Abstract
To obtain a tumor cell-selectivity of methyl-β-cyclodextrin (M-β-CyD), we newly synthesized folate-appended M-β-CyD (FA-M-β-CyD) and evaluated the potential of FA-M-β-CyD as a novel anticancer agent in vitro and in vivo. Potent antitumor activity and cellular association of FA-M-β-CyD were higher than those of M-β-CyD in KB cells, folate receptor (FR)-positive cells. FA-M-β-CyD drastically inhibited the tumor growth after intratumoral or intravenous injection to FR-positive Colon-26 cells-bearing mice. The antitumor activity of FA-M-β-CyD was comparable and superior to that of doxorubicin after both intratumoral and intravenous administrations, respectively, at the same dose, in the tumor-bearing mice. All of the tumor-bearing mice after an intravenous injection of FA-M-β-CyD survived for at least more than 140 days. Importantly, an intravenous administration of FA-M-β-CyD to tumor-bearing mice did not show any significant change in blood chemistry values. These results strongly suggest that FA-M-β-CyD has the potential as a novel anticancer agent.
Similar content being viewed by others
Introduction
Chemotherapy is often used to treat cancer and it is expected to destroy the tumor cells for maximum treatment efficacy, while minimizing side effects to other organs1. However, conventional anticancer agents often have some unexpected limitations such as poor distribution, tissue damage and lack of target specificity. In order to overcome these problems, the drug delivery technique to tumor cells has attracted considerable attention. To give an active targeting-ability to a drug carrier, chemical modification by tumor targeting ligands is known, e.g. antibody2,3,4,5, sugar6, folic acid (FA)7,8,9,10,11, transferrin12,13,14, epidermal growth factor15,16 and Arg-Gly-Asp-Ala-Pro-Arg-Pro-Gly peptide17.
Recently, FA has emerged as a prominent targeting moiety capable of specific interaction with cells expressing the folate receptor (FR)18. FR consists of a high affinity folate binding protein (FBP) (Kd : 10−9−10−10 M) and is attached to the plasma membrane through a glycosylphosphatidylinositol (GPI) anchor19. FR is overexpressed in many human tumor cells, including malignancies of the ovary, brain, kidney, breast, myeloid cells and lung and has little expression in normal tissues9,20,21,22. This overexpression of FR provides tumor cells with increased amounts of the FA essential for DNA synthesis and seems to aid in aggressive tumor growth. In patients diagnosed with cancer, the overexpression of FR isoform α (FR-α) correlates with a higher histological grade and more advanced stage of the disease23. Therefore, FR is one of the strongest candidates, both as an attractive marker and a target molecule for diagnosis and therapy of cancer24.
Cyclodextrins (CyDs) and their hydrophilic derivatives form inclusion complexes with hydrophobic molecules. CyDs can improve the solubility, dissolution rate and bioavailability of the drugs and so the widespread use of CyDs is well known within the pharmaceutical field25,26. CyDs have been reported to interact with cell membrane constituents such as cholesterol and phospholipids, resulting in the induction of hemolysis of human and rabbit red blood cells at high concentrations of CyDs27,28,29. Additionally, methyl-β-cyclodextrin (M-β-CyD) is acknowledged to disrupt the structures of lipid rafts and caveolae30,31, which are lipid microdomains formed by lateral assemblies of cholesterol and sphingolipids in the cell membrane, through the extraction of cholesterol from the microdomains32. Furthermore, we demonstrated that 2,6-di-O-methyl-β-cyclodextrin (DM-β-CyD) induced apoptosis through the inhibition of PI3K-Akt-Bad pathway, leading to cholesterol depletion from lipid rafts in NR8383 cells, a rat alveolar macrophage cell line33. Notably, Grosse et al. reported that intraperitoneal administration of M-β-CyD showed signs of antitumor activity in human tumor xenografted athymic nude mice34. However, parenteral use of M-β-CyD is not allowed in humans35, because the antitumor activity of M-β-CyD lacks tumor cell-selectivity.
Most recently, in an attempt to confer a tumor-selective cytotoxic activity to M-β-CyD, we newly synthesized folate-appended M-β-CyD (FA-M-β-CyD, Fig. 1) having an average degree of substitution of FA (DSF) of 1.036. FA-M-β-CyD possesses several advantages as an antitumor agent compared to antibody drugs: 1) the physicochemical stability is high, 2) the pharmacokinetics after intravenous administration is rarely affected by serum proteins due to its low molecular weight compound, 3) the batch difference in bioactivity does not occur as it is a chemically synthesized product and 4) the cost performance is superior to that of biosynthesis products. However, the tumor cell-specific cytotoxic activity of FA-M-β-CyD in vitro and in vivo still remains unclear. Therefore, in the present study, we investigated whether FA-M-β-CyD provides the FR-expressing cell-selective antitumor activity and its safe profile in vitro and in vivo or not.
Results
Antitumor activity of FA-M-β-CyD
To clarify the FR-selective antitumor activity of FA-M-β-CyD (Fig. 1), we evaluated antitumor activity of FA-M-β-CyD in KB cells, FR-positive cells and A549 cells, FR-negative cells. FA-M-β-CyD displayed potent antitumor activity, compared to M-β-CyD in KB cells (Fig. 2A), but not in A549 cells (Fig. 2B). In contrast, DM-β-CyD showed significant antitumor activity in both KB cells and A549 cells (Fig. 2). Additionally, in Colon-26 cells (FR-positive), FA-M-β-CyD showed potent antitumor activity, compared to M-β-CyD (Fig. 2C). Meanwhile, the antitumor activity of FA-M-β-CyD was significantly attenuated in FR knockdown-KB cells produced by treatment with FR-α siRNA (Fig. 2D). These results suggest that FA-M-β-CyD has FR-expressing cell-selective antitumor activity.
Next, we investigated the effect of FA, as a competitor of FR, on antitumor activity of β-CyDs in KB cells. The antitumor activity of FA-M-β-CyD, but not β-CyD, M-β-CyD or DM-β-CyD, was significantly inhibited by the addition of FA (Fig. 3). These results suggest the occurrence of FR-mediated antitumor activity of FA-M-β-CyD.
Effects of M-β-CyDs on caspase 3/7 activity
Activation of caspase 3/7 is considered an essential event during apoptosis. To investigate whether FA-M-β-CyD-induced cell death is accompanied by apoptotic feature, we next examined the caspase 3/7 activity in KB cells after treatment with CyDs using the CellEvent® Caspase-3/7 Green Detection Reagent (Fig. 4). This caspase 3/7 detection reagent is intrinsically non-fluorescent as the DEVD peptide inhibits the ability of the dye to bind to DNA. However, after activation of caspase 3/7 in apoptotic cells, the DEVD peptide is cleaved and enabled the dye to bind to DNA and produce a bright, fluorogenic response. Treatment of KB cells with DM-β-CyD, but not M-β-CyD or FA-M-β-CyD, for 2 h, caused caspase-3/7 activation (Fig. 4). These results suggest that FA-M-β-CyD caused cell death in KB cells in an apoptosis- independent pathway.
Cellular association of FA-M-β-CyD
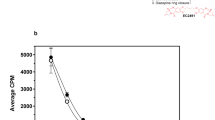
To gain insight into the mechanism for the FR-mediated antitumor activity of FA-M-β-CyD, we examined the cellular association of FA-M-β-CyD after treatment for 1 h with KB cells and A549 cells (Fig. 5). In contrast to the general belief that CyDs are unable to enter cells, the cellular association of FA-M-β-CyD with KB cells was significantly higher than that with A549 cells (Fig. 5A). As expected, M-β-CyD was only very slightly associated with KB cells (Fig. 5B). These results suggest that cellular association of FA-M-β-CyD could be mediated by FR on KB cells.
Cellular association of FA-M-β-CyD and TRITC-M-β-CyD.
(A) Cells were incubated with FA-M-β-CyD for 1 h. The concentration of FA-M-β-CyD associated with cells was determined by a fluorescence spectrophotometer. Each value represents the mean±S.E.M. of 3–4 experiments. *p < 0.05, compared with A549 cells. (B) KB cells were incubated with TRITC-M-β-CyD for 1 h. The fluorescence intensity of TRITC associated with KB cells was determined by a flow cytometer.
Effects of β-CyDs on cholesterol efflux from tumor cells
Lipid rafts are mainly composed of cholesterol and sphingolipids in the cell membranes and contain various signal transduction molecules including growth factor receptors37. We previously reported that CyDs showed hemolytic activity at high concentration through the extraction of cell membrane components such as cholesterol and phospholipids from lipid rafts27,29. Furthermore, we demonstrated that DM-β-CyD induces apoptosis through cholesterol depletion in NR8383 cells, a rat alveolar macrophage cell line33. Therefore, to reveal whether cell death induced by FA-M-β-CyD is apoptosis through cholesterol depletion in FR-positive cells, we investigated the effects of CyDs on the release of cholesterol from KB cells and A549 cells. The amount of cholesterol released in the culture medium after incubation with 5 mM CyDs for 1 h was determined by Cholesterol-test Wako® (Fig. 6). The extent of cholesterol extracted by treatment with FA-M-β-CyD was significantly higher than that with β-CyD, M-β-CyD and DM-β-CyD in KB cells (Fig. 6A). Similar results were observed in A549 cells (Fig. 6B), although FA-M-β-CyD did not display antitumor activity under the same conditions. Furthermore, we confirmed that FA-M-β-CyD reduced in neither DNA content nor mitochondrial transmembrane potential in KB cells (manuscript in preparation). Taken together, these results suggest that the extraction ability of FA-M-β-CyD on cholesterol from plasma membranes is not associated with antitumor activity and the mechanism of antitumor activity of FA-M-β-CyD is not apoptosis in KB cells.
Effects of β-CyDs on efflux of cholesterol from KB cells (FR (+)) and A549 Cells (FR (−)).
(A) KB cells and (B) A549 cells were incubated with β-CyDs (5 mM) for 1 h. Each value represents the mean±S.E.M. of 4–5 experiments. *p < 0.05, compared with control. †p < 0.05, compared with M-β-CyD. ‡p < 0.05, compared with DM-β-CyD.
Hemolytic activity
As described above, FA-M-β-CyD extracted cholesterol from plasma membranes of KB cells, which suggests high hemolytic activity. One of the most substantial requirements for drugs is that they have either no or acceptably low levels of local irritancy. The studies with isolated rabbit red blood cells (RRBC), which have no nucleus, mitochondria, endoplasmic reticulum or other organelles, may provide a simple and reliable measure to determine the local irritancy induced by CyDs. Therefore, we investigated hemolytic activity of FA-M-β-CyD in order to estimate its local irritation. Herein, RRBC do not express FR on plasma cell membranes. Figure 7 shows hemolytic profiles of β-CyD, M-β-CyD, DM-β-CyD and FA-M-β-CyD. It is apparent that the hemolytic activity of FA-M-β-CyD was weaker than those of β-CyD, M-β-CyD and DM-β-CyD. For example, the hemolysis onset at about 0.5 mM, 1 mM, 3 mM and 8 mM and the concentrations to induce 50% hemolysis were about 1.3 mM, 3.5 mM, 5.7 mM and 10.9 mM for DM-β-CyD, M-β-CyD, β-CyD and FA-M-β-CyD, respectively. These results suggest that local irritancy of FA-M-β-CyD is quite low.
Hemolytic activity of β-CyDs on RRBC.
The RRBC suspension (0.1 mL) was added to the PBS (pH 7.4, 1 mL) containing β-CyDs at various concentrations. The mixture was incubated for 30 min at 37°C. The release of hemoglobin from the RRBC was determined by a UV spectrophotometer at 543 nm. Each point represents the mean±S.E.M. of 3 experiments.
Antitumor Activity of FA-M-β-CyD in tumor-bearing mice
To investigate antitumor activity of FA-M-β-CyD in vivo, we injected FA-M-β-CyD solution intratumorally or intravenously to tumor-bearing mice. Herein, we confirmed that Colon-26 cells express FR using a RT-PCR method. As shown in Fig. 8A, an intratumoral injection of the FA-M-β-CyD at a dose of 30 mg/kg significantly inhibited the tumor growth, compared to that of control (5% mannitol solution). The similar results of the inhibitory effects on tumor growth were observed in both the doxorubicin and the M-β-CyD systems. To make sure there were no side effects, the body weight of mice after an intratumoral injection of FA-M-β-CyD or M-β-CyD was slightly increased as the time passed (Fig. 8B). However, in the doxorubicin system, the body weight of mice did not increase up to 30 days after intratumoral injection, probably due to the side effects of doxorubicin (Fig. 8B). These results suggest that FA-M-β-CyD has the potent antitumor activity after intratumoral injection to tumor-bearing mice with less systemic side effect.
Next, we investigated the effects of an intravenous injection of FA-M-β-CyD (5 mg/kg) to tumor-bearing mice on tumor growth, body weight change and survival rate (Fig. 9). As shown in Figure 9A, an intravenous injection of doxorubicin or M-β-CyD slightly suppressed the tumor growth. Remarkably, FA-M-β-CyD drastically inhibited the tumor growth after an intravenous injection (Fig. 9A). Furthermore, the tumor inoculated subcutaneously completely disappeared after treatment with FA-M-β-CyD (Fig. 9B). Surprisingly, all of the tumor-bearing mice after intravenous injection of FA-M-β-CyD survived for at least 140 days without any relapse, while the mice treated with doxorubicin and M-β-CyD died of sickness within 70 days (Fig. 9C). Additionally, the body weight of mice after an intravenous injection of FA-M-β-CyD was increased slightly as the time passed, suggesting that FA-M-β-CyD does not have any significant side effect (Fig. 9D). In terms of blood chemistry data, doxorubicin tended to elevate the alanine aminotransferase (ALT) and lactate dehydrogenase (LDH) values and M-β-CyD significantly increased blood urea nitrogen (BUN), aspartate aminotransferase (AST) and LDH levels, compared to control, suggesting induction of systemic side effects of doxorubicin and M-β-CyD. Strikingly, no significant changes in the blood chemistry values such as creatinine (CRE), BUN, AST, ALT and LDH were observed 24 h after an intravenous injection of FA-M-β-CyD, compared to control (5% mannitol solution) at the same dose as doxorubicin and M-β-CyD (Table 1). These results strongly suggest that FA-M-β-CyD has the potential as a novel antitumor agent with negligible systemic side effects even after intravenous injection.
Effects of intravenous administration of FA-M-β-CyD on tumor growth (A, B), survival rate (C) and body weight (D) in tumor-bearing mice.
Each point represents the mean±S.E.M. of 5 experiments. Each line represents the survival of 5–7 experiments. *p < 0.05, compared with control (5% mannitol solution). †p < 0.05, compared with doxorubicin. ‡p < 0.05, compared with M-β-CyD.
Discussion
In the present study, we revealed that FA-M-β-CyD showed a FR-expressing cell-selective in vitro antitumor activity through FR-mediated endocytosis. In addition, FA-M-β-CyD had great inhibitory effects on tumor growth after both intratumoral and intravenous injections to FR-positive tumor cells-bearing mice, without any significant change in blood chemistry values.
Having targeting ability is one of the most important factors for antitumor agents to provide not only potent antitumor activity but also to reduce side effects for cancer treatment. FA-M-β-CyD showed FR-expressing cell-selective antitumor activity. In fact, FA-M-β-CyD elicited cytotoxic activity to KB cells rather than A549 cells (Fig. 2). In addition, the antitumor activity of FA-M-β-CyD was significantly inhibited by the addition of FA, suggesting that FR-mediated endocytosis is important for the induction of antitumor activity by FA-M-β-CyD (Fig. 3). GPI-anchored proteins including FR were acknowledged to be endocytosed through clathrin-independent carrier/GPI-anchored proteins enriched early endosomal compartment (CLIC/GEEC)38. Therefore, FA-M-β-CyD may be endocytosed through CLIC/GEEC after the recognition by FR on KB cells. Three isoforms of the FR, FR-α, FR-β and FR-γ, have been identified and cloned to date. The FR-α levels are high in specific malignant tumors of epithelial origin, compared to normal cells and are positively associated with tumor stage and grade23,39. Generally, FR-α recognizes the α-carboxy group of folate and internalizes folate to confer a growth advantage to the tumor40. Recently, Guaragna et al. demonstrated that the conjugation of anticancer agent chlorambucil to γ-carboxy group of FA via suitable linkers enhanced the selective drug delivery to the FR-expressing tumor cells41. Furthermore, we previously reported that FA-M-β-CyD was synthesized by a condensation reaction between γ-carboxy group of FA and amino group of NH2-M-β-CyD36. Therefore, it is suggested that the α-carboxy group of FA in FA-M-β-CyD is recognized by FR-α in KB cells (FR-positive) and internalized by CLIC/GEEC endocytosis. In fact, the cellular association of FA-M-β-CyD with KB cells was significantly higher than that with A549 cells (FR-negative) (Fig. 5A), strongly suggesting the potential of FA-M-β-CyD as a FR-expressing cell-selective anticancer agent. However, further elaborate studies on cellular uptake of FA-M-β-CyD are required using FR-α-deficient KB cells.
In spite of the impressive treatment developed, few options for cancer cells are available. A number of promising agents with multiple mechanisms of action are under investigation. Recent studies exploring the cell death machinery have led to the discovery of alternative pathways for modulating cell death and also novel compounds inducing cancer cell demise42. Among cell death mechanisms, apoptosis plays essential roles in cell survival, growth and tumorigenesis43. M-β-CyD is often used to disrupt lipid rafts because of its ability to deplete cholesterol stores on cell membranes30,31. A number of studies have also demonstrated that the disruption of lipid rafts by M-β-CyD can harm cancer cells and cause cell death. In human epidermoid carcinoma cells, for example, cholesterol depletion by M-β-CyD induced apoptosis and caveolae internalization44. Furthermore, we previously demonstrated that DM-β-CyD induced apoptosis through the inhibition of the PI3K-Akt-Bad pathway, leading to cholesterol depletion from lipid rafts in NR8383 cells33. We also confirmed that DM-β-CyD induced apoptosis in KB cells (Fig. 4), probably due to the cholesterol depletion, leading to a decrease in not only DNA content but also mitochondrial transmembrane potential. In the present study, 5 mM FA-M-β-CyD released significant amount of cholesterol from KB cells and A549 cells to culture medium, compared to that of DM-β-CyD (Fig. 6). However, FA-M-β-CyD caused cell death without lowering the DNA content and mitochondrial transmembrane potential (manuscript in preparation) and also activation of caspase 3/7 (Fig. 4), indicating that cell death induced by FA-M-β-CyD in KB cells is not involved in apoptosis. Additionally, FA-M-β-CyD did not induce cell death in A549 cells even through its potent cholesterol depletion ability, compared to the other β-CyDs, under the present experimental conditions (Fig. 2B and Fig. 6). Meanwhile, M-β-CyD elicited apoptosis in A549 cells through not only lowering DNA content but also reducing mitochondrial transmembrane potential (Supplementary Fig. 1). Collectively, these results suggest that the extraction of cholesterol from plasma membranes by FA-M-β-CyD is not associated with the induction of cell death.
Most importantly, our most recent study demonstrated that FA-M-β-CyD induces autophagy, not apoptosis, in KB cells. That is, FA-M-β-CyD was found to elevate the expression of LC3-II, an autophagosome marker, in autophagic membranes in KB cells, through its FR-mediated cellular uptake (manuscript in preparation). An implication of this finding is that FA-M-β-CyD may be an antitumor drug with a curious mechanism. However, it still remains unclear whether FA-M-β-CyD affects the dephosphorylation and inactivation of mammalian target of rapamaycin (mTOR), which induces autophagy and the activation of class III PI3K, which is involved in autophagosome formation. Clearly, the present study needs to be followed by further studies on the mechanism of autophagy induced by FA-M-β-CyD.
It should be noted that the in vivo antitumor activity of FA-M-β-CyD was superior to that of M-β-CyD and doxorubicin after an intravenous injection to FR-positive Colon-26 cells-bearing mice (Fig. 9). Generally, the blood vessels of cancerous tumors are leaky45,46 and poorly organized47,48. This can increase the interstitial fluid pressure inside tumors and reduce blood supply to them, which impairs drug delivery49,50. Most recently, Chauhan et al. reported that the nanomedicines (diameter, 12 nm) are ideal in cancer therapy due to their superior tumor penetration, rather than those with a larger size of nanoparticles (diameter, 125 nm)51. Herein, the particle size of FA-M-β-CyD was less than 10 nm, suggesting that FA-M-β-CyD could penetrate into interstitial space of tumor tissues via blood vessels of cancerous tumors after an intravenous injection. Following that, FA-M-β-CyD may actively enter tumor cells through FR-mediated endocytosis to exert potent antitumor effects. To validate the in vitro findings that FA-M-β-CyD can induce autophagy rather than apoptosis in FR-expressing tumor cells, further elaborate studies such as histological analysis such as TUNEL assay with immunohistochemistry and in vivo antitumor activity against FR-negative tumor cells-bearing mice are in progress.
Intriguingly, an intravenous administration of FA-M-β-CyD to Colon-26 cells-bearing mice did not show any significant change in blood chemistry values (Table 1). Grosse et al. reported that the intraperitoneal injections of M-β-CyD (800 mg/kg) once a week significantly suppressed tumor growth of MCF7 cells xenograft in nude mice, compared to those of doxorubicin (2 mg/kg)34. However, due to the lack of target specificity, M-β-CyD may have the possibility to cause cytotoxicity not only to tumor cells but also to normal cells. Actually, the LDH value of M-β-CyD was significantly higher than that of control, suggesting the induction of the systemic side effects (Table 1). Furthermore, M-β-CyD was reported to be accumulated in the kidney34, through glomerular filtration after intraperitoneal injection to tumor-bearing mice, resulting in risk of renal disorder. Actually, in the present study, M-β-CyD elevated the value of BUN 24 h after an intravenous injection, compared to control (Table 1). In sharp contrast, FA-M-β-CyD did not change the values of BUN and CRE, suggesting that pharmacokinetic profile of FA-M-β-CyD may differ somewhat from that of M-β-CyD. So, further elaborate study on pharmacokinetics of FA-M-β-CyD following intravenous administration is in progress. Besides, the hematological toxicity caused by FA-M-β-CyD after an intravenous administration is assumed to be low, because FA-M-β-CyD, which is recognized by FR-α through α-carboxy moiety of FA, does not appear to associate with hematopoietic cells expressing FR-β. Therefore, these lines of evidence make it tempting to speculate that FA-M-β-CyD provides the drastic antitumor activity in vivo with its high safety profiles.
In conclusion, in the present study, we evaluated the potential of FA-M-β-CyD as a novel anticancer agent in vitro and in vivo. FA-M-β-CyD displayed potent antitumor activity in vitro, compared to M-β-CyD in KB cells, FR-positive cells, but not in A549 cells, FR-negative cells. The cellular association of FA-M-β-CyD with KB cells was much higher than that with A549 cells. Furthermore, FA-M-β-CyD drastically inhibited tumor growth after an intratumoral or intravenous injection to tumor-bearing mice, compared to doxorubicin and M-β-CyD, without any significant change in blood chemistry values after an intravenous administration. These results strongly suggest that FA-M-β-CyD has the potential as a novel antitumor agent.
Methods
Materials
β-CyD was donated by Nihon Shokuhin Kako (Tokyo, Japan). M-β-CyD with an average degree of substitution (DS) of methyl group of 12.2 was obtained from Junsei Chemical (Tokyo, Japan). DM-β-CyD was purchased from Wako Pure Chemical Industries (Osaka, Japan). FA was purchased from Nakalai Tesque (Kyoto, Japan). RPMI-1640 culture medium (FA-free) was purchased from GIBCO (Tokyo, Japan). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Nissui Pharmaceuticals (Tokyo, Japan) and Nichirei (Tokyo, Japan), respectively. Tetramethylrhodamine isothiocyanate (TRITC) was obtained from Funakoshi (Tokyo, Japan). FR-α siRNA (sc-39969) was purchased from Santa Cruz Biotechnology (CA, USA). LipofectamineTM2000 reagent was obtained from Invitrogen (Tokyo, Japan). Doxorubicin was donated by Daiichi Sankyo (Tokyo, Japan). All other chemicals and solvents were of analytical reagent grade and deionized double-distilled water was used throughout the study.
Cell culture
A549 cells, a human lung epithelium cell line and colon-26 cells, a mouse colon carcinoma cell line, were cultured as reported previously36. KB cells, a human oral squamous carcinoma cell line, were grown in a RPMI-1640 culture medium (FA-free) containing penicillin (1×105 mU/mL) and streptomycin (0.1 mg/mL) supplemented with 10% FBS at 37°C in a humidified 5% CO2 and 95% air atmosphere. FR knockdown KB cells were prepared by the transfection with the FR-α siRNA complex with LipofectamineTM2000.
In vitro antitumor activity
In vitro antitumor activity was assayed by the WST-1 method (a Cell Counting Kit, Wako Pure Chemical Industries, Osaka, Japan), as reported previously52,53. Briefly, KB, A549 and Colon-26 cells were seeded at 2×104 cells onto 96-well microplate (Iwaki, Tokyo, Japan) and FR knockdown KB cells were seeded at 1×105 cells onto 24-well microplate (Iwaki, Tokyo, Japan) and then incubated for 24 h in a humidified atmosphere of 5% CO2 and 95% air at 37°C. Cells were washed twice with RPMI-1640 culture medium (FA-free) and then incubated with 150 μL of RPMI-1640 culture medium (FA-free) containing 0–10 mM β-CyDs or Tween 20 for 2 h at 37°C. For the competition study, cells were pretreated with RPMI-1640 culture medium containing 1 mM FA for 1 h and then incubated with RPMI-1640 culture medium containing 1 mM FA and 0–10 mM β-CyDs for 2 h. After washing twice with phosphate-buffered saline (PBS, pH 7.4) to remove β-CyDs, 100 μL of fresh Hanks' balanced salt solution (HBSS, pH 7.4) and 10 μL of WST-1 reagent were added to the plates and incubated for 30 min at 37°C. The absorbance at 450 nm against a reference wavelength of 630 nm was measured with a microplate reader (Bio-Rad Model 550, Tokyo, Japan).
Activation of caspase 3/7
KB cells (1×106/35 mm dish) were incubated with 5 mM M-β-CyDs for 2 h. After washing twice with 1 mL of RPMI-1640 (folic acid-free) medium to remove the samples, cells were add to 10 μM CellEventTM Caspase-3/7 Green Detection Reagent (Invitrogen, Tokyo, Japan) and incubated at 37°C for 30 min. The cells were washed with RPMI-1640 (folic acid-free) medium twice and added 1 mL of RPMI-1640 (folic acid-free) medium. The cells were observed by a fluorescence microscope (KEYENCE Biozero BZ-8000, Tokyo, Japan).
Cellular association of M-β-CyD
KB cells and A549 cells (1×106/35 mm dish) were incubated with 1 mL of RPMI-1640 culture medium (FA-free) and DMEM, respectively, containing 10 μM of TRITC-labeled M-β-CyD at 37°C for 1 h. After washing twice with 1 mL of PBS (pH 7.4) to remove the sample and immediately scraped with 1 mL of PBS (pH 7.4). The cells were collected and filtered through nylon mesh. Data were collected for 1×104 cells on a FACSCalibur flow cytometer using CellQuest software (Becton-Dickinson, Mountain View, CA).
Cellular association of FA-M-β-CyD
KB cells (1×106/35 mm dish) were incubated with 1 mL of RPMI-1640 culture medium (FA-free) containing 1 mM FA-M-β-CyD at 37°C for 1 h. After washing twice with 1 mL of PBS to remove the sample, cells were lysed by the addition of 1 mL of 1 N NaOH. The cellular association of FA-M-β-CyD was determined by a fluorescence spectrometry (Ex : 286 nm, Em : 350 nm, Hitachi F-4500, Tokyo, Japan).
Determination of cholesterol in the culture medium
KB cells or A549 cells (1×106/35 mm dish) were incubated with 5 mM β-CyDs in RPMI-1640 culture medium (FA-free) or DMEM, respectively, at 37°C for 1 h. After centrifugation (10,000 rpm, 5 min) of the culture medium, the supernatant was recovered. Total cholesterol in the culture medium was determined using a Cholesterol-test Wako® (Wako Pure Chemical Industries, Osaka, Japan).
Hemolytic activity
RRBC were isolated from Japanese white male rabbits (Kyudo, Tosu, Japan) as described previously27,29. Isolated RRBC were centrifuged at 3,000 rpm for 5 min and washed three times with PBS (pH 7.4). Five percents of RRBC suspension in PBS were incubated with 2 mL of PBS (pH 7.4) containing β-CyDs at 37°C for 30 min. After centrifugation at 3,000 rpm for 10 min, the optical density of the supernatant was measured at 543 nm. The results were expressed as a percent of total hemolysis, which was obtained when RRBC were incubated in water only. All hemolytic assays were carried out on the same day of blood collection.
Evaluation of antitumor effect of FA-M-β-CyD
Four-weeks-old BALB/c male mice (ca. 20 g) were subcutaneously injected the suspension containing Colon-26 cells (5×105 cells/100 μL), FR-expressing cells. About 10 days later, the mannitol solution (5%) dissolved with doxorubicin, M-β-CyD or FA-M-β-CyD was administered by the single intratumoral or intravenous injection to tumor bearing mice. The concentrations of doxorubicin, M-β-CyD and FA-M-β-CyD were 30 mg/kg for intratumoral injection and 5 mg/kg for intravenous injection. The tumor volumes were determined by the equation (Volume = LW2/2), where L is the longest dimension parallel to the skin surface and W is the dimension perpendicular to L and parallel to the surface. The body weight changes and survival rates of tumor-bearing mice were monitored for 30 days and 140 days, respectively. Animal experiments were approved by the Ethics Committee for Animal Care and Use of Kumamoto University (Approval ID: 24-286).
Determination of blood chemistry values
Blood samples were taken from the vital artery 24 h after an intravenous injection of the mannitol solution (5%) dissolved with doxorubicin (5 mg/kg), M-β-CyD (5 mg/kg) or FA-M-β-CyD (5 mg/kg) to tumor-bearing mice. After centrifugation, serum was collected and the blood chemistry values of CRE, BUN, AST, ALT and LDH were determined by a clinical chemistry analyzer, JCA-BM2250 (JEOL, Tokyo, Japan).
Data analysis
Data are given as the mean±S.E.M. Statistical significance of mean coefficients for the studies was performed by analysis of variance followed by Scheffe's test. p-Values for significance were set at 0.05.
References
Wong, H. L., Bendayan, R., Rauth, A. M., Li, Y. & Wu, X. Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 59, 491–504 (2007).
Harata, M. et al. CD19-targeting liposomes containing imatinib efficiently kill Philadelphia chromosome-positive acute lymphoblastic leukemia cells. Blood 104, 1442–1449 (2004).
Lukyanov, A. N., Elbayoumi, T. A., Chakilam, A. R. & Torchilin, V. P. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J. Control. Release 100, 135–144 (2004).
Park, J. W. et al. Tumor targeting using anti-her2 immunoliposomes. J. Control. Release 74, 95–113 (2001).
Pastorino, F. et al. Doxorubicin-loaded Fab' fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 63, 86–92 (2003).
Roche, A. C. et al. Glycofection: facilitated gene transfer by cationic glycopolymers. Cell Mol. Life Sci. 60, 288–297 (2003).
Chen, H., Ahn, R., Van den Bossche, J., Thompson, D. H. & O'Halloran, T. V. Folate-mediated intracellular drug delivery increases the anticancer efficacy of nanoparticulate formulation of arsenic trioxide. Mol. Cancer Ther. 8, 1955–1963 (2009).
Gabizon, A. et al. Improved therapeutic activity of folate-targeted liposomal doxorubicin in folate receptor-expressing tumor models. Cancer Chemother. Pharmacol. 66, 43–52 (2010).
Lu, Y. & Low, P. S. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv. Drug Deliv. Rev. 54, 675–693 (2002).
Mi, Y., Liu, Y. & Feng, S. S. Formulation of Docetaxel by folic acid-conjugated d-α-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS(2k)) micelles for targeted and synergistic chemotherapy. Biomaterials 32, 4058–4066 (2011).
Nukolova, N. V., Oberoi, H. S., Cohen, S. M., Kabanov, A. V. & Bronich, T. K. Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials 32, 5417–5426 (2011).
Hayakawa, K. et al. Sensitivity to apoptosis signal, clearance rate and ultrastructure of fas ligand-induced apoptosis in in vivo adult cardiac cells. Circulation 105, 3039–3045 (2002).
Kobayashi, T. et al. Effect of transferrin receptor-targeted liposomal doxorubicin in P-glycoprotein-mediated drug resistant tumor cells. Int. J. Pharm. 329, 94–102 (2007).
Miyajima, Y. et al. Transferrin-loaded nido-carborane liposomes: tumor-targeting boron delivery system for neutron capture therapy. Bioconjug. Chem. 17, 1314–1320 (2006).
Kim, I. Y. et al. Antitumor activity of EGFR targeted pH-sensitive immunoliposomes encapsulating gemcitabine in A549 xenograft nude mice. J. Control. Release 140, 55–60 (2009).
Mamot, C. et al. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 63, 3154–3161 (2003).
Schiffelers, R. M. et al. Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J. Control. Release 91, 115–122 (2003).
Low, P. S. & Kularatne, S. A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 13, 256–262 (2009).
Antony, A. C. The biological chemistry of folate receptors. Blood 79, 2807–2820 (1992).
Limmon, G. V. et al. Scavenger receptor class-A is a novel cell surface receptor for double-stranded RNA. FASEB J. 22, 159–167 (2008).
Parker, N. et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 338, 284–293 (2005).
Ross, J. F., Chaudhuri, P. K. & Ratnam, M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer 73, 2432–2443 (1994).
Toffoli, G. et al. Overexpression of folate binding protein in ovarian cancers. Int. J. Cancer 74, 193–198 (1997).
Low, P. S., Henne, W. A. & Doorneweerd, D. D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 41, 120–129 (2008).
Szente, L. & Szejtli, J. Highly soluble cyclodextrin derivatives: chemistry, properties and trends in development. Adv. Drug Deliv. Rev. 36, 17–28 (1999).
Uekama, K. & Otagiri, M. Cyclodextrins in drug carrier systems. Crit. Rev. Ther. Drug Carrier Syst. 3, 1–40 (1987).
Motoyama, K. et al. Effect of 2,6-di-O-methyl-α-cyclodextrin on hemolysis and morphological change in rabbit's red blood cells. Eur. J. Pharm. Sci. 29, 111–119 (2006).
Motoyama, K. et al. Involvement of lipid rafts of rabbit red blood cells in morphological changes induced by methylated β-cyclodextrins. Biol. Pharm. Bull. 32, 700–705 (2009).
Ohtani, Y., Irie, T., Uekama, K., Fukunaga, K. & Pitha, J. Differential effects of α-, β- and γ-cyclodextrins on human erythrocytes. Eur. J. Biochem. 186, 17–22 (1989).
Galbiati, F., Razani, B. & Lisanti, M. P. Emerging themes in lipid rafts and caveolae. Cell 106, 403–411 (2001).
Simons, K. & Ehehalt, R. Cholesterol, lipid rafts and disease. J. Clin. Invest. 110, 597–603 (2002).
Anderson, R. G. & Jacobson, K. A role for lipid shells in targeting proteins to caveolae, rafts and other lipid domains. Science 296, 1821–1825 (2002).
Motoyama, K. et al. Involvement of PI3K-Akt-Bad pathway in apoptosis induced by 2,6-di-O-methyl-β-cyclodextrin, not 2,6-di-O-methyl-α-cyclodextrin, through cholesterol depletion from lipid rafts on plasma membranes in cells. Eur. J. Pharm. Sci. 38, 249–261 (2009).
Grosse, P. Y., Bressolle, F. & Pinguet, F. Antiproliferative effect of methyl-β-cyclodextrin in vitro and in human tumour xenografted athymic nude mice. Br. J. Cancer 78, 1165–1169 (1998).
Loftsson, T. & Brewster, M. E. Pharmaceutical applications of cyclodextrins: basic science and product development. J. Pharm. Pharmacol. 62, 1607–1621 (2010).
Onodera, R., Motoyama, K. & Arima, H. Design and Evaluation of Folate-appended Methyl-β-cyclodextrin as a New Antitumor Agent. J. Incl. Phenom. Macrocycl. Chem. 70, 321–326 (2011).
Le Roy, C. & Wrana, J. L. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 6, 112–126 (2005).
Doherty, G. J. & McMahon, H. T. Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 (2009).
Kelemen, L. E. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int. J. Cancer 119, 243–250 (2006).
Dhar, S., Liu, Z., Thomale, J., Dai, H. & Lippard, S. J. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J. Am. Chem. Soc. 130, 11467–11476 (2008).
Guaragna, A. et al. Synthesis and evaluation of folate-based chlorambucil delivery systems for tumor-targeted chemotherapy. Bioconjug. Chem. 23, 84–96 (2012).
Long, J. S. & Ryan, K. M. New frontiers in promoting tumour cell death: targeting apoptosis, necroptosis and autophagy. Oncogene (2012).
Tan, M. L., Ooi, J. P., Ismail, N., Moad, A. I. & Muhammad, T. S. Programmed cell death pathways and current antitumor targets. Pharm. Res. 26, 1547–1560 (2009).
Park, E. K. et al. Cholesterol depletion induces anoikis-like apoptosis via FAK down-regulation and caveolae internalization. J. Pathol. 218, 337–349 (2009).
Hobbs, S. K. et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. U.S.A. 95, 4607–4612 (1998).
Nagy, J. A., Dvorak, A. M. & Dvorak, H. F. VEGF-A and the induction of pathological angiogenesis. Annu. Rev. Pathol. 2, 251–275 (2007).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Nagy, J. A., Chang, S. H., Dvorak, A. M. & Dvorak, H. F. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer 100, 865–869 (2009).
Chauhan, V. P., Stylianopoulos, T., Boucher, Y. & Jain, R. K. Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annu. Rev. Chem. Biomol. Eng. 2, 281–298 (2011).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Chauhan, V. P. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 7, 383–388 (2012).
Hamasaki, K., Kogure, K. & Ohwada, K. A biological method for the quantitative measurement of tetrodotoxin (TTX): tissue culture bioassay in combination with a water-soluble tetrazolium salt. Toxicon. 34, 490–495 (1996).
Ono, N., Arima, H., Hirayama, F. & Uekama, K. A moderate interaction of maltosyl-α-cyclodextrin with Caco-2 cells in comparison with the parent cyclodextrin. Biol. Pharm. Bull. 24, 395–402 (2001).
Acknowledgements
This work was partially supported by a Grant-in-Aid for Young Scientists (B) from Japan Society for the Promotion of Science (22790040) and by a Grant-in-Aid for Third Term Comprehensive Control Research for Cancer from Ministry of Health Labour and Welfare (24100701).
Author information
Authors and Affiliations
Contributions
R.O., K.M., A.O. and T.H. performed the experiments. R.O., K.M., T.H. and H.A. analysed and interpreted the data. R.O., K.M., T.H. and H.A. planned the research. K.M., R.O. and H.A. wrote the manuscript. H.A. supervised the work. All authors discussed the results.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Figure 1
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Onodera, R., Motoyama, K., Okamatsu, A. et al. Potential use of Folate-appended Methyl-β-Cyclodextrin as an Anticancer Agent. Sci Rep 3, 1104 (2013). https://doi.org/10.1038/srep01104
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep01104
This article is cited by
-
The effect of folate-appended methyl-β-cyclodextrin increases on survival rates in a peritoneal dissemination mouse models of human ovarian cancer
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2022)
-
Magnetic Nanoparticles in Targeted Drug Delivery: a Review
Journal of Superconductivity and Novel Magnetism (2021)
-
Chlorpromazine eliminates acute myeloid leukemia cells by perturbing subcellular localization of FLT3-ITD and KIT-D816V
Nature Communications (2020)
-
Methyl-β-cyclodextrin, an actin depolymerizer augments the antiproliferative potential of microtubule-targeting agents
Scientific Reports (2019)
-
Involvement of mitophagy-mediated cell death in colon cancer cells by folate-appended methyl-β-cyclodextrin
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.