Abstract
We report the observation of an unusual phase assembly behavior during the growth of hexagonal LuFeO3 thin films which resulted in the formation of epitaxial Fe3O4 nanolayers. The magnetite layers were up to 5 nm thick and grew under the conditions at which Fe2O3 is thermodynamically stable. These Fe3O4 nanolayers act as buffer layers promoting a highly epitaxial growth of the hexagonal LuFeO3 thin film up to 150 nm thick. Using scanning transmission electron microscopy, we show that the interface between (001) LuFeO3 and (111) Fe3O4 can be reconstructed in two ways depending on the sequence in which these compounds grow on each other. We suggest the polarity of the interface is the reason behind the observed interface reconstruction and epitaxial stabilization of magnetite.
Similar content being viewed by others
Introduction
Interface engineering has recently become one of the most exciting topics of solid state sciences owing to numerous physical phenomena taking place at interfaces such as magnetism, superconductivity and magnetoelectricity. These interface phenomena depend on epitaxy, strain and reconstruction between the two phases forming the interface, as well as on their polarity, spin, orbital and electronic band structures1,2. These fascinating phenomena have been observed mainly at interfaces between perovskite oxides because their bulk properties are well-known, allowing for a relatively easy control of the physics at their interface3. Among the important aspects of interface engineering that are poorly explored are the polarity and reconstruction of the crystal surfaces and their influence on the physical properties of more complex interfaces4,5. Polarity is known to often cause a surface reconstruction when the polar catastrophe is not mitigated by absorption, yet its effect on the structure of interfaces is difficult to explore experimentally. Moreover, the polarity of the crystal surfaces has been also reported to strongly influence the growth of thin films on such surfaces, inducing a polymorphic phase transition or a phase separation on the polar substrates6,7,8.
Here we report an interesting phenomenon of spontaneous formation of magnetite nanolayers in thin films of hexagonal LuFeO3 and describe the interface between these two phases. Hexagonal orthoferrites RFeO3 (R is a rare earth element), the structural analogues of multiferroic hexagonal RMnO3,9,10 have been mainly obtained by epitaxial stabilization in thin-film state and possess a non-centrosymmetric (space group P63cm) crystal structure that exhibits ferroelectricity along with weak ferromagnetism.11,12,13
Results
Hexagonal LuFeO3 thin film was grown c-oriented on the (111) cut of cubic ZrO2(Y2O3) as confirmed by a detailed X-ray diffraction (XRD) study (see Supplementary material, Figure S1). In the in-plane XRD patterns of the films that were thicker than 50 nm the (110) LuFeO3 peak appeared in addition to the (300) peak. The additional in-plane orientation results from a 30° rotation of the unit cell of hexagonal RFeO3 around the c axis and has not been observed yet in thin films of hexagonal orthophases (RMO3, M = Al, Mn, Fe, Ga, In). To clarify the origin of the additional orientation, we performed a scanning transmission electron microscopy (STEM) study. In our earlier studies we reported the maximum thickness of epitaxially stabilized hexagonal RFeO3 and RMnO3 thin films to be approximately 70 nm.12,14 However, STEM imaging showed that the thickness of our film exceeded ~150 nm (see Figure S3(a,b) in Supplementary material). Furthermore, low- and high-resolution high-angle annular dark-field (HAADF) STEM imaging revealed the presence of intergrown Fe3O4 nanolayers in the LuFeO3 film (Figure 1). The magnetite layers grew within the LuFeO3 film matrix parallel to the substrate surface and were up to 5 nm thick. Two types of Fe3O4 layers can be distinguished: continuous (at least several µm long) and isolated island-like (~50 nm long) ones shown in Figure 1(b) and (c), respectively. The continuous Fe3O4 layers change the in-plane orientation of the upper LuFeO3 layer by 30° and the isolated layers do not reorient the hexagonal LuFeO3 layers grown on top of it (Figure S2 in Supplementary material).
(a) Low-resolution HAADF STEM of hexagonal LuFeO3 thin film grown on ZrO2(Y2O3) substrate; black arrows indicate Fe3O4 nanolayers.High-resolution HAADF STEM images of a continuous nanolayer (b) that imposes the reorientation of LuFeO3 and an isolated nanolayer (c) that does not reorient LuFeO3. The framed inset in (c) represents the simulated HAADF STEM image of Fe3O4; white dots in (c) are real atomic positions of Fe atoms in the magnetite structure. The (b) image was taken from the region, where the underlying LuFeO3 layer is not reoriented, while the same orthoferrite sublayer in the (c) image is reoriented by the previous Fe3O4 nanolayers.
At first sight a reasonable explanation of our observations seems to lie in epitaxial stabilization of Fe3O4 (space group Fd-3m, a = 8.396 Å) on top of the (001) surface of RFeO3 (Figure S5 in Supplementary material). The a lattice parameter of hexagonal LuFeO3 is 5.90 Å, which is very close to half of the distance amagnetite√2 = 11.874 Å between the oxygen positions on the (111) surface of Fe3O4. Such a close lattice match at the interface between hexagonal LuFeO3 and Fe3O4 favors epitaxial growth. However, according to the temperature-pressure diagram of Fe-O system15, under the conditions used in our MOCVD process Fe2O3 is the thermodynamically stable phase. The partial oxygen pressure was at least 103 times higher than needed for the equilibrium phase formation of the magnetite phase. In pulsed-laser deposition experiments carried out by Liu et al., it was shown that magnetite phase appears in the thin films of the Lu-Fe-O system only in the temperature range of 700–900 °C and under extremely low pressure of 10−10 atm16. It suggests that in our case (T = 900 °C, p(O2)≈10−3 atm) a more complex mechanism rather than epitaxial stabilization alone took place during the film growth.
Similar phenomena have been described in the literature. Nanoinclusions of metalllic iron (Fe0) were found both at the interface and within the film of (111) Fe3O4 epitaxial films grown on the polar surface of (111) MgO substrates7. Analogously, Fe0 and FeO inclusions formed in (111) Fe3O4 films grown on (0001) Al2O3 substrates17. Such an unusual phase behavior was observed neither in (001) Fe3O4 films on (001) MgO, nor in (111) Fe3O4 films on (111) Pt substrates18,19. Recently, Lazarov et al reported the formation of a single Fe3O4 nanolayer on a surface of reconstruction-stabilized (111) MgO substrates in thin films of the hematite phase (α-Fe2O3)6. On the contrary, when the unreconstructed (111) surface of MgO was hydrogen-stabilized, the magnetite phase was not detected in Fe2O3 thin films.6 Thus, the polarity of the oxide surface and its reconstruction may play a crucial role in the process of phase stabilization. In this study we did not find magnetite nanolayers grown directly on the non-polar (111) surface of the ZrO2(Y2O3) substrate. To explore the distribution of cations at the oxide interface we performed a detailed HAADF STEM imaging and atomically resolved mapping of chemical elements by energy-dispersive x-ray spectroscopy.
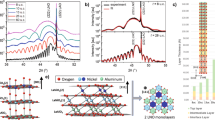
According to the mapping results (Figure 2(b–c)), no noticeable doping of Fe3O4 by lutetium was observed and the interface was chemically abrupt in most cases. The most intriguing result is the interface reconstruction along the entire interface that is designated by Lu atoms belonging to both Fe3O4 and LuFeO3 structures at the interface. In Figure 2(a) yellow arrows point at the Lu sites occupied by Fe atoms and green circles mark similar sites in LuFeO3, which are occupied by Lu atoms. The atoms at the interface form the Fe3O4 motif, yet with a periodic substitution of Fe by Lu (the in-plane period is 2Lu+1Fe atoms). The top layer of the alternating two Lu and one Fe atoms appears to be isostructural to Fe3O4 and becomes the first atomic layer of the magnetite phase grown on LuFeO3 (Figure 2(a–d)). A model interface between LuFeO3 and Fe3O4 and the sequence of ions perpendicular to the substrate are shown in Figure 2(e). The shift of some of Lu ions along the c axis is characteristic of hexagonal LuMO3 (M = Fe, Mn) with the P63cm space group (in paraelectric state hexagonal RMO3 belongs to the centrosymmetric P63/mmc space group). One would expect the Lu atoms to be shifted along the [001] direction to be present at the reconstructed interface, because in this case the shifted Lu3+ ions would find perfectly matched sites within the Fe3O4 crystal structure. However, this scenario does not take place at the interface between Fe3O4 grown on top of LuFeO3, where the interface reconstruction is accomplished by the downward shift of Lu3+ ions (Lu4 and Lu5 in Figure 2(e)). Only when the Fe3O4 layer thickness reaches ~5 nm does hexagonal LuFeO3 grow on top of it in a manner expected from our speculation above: Lu ions that are shifted downward (Lu3 in Figure 2(e)) participate in the formation of the reconstructed interface and the growth of LuFeO3 proceeds.
(a) HAADF STEM image of the reconstructed interface between LuFeO3 and Fe3O4.Open green circles mark the present Lu atoms in LuFeO3 structure and yellow circles show the corresponding position at the interface, where Fe is located. (b–c) Corresponding atomic resolution EDX maps of Lu and Fe, open yellow circles mark the same position with Fe. (d) Combined HAADF STEM image and Fe+Lu map, where the colors of Lu and Fe remain the same as in (b–c). The scale bar is 1 nm. (e) A model interface between LuFeO3 and Fe3O4 and the sequence of atomic layers forming polar (001) LuFeO3 and (111) Fe3O4 surfaces. Black rectangles are the unit cells. "Plane 1" and "Plane 2" are two planes, the top views of which are shown in Figure 3. Magnified area on the right shows distances calculated from STEM images (error is 0.1 Å). (f) The profiles of Lu Lβ and Fe Kα intensities perpendicular to the interface, where each peak correlates well with atomic planes in the interface area (dotted lines).
Using the distribution of atoms along the [010] direction of LuFeO3, schemes of the individual reconstructed surfaces of Fe3O4 and hexagonal LuFeO3 at the interface were created (Figure 3). The reconstruction of the Fe3O4 surface is described as Fe3O4−(111) . On the contrary, LuFeO3 has a (1×1) surface reconstruction without the rotation of the "surface" unit cell relative to the "bulk" crystal structure. Based on a high-resolution HAADF STEM imaging, the distances between atomic planes/columns were calculated (red-framed zoomed structure in Figure 2(e)). The Fe1–Fe2 distance is 1.8 Å, being identical to that in bulk Fe3O4. The Lu1–Lu2 distance is very close to the Fe1–Fe2 distance (1.9 Å), yet the distance between Lu1 and Fe2 is rather elongated (2.3 Å). The Lu1 atom is shifted towards Fe3, shortening the Lu1–Fe3 distance to 3.0 Å (the bulk value is 3.2 Å) and noticeably increasing the Fe2–Lu1 distance. Figure 2(f) shows the profiles of Lu and Fe distribution perpendicular to the interface, from which one can easily deduce the sequence of different atomic planes. Intriguing is the fact that Lu3+ ions (labeled "Lu2") take the position of Fe2+/3+ in Fe3O4, which suggests an electron doping may occur in the interface area (if it is not compensated by oxygen vacancies). The possibility of the surface-reconstruction-induced carrier doping at the interface requires further studies.
. On the contrary, LuFeO3 has a (1×1) surface reconstruction without the rotation of the "surface" unit cell relative to the "bulk" crystal structure. Based on a high-resolution HAADF STEM imaging, the distances between atomic planes/columns were calculated (red-framed zoomed structure in Figure 2(e)). The Fe1–Fe2 distance is 1.8 Å, being identical to that in bulk Fe3O4. The Lu1–Lu2 distance is very close to the Fe1–Fe2 distance (1.9 Å), yet the distance between Lu1 and Fe2 is rather elongated (2.3 Å). The Lu1 atom is shifted towards Fe3, shortening the Lu1–Fe3 distance to 3.0 Å (the bulk value is 3.2 Å) and noticeably increasing the Fe2–Lu1 distance. Figure 2(f) shows the profiles of Lu and Fe distribution perpendicular to the interface, from which one can easily deduce the sequence of different atomic planes. Intriguing is the fact that Lu3+ ions (labeled "Lu2") take the position of Fe2+/3+ in Fe3O4, which suggests an electron doping may occur in the interface area (if it is not compensated by oxygen vacancies). The possibility of the surface-reconstruction-induced carrier doping at the interface requires further studies.
Top view of the reconstructed surfaces of (a) LuFeO3 and (b) Fe3O4 at the interface (respectively, "Plane 1" and "Plane 2" in Figure 2(e); colors of atoms are the same).
Orange lines represent the unit cell of the main crystal and red lines mark the "unit cell" of the reconstructed surface. The arrows show unit vectors of the structures. The structures were drawn using VESTA software22.
Discussion
We suppose that, despite the perfect lattice match of the (111) Fe3O4 and (001) LuFeO3 crystal planes, the reconstruction that takes place at the interface between these surfaces is driven by their polarity. To underpin our assumptions, we should take into account the following facts. First, based on a complete structural similarity of hexagonal orthoferrites to hexagonal manganites one can expect the presence of spontaneous polarization along the c axis in hexagonal RFeO3, which has recently been proved in13. Second, the sequence of atomic layers in LuFeO3 along the [001] direction includes a polar fragment O-2/3 Lu-1/3 Lu-O that makes the (001) surface of the hexagonal phase to be of the type 3 according to Tasker's notation20. Third, the growth of magnetite was observed only for the (001) surface of LuFeO3 and not for the non-polar (111) surface of ZrO2(Y2O3). As a result, in our scenario after the reconstruction of the oxide interface a following formation of magnetite layers was driven by epitaxial stabilization up to the thickness when the polar catastrophe of (111) Fe3O4 made it impossible for magnetite to grow any further under the deposition conditions used. Hence, in such a layered composite both its components form in situ and assist each other via the process that occurs in two stages - compensation of surface polarity of the (001) LuFeO3 sublayer and epitaxial stabilization of the (111) Fe3O4 nanolayer (and vice versa). Our results are important for the growing field of interface engineering, where magnetite, which has already found various technological applications, can be used as a spintronic material with a high tunneling magnetoresistance21. Also, it is worth noting that in our films the ferroelectric hexagonal LuFeO3 is combined with ferrimagnetic Fe3O4, thus forming a layered multiferroic composite in situ (no layer-by-layer deposition is required) with both phases perfectly tailored through the reconstructed interface. Moreover, in layer-by-layer deposition (using, for example, pulse laser deposition) one can obtain a multiferroic superlattice with the same perfect epitaxial growth of each layer.
In conclusion, the in situ formation of Fe3O4 in the hexagonal LuFeO3 thin films in the form of continuous layers (~5 nm thick) that preserved the stability of the epitaxial hexagonal phase, simultaneously changing its in-plane orientation, was observed in MOCVD process. The formation of magnetite occurred only on the polar (001) surface of LuFeO3 under the conditions, under which Fe2O3 phase is thermodynamically stable. A peculiar structural reconstruction along the interface between the (111) Fe3O4 and (001) LuFeO3 surfaces is found and analyzed. Most likely, the polarity of the (001) surface of ferroelectric hexagonal LuFeO3 induces the interface reconstruction during growth and the subsequent epitaxial stabilization of nanolayered magnetite, which is consistent with other recent studies. The interface reconstruction turns out to be one of the universal mechanisms of the stabilization of polar surfaces.
Methods
Thin films of hexagonal LuFeO3 were grown on single-crystalline (111) ZrO2(Y2O3) substrates at 900°C using MOCVD with a flash evaporation at 250°C of a mixture of Lu(thd)3 and Fe(thd)3 (thd = 2,2,6,6-tetramethylheptane-3,5-dionate) precursors. The total pressure was 5 mbar, the oxygen partial pressure was 1-2 mbar. The growth rate was 5 nm/min, which was small enough to ensure the surface-diffusion-driven growth. After the deposition the same oxygen partial pressure was used for the in-situ post-deposition annealing carried out for 20-30 min at 900°C and the subsequent cooling process. More information regarding the chemical equilibrium achieved in MOCVD process can be found in Supplementary material. X-ray diffraction (XRD) study of thin films was performed using Rigaku SmartLab 5-circle X-ray diffractometer. Scanning transmission electron microscopy (STEM) study was performed using probe-corrected FEI Titan 80-300 S/TEM operating at 300 kV. Atomic-resolution maps of chemical elements were obtained by energy-dispersive x-ray spectroscopy (AR EDS) using a high-sensitivity Super-X EDX detector system in a probe-corrected FEI Titan G2 60-200 S/TEM with X-FEG source (ChemiSTEM technology). The Lu Lâ and Fe Ká lines were identified on the spectra and used in the mapping procedure.
References
Bibes, M., Villegas, J. E. & Barthelemy, A. Ultrathin oxide films and interfaces for electronics and spintronics. Advances in Physics 60, 5–84 (2011).
Zubko, P., Gariglio, S., Gabay, M., Ghosez, P. & Triscone, J.-M. Interface Physics in Complex Oxide Heterostructures. Annual Review of Condensed Matter Physics 2, 141–165 (2011).
Hwang, H. Y. et al. Emergent phenomena at oxide interfaces. Nature Materials 11, 103–113 (2012).
Goniakowski, J., Finocchi, F. & Noguera, C. Polarity of oxide surfaces and nanostructures. Reports on Progress in Physics 71, 016501 (2008).
Noguera, C. Polar oxide surfaces. Journal of Physics: Condensed Matter 12, R367 (2000).
Cheung, S. H. et al. Effects of unreconstructed and reconstructed polar surface terminations on growth, structure and magnetic properties of hematite films. Phys. Rev. B 85, 045405 (2012).
Lazarov, V. K., Chambers, S. A. & Gajdardziska-Josifovska, M. Polar Oxide Interface Stabilization by Formation of Metallic Nanocrystals. Phys. Rev. Lett. 90, 216108 (2003).
Lazarov, V. K. et al. Selected Growth of Cubic and Hexagonal GaN Epitaxial Films on Polar MgO(111). Phys. Rev. Lett. 94, 216101 (2005).
Lottermoser, T. et al. Magnetic phase control by an electric field. Nature 430, 541–544 (2004).
Van Aken, B., Palstra, T., Filippetti, A. & Spaldin, N. The origin of ferroelectricity in magnetoelectric YMnO3 . Nature materials 3, 164–170 (2004).
Akbashev, A. R., Semisalova, A. S., Perov, N. S. & Kaul, A. R. Weak ferromagnetism in hexagonal orthoferrites RFeO3 (R = Lu, Er-Tb). Applied Physics Letters 99, 122502 (2011).
Bosak, A. A., Dubourdieu, C., Senateur, J.-P., Gorbenko, O. Y. & Kaul, A. R. Epitaxial stabilization of hexagonal RMnO3 (R = Eu-Dy) manganites. J. Mater. Chem. 12, 800–801 (2002).
Jeong, Y. K. et al. Structurally Tailored Hexagonal Ferroelectricity and Multiferroism in Epitaxial YbFeO3 Thin-Film Heterostructures. Journal of the American Chemical Society 134, 1450–1453 (2012).
Bossak, A. A. et al. XRD and HREM Studies of Epitaxially Stabilized Hexagonal Orthoferrites RFeO3 (R = Eu-Lu). Chemistry of Materials 16, 1751–1755 (2004).
Schlueter, C., Lubbe, M., Gigler, A. M. & Moritz, W. Growth of iron oxides on Ag(111) - Reversible Fe2O3/Fe3O4 transformation. Surface Science 605, 1986–1993 (2011).
Liu, J., Wang, Y. & Dai, J. Y. Structural and dielectric properties of LuFe2O4 thin films grown by pulsed-laser deposition. Thin Solid Films 518, 6909–6914 (2010).
Farrow, R. F. C. et al. Nanoscale phase separation in Fe3O4(111) films on sapphire(0001) and phase stability of Fe3O4(001) films on MgO(001) grown by oxygen-plasma-assisted molecular beam epitaxy. Journal of Applied Physics 93, 5626–5636 (2003).
Chambers, S. A., Thevuthasan, S. & Joyce, S. A. Surface structure of MBE-grown Fe3O4(001) by X-ray photoelectron diffraction and scanning tunneling microscopy. Surface Science 450, L273–L279 (2000).
Weiss, W., Barbieri, A., Van Hove, M. A. & Somorjai, G. A. Surface structure determination of an oxide film grown on a foreign substrate: Fe3O4 multilayer on Pt(111) identified by low energy electron diffraction. Phys. Rev. Lett. 71, 1848–1851 (1993).
Tasker, P. W. The stability of ionic crystal surfaces. Journal of Physics C: Solid State Physics 12, 4977 (1979).
Sorenson, T. A., Morton, S. A., Waddill, G. D. & Switzer, J. A. Epitaxial Electrodeposition of Fe3O4 Thin Films on the Low-Index Planes of Gold. Journal of the American Chemical Society 124, 7604–7609 (2002).
Momma, K. & Izumi, F. VESTA3 for three-dimensional visualization of crystal, volumetric and morphology data.Journal of Applied Crystallography 44, 1272–1276 (2011).
Acknowledgements
This work was supported by the Russian Foundation of Basic Research (Project No. 10-03-00964).
Author information
Authors and Affiliations
Contributions
A.A. planned and performed the deposition of thin films. V.A. measured XRD. A.V. and V.R. performed preliminary HRTEM experiments and S.L. carried out the atomic-resolution EDX measurements. A.A. interpreted the data and with the help of A.K. proposed the model describing the results. The manuscript was written by A.A. and A.K. with critical comments made by all co-authors.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Akbashev, A., Roddatis, V., Vasiliev, A. et al. Reconstruction of the polar interface between hexagonal LuFeO3 and intergrown Fe3O4 nanolayers. Sci Rep 2, 672 (2012). https://doi.org/10.1038/srep00672
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00672
This article is cited by
-
Polarity compensation in ultra-thin films of complex oxides: The case of a perovskite nickelate
Scientific Reports (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.