that the presence of donor-like oxygen vacancies (Vo) located within the wide bandgap of AOS (ref. 6), which is also critical for the high mobility7,8, leads to slow decay of photogenerated current when light and negative gate bias are applied and hence significantly compromises device stability by shifting the threshold voltage9,10,11. This negative-bias illumination (NBI) instability makes AOS remain conductive for several hours/days, even in the absence of light. Therefore, such slow recovery also limits the switching speed of TFTs and the frame rates in flat-panel displays (FPDs)12. Although the approaches to resolve the NBI instability by applying short positive gate pulses inducing electron accumulation and accelerating the recombination with ionized Vo sites have been recently reported, they can only be applied to three-terminal devices along with the complex bias scheme12,13. Therefore, a more fundamental solution, which could be adapted to a variety of oxide systems and device architectures, is in high demand.
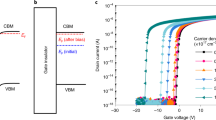
In this work, through the careful consideration of the electronic band structure of the most well-known oxide semiconductor ZnO, where problematic Vo levels are located near its valence band maximum (VBM), we have hypothesized that the NBI instability could be suppressed via the control over the valence band by the substitution of non-oxide anions for the oxygen anions that contribute to the top portion of valence band. Therefore, it is believed that the larger size anions (N3−, S2−, Se2− and so on; refs 14,15,16,17,18) with higher p orbital energy than oxygen may deactivate or remove oxygen vacancies by increasing the valence band energy, as shown in Fig. 1a.
Valence band control of oxide semiconductor to deactivate the oxygen vacancy and high-mobility properties of amorphous-like ZnON.
(a) Schematic band diagrams of ZnO (optical bandgap = 3.2 eV), ZnON (1.3 eV) and Zn3N2 (1.0 eV) and ZnON (1.3 eV). The persistent photocurrent (PPC) under negative gate bias, which is related to donor-like oxygen vacancies (Vo) located within the wide bandgap, is found in the typical oxide semiconductor ZnO. On the other hand, such an effect is not expected in ZnON, where the nitrogen anions with higher p orbital energy than oxygen may passivate or remove Vo by increasing the valence band maximum (VBM) energy level. Zn3N2 exhibits conductor-like characteristics, not semiconducting due to the large number of electron carriers (> 1019 cm−3; ref. 21). (b) Relationship between Hall mobility and carrier concentration for ZnON films. Data on a-IGZO films are shown for comparison (ref. 1). (c) Conduction bands of c-ZnO, c-In2O3, c-Zn3N2 and a-ZnON are plotted along kx with the conduction band minimum (CBM) aligned at 0 eV. Inset: The model unit cell of a-ZnON containing 60 Zn, 24 O and 24 N atoms obtained from the melt-quench simulations.
Here, we present a combined experimental and theoretical study on Zn3N2-alloyed ZnO (or ZnON) to elucidate the possibility of tuning the valence band to deactivate the deep Vo level in oxide semiconductors. Through the substitutional alloying with nitrogen anions, we successfully increase the VBM by as high as ∼1.6 eV compared to pure ZnO, which is sufficiently higher than the theoretically reported Vo levels (located within 1 eV above VBM; ref. 19) in the ZnO bandgap. To verify our strategy, we fabricated and electrically tested the TFTs that use amorphous-like ZnON films as the active channel layer. While having high field-effect mobility exceeding 30 cm2V−1s−1, the TFTs based on ZnON exhibit little signature of the NBI instability and also excellent device stability under illumination with visible light, compared to conventional AOS-devices with similar mobility values. As such, the device may rival a-Si:H TFTs that have been widely adopted in active-matrix LCDs in terms of long-term stability.
Results
Films of ZnON were prepared by reactive radio-frequency magnetron sputtering using a Zn target in oxygen and nitrogen atmosphere, which was developed by Ye et al. (ref. 20). In order to control anion contents within the film, oxygen partial pressure in the chamber was varied between 3.6–28.1 mPa during deposition. The composition of the grown sample was measured by Rutherford backscattering spectroscopy and the oxygen (nitrogen) content was found to increase (decrease) with the oxygen partial pressure, taking up below 5 to 40% of the total atoms while the Zn fraction was kept nearly constant at ∼55% (see Supplementary Fig. S1a). All grown films were n-type semiconductors when characterized by Hall measurements. The carrier concentration significantly decreases from ∼3.0 × 1018 to ∼2.0 × 1016 cm−3 as the oxygen partial pressure increases, indicating that oxygen may act as a carrier suppressor (Supplementary Fig. S1b). The Hall mobility increases as the carrier concentration increases, which might be understood by the percolation model similar to the case of a-IGZO (ref. 1) and large Hall mobilities (≥ 50 cm2V−1s−1) were obtained at carrier concentrations ≥ 1017 cm−3 (Fig. 1b). It is noted that the Hall mobility of ZnON increases with the carrier concentration more rapidly than for a-IGZO. This could be attributed to the relatively low potential barriers above the conduction band edge of ZnON that is merely composed of single Zn2+ ions, unlike the random distribution of multiple cations (In3+, Zn2+ and Ga3+) in a-IGZO (ref. 20). For all subsequent experiments, the film made at 14 mPa was used as the film shows high Hall mobility values (∼50 cm2V−1s−1) and reasonably low carrier concentrations of ∼1017 cm−3. The chemical composition of the film is Zn:N:O = 56.3:21.7:22.0 (in atomic percent).
From grazing incidence angle X-ray diffraction (GIAXRD; Supplementary Fig. S2a), the ZnON films are confirmed to retain the amorphous-like structure up to an annealing temperature of 350°C, which corresponds to the maximum process temperature (i.e., for the gate insulator growth before ZnON deposition) for our TFT fabrication. It is believed that amorphous-like phase of ZnON is promoted by the competition between reactions for the growth of cubic Zn3N2 and for growth of hexagonal ZnO in a reactive sputtering process20. Further transmission electron microscopy (TEM) images reveal the presence of a small fraction of nanocrystallites randomly dispersed in the amorphous matrix (Supplementary Fig. S2b). The films have a smooth surface topography with no discernible grain boundaries and a small root mean square surface roughness of 0.7 nm (Supplementary Fig. S2c). The X-ray photoelectron spectroscopy (XPS) data in Supplementary Fig. S3 show the existence of N-Zn and O-Zn bonds, confirming the formation of an oxynitride compound21,22. The additional small O 1s peak at 531.4 eV is attributed to OH− impurities which may originate from unintentional moisture adsorption before ex-situ XPS analysis, as the feature is more pronounced in the O 1s spectra collected at the top surface prior to Ar sputtering.
The insets of Fig. 2a show photographs of ZnON and ZnO films on glass substrates. While ZnO is highly transparent, ZnON has a darker color due to the enhanced absorption of light in the visible wavelength range (photon energy, hν = 1.65 ∼ 3.18 eV). Indeed, the optical bandgap estimated from the Tauc model is ∼1.3 eV for ZnON (Fig. 2a), which is drastically reduced from 3.2 eV of ZnO and is slightly larger than that (∼1.0 eV) of Zn3N2 (ref. 22,23), suggesting that ZnON is a narrow band gap semiconductor. It is also noted that there is a large Urbach tail in ZnO, which suggests the presence of a large density of subgap defect states mostly originating from Vo (ref. 12). Figure 2b shows the XPS spectra of the bandgap region near the valence band maximum (VBM). The VBM energy level of the ZnON film shifts upward by ∼1.6 eV relative to ZnO, which is similar to the optical bandgap difference (∼1.9 eV).
Optical properties and electronic structures of ZnON.
(a) Tauc plot; (αhν)2 as a function of photon energy hν, where ν is the photon frequency, indicating the optical bandgap (Eg) for ZnON (1.3 eV) and ZnO (3.2 eV) films. Insets: Photographs of ZnON and ZnO samples intuitively showing the effect of nitrogen incorporation on the bandgap reduction. (b) XPS spectra of the bandgap region for ZnO and ZnON films. The valence band offsets are determined by extrapolating the straight line regions of the onset spectra to the baseline. (c) Calculated total electronic DOS (TDOS) and projection onto the Zn, O and N atoms for a-ZnON. The VBM energy level is set to 0 eV.
To understand the electronic structure of amorphous-like ZnON in comparison with conventional oxide/nitride semiconductors, first-principles calculations based on density functional theory (DFT) were performed. Amorphous structures were obtained through melt-quench simulations. To prevent the formation of N2 molecules during the melting stage, the structure was melted briefly at 2000 K. (See Methods section for the full computational details.) The inset of Fig. 1c shows atomic structures of an amorphous ZnON (a-ZnON) with stoichiometry of Zn60O24N24. It is found that the local order around each atom is similar to those in crystalline ZnO (c-ZnO) or Zn3N2 (c-Zn3N2). In Fig. 1c, the conduction bands of c-ZnO, c-In2O3, c-Zn3N2 and a-ZnON are compared since electron conduction is dominant in these materials. For comparison, the conduction band minima were aligned among different materials. Even though the dominant scattering mechanism in AOS is not clarified yet, the effective mass at the conduction band bottom should be an important parameter in any mechanism. It was shown that the conduction bands of AOS can be well fitted to the dispersion relation of a massive Dirac particle in the form of  , with ε0, ε and γ as fitting parameters24. The effective masses at Γ (
, with ε0, ε and γ as fitting parameters24. The effective masses at Γ ( ) inferred from this dispersion relation are 0.27, 0.22, 0.10 and 0.19 me for c-ZnO, c-In2O3, c-Zn3N2 and a-ZnON, respectively. Therefore,
) inferred from this dispersion relation are 0.27, 0.22, 0.10 and 0.19 me for c-ZnO, c-In2O3, c-Zn3N2 and a-ZnON, respectively. Therefore,  of a-ZnON lies in between those of c-ZnO and c-Zn3N2 and is smaller than those of c-In2O3 and, presumably, AOS. Therefore, the high mobility of a-ZnON may originate from the smaller
of a-ZnON lies in between those of c-ZnO and c-Zn3N2 and is smaller than those of c-In2O3 and, presumably, AOS. Therefore, the high mobility of a-ZnON may originate from the smaller  .
.
Figure 2c shows the calculated total electronic density of states and their projection onto the Zn, O and N atoms for a-ZnON. The bandgap reduction in a-ZnON is mainly due to N states (mostly N 2p character) in valence band, which is consistent with the experimental XPS data in Fig. 2b.
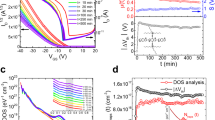
We fabricated more than 100 bottom-gate ZnON TFTs with Molybdenum (Mo) source-drain top contacts, which are the typical device structure of the AOS-TFTs (Fig. 3a). Figure 3b shows a cross-sectional TEM image of the fabricated ZnON TFT, where the gate, insulator, channel, etch stopper and source/drain layers are well defined with excellent thickness uniformity, suggesting the absence of inter-diffusion between its constituent layers. The output and transfer characteristics were electrically measured and a typical example is illustrated in Fig. 3c and d. The source-to-drain current (IDS) increases markedly with the source-to-drain voltage (VDS) at a positive gate-to-source voltage (VGS), indicating that the channel is indeed n-type (Fig. 3c). The IDS–VDS curve exhibits a clear pinch-off and current saturation without current-crowding in the low VDS region, which suggests that the TFT operation is consistent with the standard field-effect transistor theory25. The transfer curve (Fig. 3d) shows that a low off-current of the order of 10−12 A and an on-to-off current ratio ∼108 are obtained. The threshold voltage (VT) is positive (∼5.4 V), suggesting that the TFT operates in enhancement mode. The gate leakage current (IG) is orders of magnitude lower than the IDS, which guarantees that the TFT characteristics are not affected by the gate leakage. The calculated saturation field-effect mobility (μsat) reaches a high value of 42.6 cm2V−1s−1 which agrees roughly with the Hall mobility of ZnON films (∼50 cm2V−1s−1). However, a relatively large subthreshold swing (S) of ∼1.2 V/decade and a hysteresis of ∼0.7 V (Supplementary Fig. S4a) indicate that further improvements in the channel-insulator interface are in demand. In addition, it is speculated that the large S value of ZnON TFTs would have also originated from the generation of the tail states related to nitrogen vacancies with shallow defect states26.
Typical ZnON TFT characteristics.
(a) Schematic of the fabricated ZnON TFT device with an inverted staggered bottom-gate structure. A white-light LED with a tunable luminance of up to 15,400 lux was used in order to supply visible light. SiOx is a well-known insulator material with a wide band gap of 8.5-9 eV and transparent or inactive in the visible light range (photon energy, hν = 1.65 ∼ 3.18 eV). Therefore, most of the visible light goes through the etch-stopper layer and reaches the ZnON active layer. (b) Cross-sectional TEM image of the fabricated TFT structure consisting of a glass substrate, Mo gate electrode (200 nm), SiNx (350 nm)/SiOx (50 nm) gate insulator, ZnON (50 nm) for the active channel, SiOx (100 nm) etch-stopper layer and Mo (200 nm) for the source/drain electrodes. (c) Output (IDS-VDS) curves which were collected at different VGS as marked. (d) Transfer curves (IDS-VGS) acquired with a VDS of 10 V.
Next, we examine the photo-response against visible light illumination using a commercially available white-light LED (Fig. 3a). As references, conventional a-Si:H TFTs (μsat = 0.8 cm2V−1s−1, VT = 3.2 V, S = 0.9 V/decade) and a-IZO TFTs (μsat = 43.2 cm2V−1s−1, VT = −0.7 V, S = 0.2 V/decade) were fabricated (see Supplementary Fig. S5) and compared with ZnON TFTs. Figure 4a summarizes the transfer characteristics of the TFTs both in the dark and under illumination (15,400 lux). First, it is observed that a-IZO TFTs are highly sensitive to visible light and the device cannot be turned off with the application of gate voltages down to −30 V. Furthermore, the photo-current (>10−7 A) increases by more than five orders of magnitude on illumination and persists long even after the illumination has been turned off (left inset of Fig. 4a), which is related to the NBI instability6,9,10,12,13. On the other hand, ZnON TFTs exhibit inappreciable VT shift under exposure to visible light and very low photo-currents (<10−10 A; ref. 27) with magnitudes smaller than even that of a-Si:H (∼10−9 A) and do not suffer from the VT shift via the fast recombination or recovery by band-to-band transition under such a harsh illumination of 15,400 lux (typical office lighting conditions lie between 320–500 lux).
The photo-stability of ZnON TFTs. Data on a-IZO and a-Si:H TFTs are also shown for comparison.
(a) Transfer characteristics of the TFTs both in the dark and under illumination (15,400 lux). Inset shows the source-to-drain current (IDS) as a function of time as the TFTs are subjected to illumination at an intensity of 15,400 lux, turned on and off with a time interval of 60 sec. (b) Parallel negative shifts in the transfer characteristics of the TFTs under illumination (445 lux). (c) Threshold voltage shift (ΔVT) as a function of stress time under dark and illumination (445 lux). (d) ΔVT as a function of light intensity illuminating the TFTs. Inset is an enlarged graph showing ΔVT under weak illumination < 500 lux.
An important performance metric, particularly for the TFTs in FPDs, is the operational device stability under illumination2,5,7,8, i.e., when both light and electrical bias are applied simultaneously. In the dark, IZO TFTs show very stable performance with almost no VT shift (ΔVT) as a function of stress time (see Supplementary Fig. S6a), however, a huge negative VT shift (∼10 V) occurs even under weak illumination (Fig. 4b; Supplementary Fig. S6a). In contrast, illuminated ZnON TFTs operate stably with no apparent performance degradation (Fig. 4b and 4c; Supplementary Fig. S6c) and the good stability is maintained even at a maximum light intensity of 15,400 lux. Such a level of stability is comparable to that of a-Si:H TFTs (Fig. 4c and 4d; Supplementary Fig. S6b). The superior device stability of ZnON TFTs may originate from the fact that much less photo-excited carriers are generated with a higher recombination rate compared to a-IZO (see Fig. 4a).
Discussion
In the present work, we have demonstrated that the addition of non-oxide anions into oxide semiconductors can offer an effective way to circumvent NBI instability which is the most critical drawback in oxide semiconductors. In particular, we showed the typical example of oxide semiconductor ZnO, where the valence band could be controlled to remove or passivate the Vo levels by substitutional alloying with nitrogen anions. The TFT devices based on ZnON as active channel layer demonstrate the real suppression of persistent photocurrents under negative bias and excellent device stability even in the presence of visible light radiation, in contrast to conventional AOSs with similar mobilities exceeding 30 cm2V−1s−1. It is thus anticipated that our approach of controlling anions will open a new avenue to the realization of high-performance and high-stability microelectronics and optoelectronics including displays based on AOS. Additionally, the ability to suppress the adverse Vo effect may shed light on other oxide systems that suffer from Vo.
Methods
All samples were prepared on 15 cm × 15 cm glass substrates. Prior to film deposition, the substrates were chemically cleaned using aqueous mixtures of H2SO4 and H2O2, followed by deionized water rinsing. ZnON films were fabricated by reactive radio-frequency (RF) magnetron sputtering (ULVAC, SME-200U) using a mixture of N2, O2 and Ar as working gases, where the purity of the sputtering gases was 99.999%. Each gas was introduced through separate mass flow controllers. A sputtering chamber was evacuated to below 2.0 × 10−5 Pa with a turbomolecular pump and a mechanical rotary pump. A 5-inch diameter Zn (purity 99.99%) target was used and the substrates were mounted on a rotating holder at a distance of 18 cm from the target. Prior to deposition, the target was pre-sputtered with Ar ions for 30 min to remove unintentional contaminations on the surface. ZnON films were grown at a substrate temperature of 50°C with a RF sputtering power of 300 W. In order to control the anion contents in the films, the O2 partial pressure (3.6–28.1 mPa) in the chamber was varied during the deposition by adjusting the O2 flow rate (1–4 sccm) and the total gas pressure (0.4–0.8 Pa), while the N2 (100 sccm) and Ar flow rates (10 sccm) were kept constant.
The crystal structures of the films were examined by grazing incidence angle X-ray diffraction (GIAXRD) analyses using CuKα radiation (PANalytical, X'pert Pro). The microstructure and crystallinity of the films were observed in detail using transmission electron microscopy (a Cs-corrected FEI Titan operating at 300 kV) and fast Fourier transform (FFT) analysis. AFM images were obtained using a scanning probe microscope (Veeco, Dimension V) in tapping mode with Si probe tips. The film thickness was determined by a surface profilometry (KLA Tencor, P16) to be approximately 50 nm. Optical spectra were acquired with a Cary 5000 ultraviolet-visible-near-infrared spectrophotometer. In order to investigate the bonding state of the films, XPS analysis was carried out using a PHI VersaProbe XPS Microprobe, equipped with an Al X-ray source, K radiation (1486.8 eV) in ultrahigh vacuum, with a pass energy of 23.5 eV (energy resolution better than 0.77 eV as determined on a silver sample). Samples were sputtered for 1 min using Ar ions, which have a sputtering rate of 1.0 nm/min with reference to SiO2, in order to remove surface contaminations. The measured binding energies were referenced to Zn 2p3 peak at 1021.8 eV. For accurate composition analysis of Zn, O and N in the films, a high-resolution Rutherford backscattering system (KOBELCO, HRBS-V500) equipped with a magnetic sector analyzer capable of obtaining a high-resolution energy spectrum of the Medium Energy Ion Scattering Spectroscopy (MEIS) level was used. The RBS analysis was carried out with a 450 keV He+ probe beam, with an incident angle of 50° and a scattering angle of 70.5°. The electrical properties of thin films such as carrier concentration and Hall mobility were measured at room temperature using the four-probe Van der Pauw technique (nanometrics, HL5500).
ZnON TFTs with an inverted staggered bottom-gate and top-contact configuration (Figure 3a) were fabricated on glass substrates (15 cm × 15 cm) using standard semiconductor fabrication process. The integration of TFT devices was done firstly by direct-current (DC) sputter depositing a 200-nm-thick Mo gate at room temperature and subsequently growing a dielectric stack of a 350-nm-thick SiNx and a 50-nm-thick SiOx at 350°C by plasma-enhanced chemical vapor deposition (PECVD). The ZnON active layer with a thickness of 50 nm was then deposited through a reactive sputtering process as previously described, using a Zn target at a substrate temperature of 50°C. Subsequently, a 100-nm-thick SiOx etch-stopper layer was deposited at 200°C by PECVD and then, 200-nm-thick Mo was sputtered at room temperature to form the source-drain electrodes. The deposition conditions for the etch-stopper layer (i.e., SiH4/N2O = 10/500 sccm) were optimized to minimize the hydrogen incorporation into ZnON layer. All patterning was done by photolithography and appropriate use of wet or dry etching. The above transistors were then annealed in air for 1 h at 250°C. As references, a-IZO TFTs with the same device structure described above, where the cation ratio (atomic %) for the IZO active layer is In:Zn = 85:15 and a-Si:H TFTs with a back-channel-etch (BCE) structure were also fabricated.
Devices with channel width/length = 50/30 μm for ZnON and a-IZO TFTs and 15/5 μm for a-Si:H TFTs were characterized at room temperature in a vacuum chamber (CryoVac, Vacuum Probe Station) to prevent ambient effects, which can be evacuated to below 1.0 × 10−3 Pa with a semiconductor parameter analyzer (Keithley, 4200-SCS). The threshold voltage (VT), subthreshold swing (S) and saturation field-effect mobility (μsat) were extracted in compliance with the gradual channel approximation25. The μFE and VT were obtained from the transfer curves in the saturation region (VDS = +10 V) using IDS = (Cox·μsat·W/2L)·(VGS−VT)2, where Cox is the capacitance of the SiOx(50 nm)/SiNx(350 nm) gate dielectric stack (Cox = 12.5 nFcm−2). The S was taken as the minimum value of the inverse slope of the log10(IDS) versus VGS characteristics. The on-to-off current ratio was calculated from the maximum IDS divided by the minimum IDS in the transfer curve. The amount of hysteresis was defined as the difference between the threshold voltages extracted from the forward and reverse sweeps of the transfer curves. The negative gate bias stress measurements were done at room temperature with simultaneous exposure to visible light. During the stress experiments, a VGS of −20 V and a VDS of +10 V were applied to each TFT device for a total duration of 3 hours. A white-light LED with a tunable luminance of up to 15,400 lux (Sol, LED Light Source) was used in order to supply visible light. During the stress measurements, transfer curves (VDS = +10 V) were collected every 30 min to extract the VT as a function of stress time. A pristine device was used for each stress measurement. The photo-response against light illumination was also examined from the IDS as a function of time, as the light radiating the TFTs were turned on (15,400 lux) and off with a time interval of 60 sec.
We used Vienna Ab-initio Simulation Package (VASP; ref. 28) to carry out first-principles calculation within density functional theory, employing the projector-augmented wave (PAW) potential for ionic potentials29. The exchange-correlation energy between electrons are described within the hybrid functional developed by Heyd-Scuseria-Ernzerhof (HSE; ref. 30) except for the melt-quench simulation to obtain a-ZnON where the generalized-gradient-approximation (GGA; ref. 31) is used to avoid the sheer computational cost of the hybrid functional. The mixing parameters in the HSE functional were chosen to fit the experimental band gap32 and they are 0.37 (c-ZnO), 0.25 (c-In2O3), 0.29 (c-Zn3N2) and 0.33 (a-ZnON). The energy cutoff is set to 500 eV for all model systems in the present work. The k-point meshes are chosen on the basis of convergence in the total energy and force within 5 meV/atom and 5 meV/Å, respectively. For a-ZnON, we made amorphous structure using melt-quenching method. Initially, the wurtzite structure of ZnO is used and half of O atoms are replaced with N atoms to mimic our optimized Zn56.3O22N21.7 sample. Some O and N atoms are removed from the model system to satisfy the stoichiometry conditions. The supercell consists of 108 atoms; 60 Zn, 24 O and 24 N atoms. The model system was melted at 2000 K for 20 ps and then it was rapidly quenched to room temperature with the cooling rate of 100 K/ps in order to avoid the formation of N2 molecule and phase separation into Zn3N2 and ZnO.
References
Arai, T. & Sasaoka, T. Emergent oxide TFT technologies for next-generation AM-OLED displays. 2011 SID International Symposium Digest of Technical Papers 42, 710–713 (2011).
Kamiya, T., Nomura, K. & Hosono, H. Present status of amorphous In-Ga-Zn-O thin-film transistors. Sci. Technol. Adv. Mater. 11, 044305 (2010).
Nomura, K. et al. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 432, 488–492 (2004).
Nomura, K. et al. Thin-film transistor fabricated in single-crystalline transparent oxide semiconductor. Science 300, 1269–1272 (2003).
Park, J. S., Maeng, W-J., Kim, H-S. & Park, J-S. Review of recent developments in amorphous oxide semiconductor thin-film transistor devices. Thin Solid Films 520, 1679–1693 (2012).
Lany, S. & Zunger, A. Anion vacancies as a source of persistent photoconductivity in II-VI and chalcopyrite semiconductors. Phys. Rev. B 72, 035215 (2005).
Kwon, J-Y. et al. Investigation of light-induced bias instability in Hf-In-Zn-O thin film transistors: A Cation combinatorial approach. J. Electrochem. Soc. 158, H433–H437 (2011).
Kim, H-S. et al. The influence of In/Zn ratio on the performance and negative-bias instability of Hf-In-Zn-O thin-film transistors under illumination. IEEE Electron Device Lett. 32, 1251–1253 (2011).
Ghaffarzadeh, K. et al. Persistent photoconductivity in Hf-In-Zn-O thin film transistors. Appl. Phys. Lett. 97, 143510 (2010).
Ghaffarzadeh, K. et al. Instability in threshold voltage and subthreshold behavior in Hf-In-Zn-O thin film transistors induced by bias-and light-stress. Appl. Phys. Lett. 97, 113504 (2010).
Chowdhury, M. D. H., Migliorato, P. & Jang, J. Light induced instabilities in amorphous indium-gallium-zinc-oxide thin-film transistors. Appl. Phys. Lett. 97, 173506 (2010).
Jeon, S. et al. Gated three-terminal device architecture to eliminate persistent photoconductivity in oxide semiconductor photosensor arrays. Nature Mater. 11, 301–305 (2012).
Ahn, S-E. et al. Metal Oxide Thin Film Phototransistor for Remote Touch Interactive Displays. Adv. Mater. 24, 2631-2636 (2012).
Jansen, M. & Letschert, H. P. Inorganic yellow-red pigments without toxic metals. Nature 404, 980–982 (2000).
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 293, 269–271 (2001).
Khan, S. U. M., Al-Shahry, M. & Ingler Jr, W. B. Efficient Photochemical Water Splitting by a chemically Modified n-TiO2 . Science 297, 2243–2245 (2002).
Umebayashi, T., Yamaki, T., Itoh, H. & Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 81, 454–456 (2002).
Sakthivel, S. & Kisch, H. Daylight Photocatalysis by Carbon-Modified Titanium Dioxide. Angew. Chem. Int. Ed. 42, 4908–4911 (2003).
Agoston, P., Albe, K., Nieminen, R. & Puska, M. J. Intrinsic n-Type Behavior in Transparent Conducting Oxides: A Comparative Hybrid-Functional Study of In2O3, SnO2 and ZnO. Phys. Rev. Lett. 103, 245501 (2009).
Ye, Y., Lim, R. & White, J. M. High mobility amorphous zinc oxynitride semiconductor material for thin film transistors. J. Appl. Phys. 106, 074512 (2009).
Futsuhara, M., Yoshioka, K. & Takai, O. Structural, electrical and optical properties of zinc nitride thin films prepared by reactive rf magnetron sputtering. Thin Solid Films 322, 274–281 (1998).
Suda, T. & Kakishita, K. Band-gap energy and electron effective mass of polycrystalline Zn3N2 . J. Appl. Phys. 99, 076101 (2006).
Xing, G. Z. et al. Structural and electrical characteristics of high quality (100) oriented-Zn3N2 thin films grown by radio-frequency magnetron sputtering. J. Appl. Phys. 108, 083710 (2010).
Kang, Y. et al. Microscopic origin of universal quasilinear band structures of transparent conducting oxides. Phys. Rev. Lett. 108, 196404 (2012).
Sze, S. M. & Ng, K. K. Physics of Semiconductor Devices (Wiley, 2007).
Long, R., Dai, Y., Yu, L., Huang, B. & Han, S. Atomic geometry and electronic structure of defects in Zn3N2 . Thin Solid Films 516, 1297–1301 (2008).
Ahn, K-S. et al. Enhanced photoelectrochemical responses of ZnO films through Ga and N codoping. Appl. Phys. Lett. 91, 231909 (2007).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993); Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B, 49, 14251–14269 (1994).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Heyd, J., Scuseria, G. E. & Ernzerhof, M. Hybrid functional based on a screened Coulomb potential. J. Chem. Phys. 118, 8207–8215 (2003).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Park, S., Lee, B., Jeon, S. H. & Han, S. Hybrid functional study on structural and electronic properties of oxides. Curr. Appl. Phys. 11, S337–S340 (2011).
Acknowledgements
The authors are grateful to SAIT colleagues Y. Kim, Y. K. Cha and C. Y. Moon for assistance with the experiments. This work was supported by the Center for Multiscale Energy System. The computations were carried out at KISTI (KSC-2011-C3-17).
Author information
Authors and Affiliations
Contributions
H.-S.K., M.R. and S.Y.L. designed this work. H.-S.K. and S.H. wrote the manuscript. H.-S.K., J.S.P., T.S.K., K.S.S. carried out the experiments and electrical measurements. E.L., J.G.C. and H.L. contributed to TEM, XPS and RBS analysis, respectively. S.H.J. and S.H. performed the DFT calculations. All authors discussed the results and commented on the manuscript. The project was supervised by K.K.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Kim, HS., Jeon, S., Park, J. et al. Anion control as a strategy to achieve high-mobility and high-stability oxide thin-film transistors. Sci Rep 3, 1459 (2013). https://doi.org/10.1038/srep01459
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01459
This article is cited by
-
Tail state mediated conduction in zinc tin oxide thinfilm phototransistors under below bandgap optical excitation
Scientific Reports (2021)
-
Significant enhancement of the bias stability of Zn-O-N thin-film transistors via Si doping
Scientific Reports (2020)
-
Comprehensive Review on Amorphous Oxide Semiconductor Thin Film Transistor
Transactions on Electrical and Electronic Materials (2020)
-
Wearable 1 V operating thin-film transistors with solution-processed metal-oxide semiconductor and dielectric films fabricated by deep ultra-violet photo annealing at low temperature
Scientific Reports (2019)
-
Understanding electronic transport in multi-component amorphous semiconductors
CSI Transactions on ICT (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.