Abstract
Physiologically relevant steroid 5α-reductase (SRD5A) activity that is essential for dihydrotestosterone (DHT) biosynthesis in human castration-resistant prostate cancer (CRPC) has not been fully characterized yet. In this study to ascertain the potential SRD5A activity, we cultured two human CRPC cell lines, C4-2 and C4-2AT6, with the steroid precursor: 13C-[2,3,4]-androstenedione (13C-Adione) and analyzed the sequential biosynthesis of 13C-[2,3,4]-testosterone (13C-T) and 13C-[2,3,4]-DHT (13C-DHT) by liquid chromatography/mass spectrometry (LC/MS/MS). The 13C-DHT/13C-T concentration ratio detected by LC/MS/MS in C4-2AT6 cells appeared to reflect the SRD5A activity. The ratio in C4-2AT6 was significantly lower than that in C4-2. An increased concentration of DHT did not have a positive effect on cell proliferation, rather it exhibited inhibitory effects. 5α-reductase inhibitors did not have any inhibitory effect at clinically achievable concentrations. These results indicate that CRPC cells may have an unknown regulation system to protect themselves from an androgenic suppressive effect mediated by SRD5A activity.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is one of the most commonly diagnosed malignant tumors in men and the second leading cause of cancer-related deaths in the United States1. Androgen ablation is the gold standard treatment for advanced PCa. One of the most troublesome aspects of PCa is that androgen-dependent PCa inevitably progresses to highly aggressive and life-threatening castration-resistant prostate cancer (CRPC) after androgen ablation therapy2. Recurrent tumors frequently express androgen receptor (AR) target genes3, such as prostate-specific antigen (PSA) and about 30% of patients with progressive disease respond to additional hormonal manipulations4,5. These findings suggest that many recurrent prostate cancers are neither hormone refractory nor androgen independent, but maintain a clinically relevant reliance on the AR signaling axis.
More recently, intratumoral conversion of adrenal androgens and de novo steroid synthesis have been proposed as potential causes of PCa progression6,7. The reported high intratumoral testosterone and dihydrotestosterone (DHT) concentrations in CRPC patients with castrated serum androgen levels also suggested that CRPC maintains a clinically relevant reliance on the AR signaling axis. Androgen receptor activation by androgens converted from adrenal androgens or synthesized intratumorally via the de novo route has been proposed as one of the mechanisms of castration resistance7,8,9,10,11. However, DHT production by PCa has not been fully characterized yet12,13. Although 5α-reductase (5AR), which is essential for DHT biosynthesis, has been detected at the mRNA level in CRPC metastases9,10,11,14, physiologically relevant 5AR activity has not been fully demonstrated in human CRPC yet. Recent advances have shed light on the relationship between androgens and the development or the progression of PCa15,16,17,18. The use of 5α-reductase inhibitors (5ARI) to prevent progression of Pca, continues to be widely discussed17,18,19. Does progression to CRPC depend on DHT produced by 5α-reductase? Is it effective to treat CRPC using 5ARIs? The effects of finasteride or dutasteride on metastatic prostate cancer or progression to CRPC have not yet been evaluated.
We have previously reported a useful model of human CRPC20,21,22,23,24,25. Briefly, we cultured the PTEN-null, androgen receptor (AR) positive, PSA producing CRPC cell line C4-2 for more than 6 months under androgen ablation conditions and named it C4-2AT6. These cells harbor the following characteristics: aggressive angiogenic properties and elevated phosphorylated Akt expression. These two cell lines may reproduce the aspect of clinical CRPC progression and offer an excellent model system with which to study their complicated biology.
In this study, we sought to determine whether there was physiologically relevant SRD5As activity in human CRPC cell lines C4-2 and C4-2AT6. To ascertain the potential of SRD5As activity, we developed a co-culture system using the steroid precursor C13-[2,3,4]-progesterone with C4-2 and C4-2AT6 cells. We analyzed the sequential biosynthesis of androgens from each C13-precursor and found direct evidence of reduced biosynthesis of DHT in CRPC.
Results
Concentration of androgens in the supernatant of C4-2 and C4-2AT6 cells
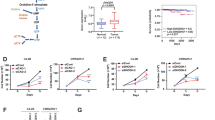
To determine whether prostate cancer cells have the ability to de novo synthesize androgen (Fig. 1A), we investigated the concentrations of testosterone (T) and dihydrotestosterone (DHT) in the supernatant of C4-2 and C4-2AT6 cells for 6 hr by LC/MS/MS analysis (Fig. 1B). In C4-2 cells, the concentration of T and DHT was 0.68 ± 0.12 and 0.46 ± 0.17 pg/mL, respectively. In C4-2AT6 cells, the concentration of T and DHT was 0.101 ± 0.01 and 0.033 ± 0.002 pg/mL, respectively. These results indicated that in C4-2 and C4-2AT6 cells 5α-reductase was active.
Detection of T and DHT in CRPC cells by LC/MS/MS analysis.
(A) T and DHT concentrations in the supernatant of C4-2 incubated for 6 hrs *** p < 0.001. (B) T and DHT concentrations in the supernatant of C4-2AT6 incubated for 6 hrs *** p < 0.001. (C) The mRNA expression of SRD5A1 and SRD5A2 in C4-2 and C4-2AT6 cells. ** p < 0.01
SRD5A1 and SRD5A2 mRNA expression in human castration-resistant prostate cancer cell lines
We quantified and compared transcripts of SRD5A1 and SRD5A2. SRD5A is a 5α-reductase essential for DHT biosynthesis. Compared with C4-2 cells, quantitative PCR (qPCR) for C4-2AT6 cells showed 1.3 ± 0.2 fold increases of the expression of SRD5A1(Fig. 1C, p < 0.01). qPCR for C4-2AT6 cells showed reduced SRD5A2 mRNA expression in C4-2AT6 cells compared to C4-2 cells (Fig. 1C, p < 0.01).
Comparison of 5α-reductase enzyme activities using 13C-[2,3,4]-androstenedione (13C- Adione)
Although 5α-reductase includes type-1 and type-2 5α-reductase26, the actual enzymatic activity in CRPC has not been elucidated yet27. To determine the activity of SRD5As, we developed a co-culture system with the C13 steroid precursor 13C-[2,3,4]-androstenedione(13C-Adione). We cultured C4-2 cells with 13C-Adione for 6 hr and examined the conversion ratio of 13C-Adione to 13C-[2,3,4]-testosterone (13C-T), as well as the concentration of 13C-[2,3,4]-dihydrotestosterone (13C-DHT) in the cultured supernatant (Fig. 2A). To compare the activity of SRD5A in CRPC cells, we estimated the concentration ratio of DHT/T (DHT/T ratio), which appeared to reflect 5AR activity in cancer cells.
(A) Simplified schematic representation of the co-culture system with 13C steroid precursors. (B) Typical selected ion recordings of the 13C-T and 13C-DHT extracted from cultured medium. (C) 13C-T and 13C-DHT concentrations in the supernatant of C4-2 and C4-2AT6 cells. *** p < 0.001. (D) DHT/T ratio appeared to reflect 5AR activity in cancer cells. C4-2AT6 cells exhibited significantly reduced 5AR activity, compared to C4-2.
13C-T and 13C-DHT were detectable by LC/MS/MS (Fig. 2B). In C4-2 cells, the concentrations of 13C-T and 13C-DHT were 5104 ± 703 and 799 ± 321 pg/mL; thus the DHT/T ratio was 0.157 ± 0.026 (Fig. 2C). In contrast, in C4-2AT6 cells, the concentrations of 13C-T and 13C-DHT were 8707 ± 2283 pg/mL and 151 ± 48.5 pg/mL, respectively and the DHT/T ratio was 0.017 ± 0.002 (Fig. 2C). The concentration of 13C-T was significantly higher in C4-2 than in C4-2AT6 (p < 0.01) and the concentration of 13C-DHT was significantly higher in C4-2AT6 than that in C4-2 (Fig. 2D, p < 0.01). In C4-2AT6 cells, the DHT/T ratio was significantly higher than that in C4-2 (p < 0.01). These results indicated that both C4-2 and C4-2AT6 cells expressed direct 5α-reductase activity and the activity was significantly higher in C4-2AT6 cells than in C4-2 cells.
Response to DHT in C4-2 and C4-2AT6 cells
The reduced DHT/T ratio and 5α-reductase activity may reflect the decreased dependence of C4-2AT6 cells proliferation on DHT, compared with C4-2 cells. To investigate the response to DHT in C4-2 and C4-2AT6 cells, we determined AR expression at the mRNA (Fig. 3A) and protein levels (Fig. 3B). C4-2AT6 cells showed an 8.1 fold increase of AR mRNA expression, accompanied by AR protein expression in the nucleus. Next, we investigated the expression of AR target gene: PSA was determined by qPCR at different concentrations of DHT at 12 h (Fig. 3C, D). The mRNA expression of PSA in C4-2 and C4-2AT6 cells increased in a dose-dependent manner. To determine whether C4-2 or C4-2AT6 cells show a proliferative response to DHT, we investigated and compared the viability of cancer cells treated with DHT at various concentrations for 96 h (Fig. 3E, F, G). C4-2 cells showed no significantly response to DHT when treated for 96 h at 10−14 M to 10−12 M DHT (Fig. 3E, G, p < 0.05). When treated with higher concentrations of 10−8 M DHT, C4-2 cells showed significantly decreased cell viability compared with that at lower DHT concentrations (Fig. 3E, G). In C4-2AT6 cells, treatment with 10−8 M DHT showed significantly decreased cell viability compared with that at lower concentrations of DHT (Fig. 3F, G). When treated with 10−10 M DHT, C4-2AT6 cells showed significant decreased cell viability compared with that at lower DHT concentrations (Fig. 3F, G, p < 0.05). Increasing the concentration of DHT did not have a positive effect on cell proliferation, rather the inhibitory effects on C4-2AT6 cells were more marked than those on C4-2 cells treated with the same DHT concentration (Fig. 3G).
At high concentrations DHT exhibited inhibitory effects on C4-2AT6 cells.
(A) mRNA expression of AR in C4-2 and C4-2AT6 cells. *** p < 0.001. (B) Western blot analysis of AR expression in the nucleus in C4-2 and C4-2AT6 cells. (C) mRNA expression of PSA in C4-2 cells increased in a dose-dependent manner. (D) mRNA expression of PSA in C4-2AT6 cells increased in a dose-dependent manner. *p < 0.05, *** p < 0.001. (E) WST cell viability assay exhibited no significant proliferative response to DHT at 10−14 M to 10−12 M DHT concentration in C4-2 cells. DHT treatment at a concentration of 10−8 M resulted in significant decreased cell viability. *p < 0.05. (F) When treated with 10−10 M DHT or 10−8 M DHT, C4-2AT6 cells showed significant decreased cell viability compared with that at lower DHT concentrations. *p < 0.01. (G) Response to DHT in C4-2 and C4-2AT6 cells detected by phase contrast field.
Is it effective to treat CRPC using 5α-reductase inhibitors?
To determine whether inhibition of 5AR activity with 5-ARIs: dutasteride or finasteride, showed some efficacy in C4-2 or C4-2AT6 cells, we investigated cell viability in cells treated with various concentrations of 5-ARIs for 96 h (Fig. 4A, B). It has been reported that 5ARIs exhibit some inhibitory actions in LNCaP cells28,29,30, therefore, we used the LNCaP cell line as a control in our experiments to compare the effects of 5ARIs on prostate cancer cell lines. As previously shown, dutasteride or finasteride exhibited significant inhibitory actions in LNCaP cells within the clinically achievable 5ARI concentration of 10 nM. On the other hand, these two 5ARIs did not have inhibitory effects on C4-2 and C4-2AT6 cells at clinically achievable 5ARI concentrations of <100 nM. To investigate the expression of AR target gene in response to finasteride and dutasteride in C4-2 and C4-2AT6 cells, we determined the expression of PSA, Nkx3.1 and TMPRSS2 by qPCR at different concentrations (Fig. 4C, D). qPCR analysis revealed 5ARIs had no effect on these AR taget genes in C4-2 and C4-2AT6 cells.
Efficacy of 5ARIs assessed using PCA cell lines.
The efficacy of 5ARIs in prostate cancer cells was assayed by incubation with dutasteride (A, C) or finasteride (B, D) at various concentrations for 96 h. In LNCaP cells, dutasteride and finasteride exhibited significant inhibitory actions within the clinically achievable 5ARI concentration of 10 nM. In C4-2 and C4-2AT6 cells, neither dutasteride nor finasteride inhibited cell viability at the same concentrations. *p < 0.05, **p < 0.01. (C, D) qPCR analysis of PSA, Nkx3.1, TMPRSS2 in C4-2 (C) and C4-2AT6 (D) after treated with 5ARIs. Dutasteride and finasteride did not show a significant effect on these AR target genes. (D) Dutasteride, (F) Finasteride.
Discussion
In this study, we developed a co-culture system with C13 steroid precursors to obtain direct evidence of 5α-reductase activity in CRPC cells. 13C-T and 13C-DHT were detected by LC/MS/MS. The activity of 5α-reductase changed in CRPC cells under androgen ablation. This is the first report showing direct evidence of changes in 5AR enzyme activity in CRPC cells. Moreover, we showed that the capacity of DHT to influence the proliferation of CRPC is limited, probably due to saturation effects at very low concentrations.
Studies in CRPC cancer tissue have measured intraprostatic testosterone or the active metabolite DHT in quantities sufficient to stimulate AR-mediated gene expression12,13,19. AR activation by androgens converted from adrenal androgens or synthesized intratumorally via the de novo route has been proposed as one of the mechanisms of castration resistance7,8,9,10,11. It has been reported that men with a Gleason score of >7 had lower intraprostatic dihydrotestosterone (DHT) than men with a Gleason score of <6, raising the possibility that a low-androgen environment predisposes men to development of high-grade PCa31,32,33. Although 5AR, which is essential for DHT biosynthesis, was detected at the mRNA level in CRPC metastases9,10,11,14, physiologically relevant 5AR activity in human CRPC has not yet been fully demonstrated. In this study, to ascertain potential 5AR activity, we co-cultured C4-2 and C4-2AT6 cells with the C13 steroid precursor 13C-Adione. We analyzed the sequential biosynthesis of the androgens 13C-T and 13C-DHT and obtained direct evidence of de novo sequential biosynthesis of androgens in CRPC, C4-2 and C4-2AT6 cells found to express 5AR activity. C4-2AT6 cells showed lower 5AR activities than C4-2 cells, although C4-2AT6 cells showed significantly higher SRD5A1 mRNA expression. These results indicated that 5AR activity changed under androgen ablation in CRPC cells and 5AR activity was not necessarily paralleled by SRD5As mRNA expression. To determine whether dutasteride and finasteride have the ability to inhibit the conversion into DHT in CRPC cells, we investigated the concentration of 13C-DHT after treatment with these 5ARIs. LC/MS/MS analysis was not able to detect 13C-DHT in C4-2 and C4-2AT6 cells (data not shown). These results indicate 5ARIs were able to inhibit the conversion into 13C-DHT in C4-2 and C4-2AT6 cells, although the 5ARIs did not have an anti-proliferative effect.
Recent advances have shed light on the relationship between androgens and the development or the progression of PCa15,16,17,18. The use of 5AR inhibitors to prevent progression of PCA continues to be widely discussed. Discussion has been fueled by the findings of two large randomized, placebo-controlled trials: the Prostate Cancer Prevention Trial (PCPT) with finasteride34 and the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial35. The PCPT trial was the first large-scale study to investigate the role of finasteride in the context of prostate cancer development. Tumors found in patients treated with finasteride were of a higher grade than the tumors in those administered a placebo. Gleason scores between 7 and 10 were found in 6.4% of the tumors in the finasteride group, while in only 5.1% of those in the placebo group. The REDUCE trial showed an overall reduction in the number of tumors with a Gleason score of 5–6 in patients receiving dutasteride versus those given a placebo (19.9% compared to 25.1%, respectively). However, during 3 and 4 year periods, tumors with a Gleason score of 8–10 were more frequent in the dutasteride-treated group than in the placebo group. The FDA reanalyzed these two major trials and cited the fact that the absolute incidence of tumors with Gleason scores between 8 and 10 was increased by 0.7% with finasteride and by 0.5% with dutasteride. In December 2010, the US Food and Drug Administration's Oncologic Drugs Advisory Committee voted against recommending 5-ARI for the indication to reduce PCa risk, because the risk of more aggressive tumors outweighed their potential for chemoprevention17. These observations still cannot be fully explained. It may also be possible that finasteride or dutasteride has little or no effect on more aggressive tumors with high Gleason scores. Whether 5ARI increases the rates of high-grade disease remains a matter of debate. The decision by FDA not to approve the use of 5ARIs to prevent prostate cancer indicates that further basic and clinical investigations exploring the role of 5-ARI in the development and progression of PCa are warranted.
The reduced 5-AR activity we observed in C4-2AT6 cells raised a critical question: Does the viability of C4-2AT6 cells depend on DHT? Thus, we investigated the effects of DHT on human CRPC cell proliferation. C4-2 and C4-2AT6 cells exhibited reduced cell viability when treated with DHT. C4-2AT6 cells exhibit elevated and functional AR expression and produce PSA in response to DHT in a dose-dependent manner; however, C4-2AT6 cells showed significantly lower cell viability at the same concentration of DHT than C4-2 cells. The suppressive effect of DHT on PCa cells is not limited to C4-2 or C4-2AT6 cells. Some reports showed that CRPC could be treated with androgens due to the inhibitory action of excess androgens36,37,38,39,40. Accumulating evidence has suggested that AR has a finite ability to bind to T or DHT and that at higher concentrations T or DHT has no further effect on prostate growth when all ARs are bound to T or DHT36,37,38,39,40. It has been proposed this be termed the saturation point. Due to this saturation point, excess DHT may result in the suppression of androgenic-induced proliferation of C4-2AT6 cells. CRPC cells may have an unknown regulation system to protect themselves from the androgenic suppressive effect mediated by 5AR activity.
In this study, we clearly showed reduced DHT dependence, accompanied by reduced 5α-reductase activity in CRPC cells. Moreover neither finasteride nor dutasteride showed a positive effect on CRPC cells. These results may provide the grounds for debate about 5ARI in PCa.
Methods
Cell lines and culture
C4-2 cells were obtained from UroCor (Oklahoma City,OK). C4-2 cells were routinely maintained in RPMI-1640 (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, at 37°C in a humidified atmosphere with 5% CO2. C4-2AT6 cells were established from C4-2 as previously reported24. Briefly, C4-2 cells were grown in RPMI-1640 containing 10% charcoal stripped fetal bovine serum at 37°C in a humidified 5% CO2 atmosphere. Cells were passaged upon attaining confluence during a 6 month period. We named this cell line C4-2AT6; that is, C4-2 cells subjected to androgen ablated treatment for 6 months.
Chemicals
13C-[2,3,4]-androstenedione (13C-Andro) was purchased from Hayashi Pure Chemical Ind., Ltd. (Osaka, Japan) and CDN Isotope (Quebec, Canada). Bond Elut C18 cartridge was purchased from Varian Medical Systems KK (Tokyo) and 4-dimethylaminopyridine (DAP), 2-methyl-6-nitrobenzoic anhydride (MNBAn) and picolinic acid (PA) were from Tokyo Kasei Industry. Triethylamine (TEA) was from Wako Pure Chemical Industries (Osaka). Cadenza CD C-18 column (250 mm × 3 mm I.D., 3 μm m) and CAPCELL PAK SCX UG80 pre-column (35 mm × 2 mm I.D., 5 μm) were from Intact (Kyoto, Japan) and Shiseido (Tokyo), respectively.
LC-ESI-MS/MS
For the measurement of T and DHT in cultured medium, an API-4000 triple stage quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) connected to Agilent 1100 (Agilent Technologies), HTC-PAL (CTC Analytical) and an ESI ion source were employed, as previously shown41. The column was a Cadenza CD-C18 column (150 mm × 3 mm I.D., particle size 3 μm) and used at 40°C. The mobile phase consisted of 0.1% formic acid (Solvent A) and methanol: acetonitrile (1:1, Solvent B). For gradient elution, A/B was used at 20/80 to 10/90 between 0 to 10 min, 0/100 between 10 and 12.5 min and 0/100 to 20/80 between 12.5 and 15 min. The flow rate was 0.4 mL/min. The following ESI conditions were used: spray voltage, 5,000 V; collision gas, nitrogen, 45 psi; curtain gas, 11 psi; ion source temperature, 450°C; and ion polarity, positive. For the quantification of 13C-[2,3,4]-testosterone (13C-T) and 13C-[2,3,4]-dihydrotestosterone (13C-DHT) transitions were at m/z 290/112 and 334/111, respectively. Preparation of the derivatization reagent for picolinyl ester derivatives was prepared as follows: Ten milligrams of DAP, 20 mg of MNBAn and 25 mg of PA were dissolved into 1 mL of tetrahydrofuran (THF) and then agitated. After 10 min, the reagent solution was used. Pretreatment of the cultured medium: The cultured medium was mixed with 13C-DHEA (1000 pg) as the internal standard (IS) and diethyl ether (4 mL). The organic layer was separated after the aqueous phase became frozen and the organic layer was then evaporated to dryness at 40°C under N2 gas. The residue was dissolved in 20% methanol-water and the solution was applied onto the Bond Elut C18 cartridge column that had been pre-conditioned with methanol and purified water. The cartridge was washed with purified water and then with 30% acetonitrile solution. Subsequently, the desired substances were eluted with 80% acetonitrile solution. The purified extracts were dissolved in the reagent mixture prepared as described above. TEA was added to this mixture and the resulting mixture was allowed to stand at room temperature for 30 min. After dilution of the reaction mixture with 1% acetic acid solution to stop the reaction, the resulting mixture was loaded onto the Bond Elut C18 cartridge that had been pre-conditioned with methanol and purified water. After the cartridge was washed with water and 40% acetonitrile solution, derivatives were eluted with 80% acetonitrile solution. After the solvent was evaporated to dryness using a centrifugation evaporator at 53–55°C, the residue was dissolved in 40% acetonitrile solution and a 20 μL-aliquot of the solution was subjected to LC-ESI-MS/MS. A series of treated samples (200 μL) spiked with authentic 13C-T or 13C-DHT at concentrations of 1.0, 5.0, 10, 50, 100, or 1,000 pg and with chlormadione (CHM, 1000 pg) and 13C-DHEA (1000 pg) as the internal standard were prepared. A calibration curve was obtained for 13C-T or 13C-DHT by assigning the concentration of 13C-T or 13C-DHT to x and the peak area ratio of the PA derivatives of 13C-T or 13C-DHT to that of the corresponding chlormadione to y. Subsequently, a 1/x weighting linear regression was performed to construct the calibration curve. To prepare QC samples, 13C-T or 13C-DHT standard solution at 20, 100 and 800 pg were similarly added to purified water with chlormadione. The calibration curves of individual compounds showed good linearity with correlation coefficients (r) of more than 0.998–0.999. Intra–day assay accuracy and precision were evaluated by measuring multiple replicates (n = 5) of QC samples. The accuracy and precision of 13C-T or 13C-DHT at mid level were 2–13%, respectively, while the inter-day accuracy and precision at mid level, were 88-108% and 2–15%, respectively.
Real-time quantitative PCR
Total RNA was isolated using RNeasy Mini kit (Qiagen, Hilden, Germany) and the quantity and quality were evaluated by spectrophotometry. Reverse transcription of RNA to cDNA was done using High Capacity cDNA Archive Kit (Applied Biosystems). The reaction mixture (1 μL) was then used as a template in a TaqMan Fast real-time quantitative PCR assay using Taqman Universal PCR Master Mix and the 7500 Fast Real-time PCR system (Applied Biosystems). The primers and TaqMan probe sets (TaqMan Gene Expression Assays) for SRD5A1(Hs00971643_g1), SRD5A2(Hs00165843_m1), PSA(Hs02576345_m1), Nkx3.1(Hs00171834_m1), TMPRSS2(Hs01120965_m1) and human GAPDH endogenous control (Hs99999903_m1) were purchased from Applied Biosystems (sequences not disclosed). The cycling conditions were 50°C for 10 minutes, 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and at 60°C for 1 minute.
Statistics
Experiments were carried out in two or more replicates and statistical analysis was performed by Student's t test. P values < 0.05 were considered significant.
References
Jemal, A. et al. Cancer statistics, 2009. CA Cancer J Clin 59, 225–249 (2009).
Scher, H. I. & Sawyers, C. L. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol 23, 8253–8261 (2005).
Chen, Y., Sawyers, C. L. & Scher, H. I. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol 8, 440–448 (2008).
Lam, J. S., Leppert, J. T., Vemulapalli, S. N., Shvarts, O. & Belldegrun, A. S. Secondary hormonal therapy for advanced prostate cancer. J Urol 175, 27–34 (2006).
Ryan, C. J. & Small, E. J. Role of secondary hormonal therapy in the management of recurrent prostate cancer. Urology 62 Suppl 1, 87–94 (2003).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 68, (2008).
Stanbrough, M. et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res 66, 2815–2825 (2006).
Mostaghel, E. A., Montgomery, B. & Nelson, P. S. Castration-resistant prostate cancer: targeting androgen metabolic pathways in recurrent disease. Urol Oncol 27, 251–257 (2009).
Thomas, L. N. et al. Type 1 and type 2 5alpha-reductase expression in the development and progression of prostate cancer. Eur Urol 53, 244–252 (2008).
Thomas, L. N. et al. Levels of 5alpha-reductase type 1 and type 2 are increased in localized high grade compared to low grade prostate cancer. J Urol 179, 147–151 (2008).
Thomas, L. N. et al. Differential alterations in 5alpha-reductase type 1 and type 2 levels during development and progression of prostate cancer. Prostate 63, 231–239 (2005).
Locke, J. A. et al. Steroidogenesis inhibitors alter but do not eliminate androgen synthesis mechanisms during progression to castration-resistance in LNCaP prostate xenografts. J Steroid Biochem Mol Biol 115, 126–136 (2009).
Locke, J. A. et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res 68, 6407–6415 (2008).
Rainey, W. E. & Nakamura, Y. Regulation of the adrenal androgen biosynthesis. J Steroid Biochem Mol Biol 108, 281–286 (2008).
Morgentaler, A. & Traish, A. M. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 55, 310–320 (2009).
Morgentaler, A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol 50, 935–939 (2006).
Theoret, M. R. et al. The risks and benefits of 5alpha-reductase inhibitors for prostate-cancer prevention. N Engl J Med 365, 97–99 (2011).
Walsh, P. C. Chemoprevention of prostate cancer. N Engl J Med 362, 1237–1238 (2010).
Parker, C. What (if anything) to do about low-risk prostate cancer. Lancet 379, (2012).
Shirotake, S. et al. Regulation of monocyte chemoattractant protein-1 through angiotensin II type 1 receptor in prostate cancer. Am J Pathol 180, 1008–1016 (2012).
Kosaka, T. et al. Long-term androgen ablation and docetaxel up-regulate phosphorylated Akt in castration resistant prostate cancer. J Urol 185, 2376–2381 (2011).
Hasegawa, M. et al. Low-dose docetaxel enhances the sensitivity of S-1 in a xenograft model of human castration resistant prostate cancer. Int J Cancer 130, 431–442 (2012).
Kosaka, T., Miyajima, A., Shirotake, S., Kikuchi, E. & Oya, M. Phosphorylated Akt up-regulates angiotensin II type-1 receptor expression in castration resistant prostate cancer. Prostate (2011).
Kosaka, T. et al. Ets-1 and hypoxia inducible factor-1alpha inhibition by angiotensin II type-1 receptor blockade in hormone-refractory prostate cancer. Prostate 70, 162–169 (2010).
Kosaka, T. et al. Angiotensin II type 1 receptor antagonist as an angiogenic inhibitor in prostate cancer. Prostate 67, 41–49 (2007).
Dillard, P. R., Lin, M. F. & Khan, S. A. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol 295, 115–120 (2008).
Yamaoka, M., Hara, T. & Kusaka, M. Overcoming persistent dependency on androgen signaling after progression to castration-resistant prostate cancer. Clin Cancer Res 16, 4319–4324 (2010).
Bologna, M., Muzi, P., Biordi, L., Festuccia, C. & Vicentini, C. Finasteride dose-dependently reduces the proliferation rate of the LnCap human prostatic cancer cell line in vitro. Urology 45, 282–290 (1995).
Lazier, C. B., Thomas, L. N., Douglas, R. C., Vessey, J. P. & Rittmaster, R. S. Dutasteride, the dual 5alpha-reductase inhibitor, inhibits androgen action and promotes cell death in the LNCaP prostate cancer cell line. Prostate 58, 130–144 (2004).
Xu, Y., Dalrymple, S. L., Becker, R. E., Denmeade, S. R. & Isaacs, J. T. Pharmacologic basis for the enhanced efficacy of dutasteride against prostatic cancers. Clin Cancer Res 12, 4072–4079 (2006).
Nishiyama, T., Ikarashi, T., Hashimoto, Y., Wako, K. & Takahashi, K. The change in the dihydrotestosterone level in the prostate before and after androgen deprivation therapy in connection with prostate cancer aggressiveness using the Gleason score. J Urol 178, 1282–1288; discussion 1288–1289 (2007).
Nishiyama, T., Ikarashi, T., Hashimoto, Y., Suzuki, K. & Takahashi, K. Association between the dihydrotestosterone level in the prostate and prostate cancer aggressiveness using the Gleason score. J Urol 176, 1387–1391 (2006).
Nishiyama, T., Hashimoto, Y. & Takahashi, K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res 10, 7121–7126 (2004).
Thompson, I. M. et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 349, 215–224 (2003).
Andriole, G. L. et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 362, 1192–1202 (2010).
Zhau, H. Y. et al. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci U S A 93, 15152–15157 (1996).
Cinar, B. et al. Androgen receptor mediates the reduced tumor growth, enhanced androgen responsiveness and selected target gene transactivation in a human prostate cancer cell line. Cancer Res 61, 7310–7317 (2001).
Chuu, C. P., Hiipakka, R. A., Fukuchi, J., Kokontis, J. M. & Liao, S. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res 65, 2082–2084 (2005).
Chuu, C. P. et al. Androgen suppresses proliferation of castration-resistant LNCaP 104-R2 prostate cancer cells through androgen receptor, Skp2 and c-Myc. Cancer Sci 102, 2022–2028 (2011).
Chuu, C. P. et al. Inhibition of tumor growth and progression of LNCaP prostate cancer cells in athymic mice by androgen and liver X receptor agonist. Cancer Res 66, 6482–6486 (2006).
Arai, S. et al. Effect of castration monotherapy on the levels of adrenal androgens in cancerous prostatic tissues. Steroids 76, 301–308 (2011).
Acknowledgements
We thank Dr. Seijiro Honma, Ph.D for his technical assistance concerning the LC/MS/MS and many helpful discussions. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Japan Urological Association. The funders had no role in the study design, data collection and analysis, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
T.K. performed a significant amount of the experimental work. A.M. and M.O. supervised the project. T.K. and A.M. wrote the manuscript. H.N., T.M. and E.K. prepared the figures.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Kosaka, T., Miyajima, A., Nagata, H. et al. Human castration resistant prostate cancer rather prefer to decreased 5α-reductase activity. Sci Rep 3, 1268 (2013). https://doi.org/10.1038/srep01268
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01268
This article is cited by
-
SRD5A2 gene expression inhibits cell migration and invasion in prostate cancer cell line via F-actin reorganization
Molecular and Cellular Biochemistry (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.