Abstract
The roles of multifunctional CD4 T cells in human tuberculosis are not well defined. In this study, we found that patients with tuberculosis had decreased PMA/ionomycin stimulated multifunctional CD4 T cells and increased Mycobacterium tuberculosis antigen-specific multifunctional CD4 T cells, when compared to individuals with latent tuberculosis infection and healthy controls. PMA/ionomycin stimulated IFN-γ+IL-2+TNF-α+ CD4 T cell responses were decreased in patients with smear-positive tuberculosis compared to those with smear-negative tuberculosis. The percentage of IFN-γ+IL-2+TNF-α+ CD4 T cells in smear positive tuberculosis patients negatively correlated with the grade of sputum smear Acid-Fast Bacilli and high-resolution computed tomography score. Therefore, our findings argue against the notion that Mycobacterium tuberculosis antigen-specific multifunctional Th1 responses in peripheral blood can serve as correlates of protective immunity against tuberculosis; they suggest that the decrease in PMA/ionomycin stimulated IFN-γ+IL-2+TNF-α+ CD4 T cells may be applied for clinical diagnosis of active tuberculosis.
Similar content being viewed by others
Introduction
Pulmonary tuberculosis (TB) caused by Mycobacterium tuberculosis (M. tuberculosis) is one of the most pervasive killers among infectious diseases1. It has been generally accepted that CD4 T cell mediated immunity plays an important role in control of the infectious bacillus2,3. However, the characteristics of protective immunity remain to be elucidated.
Recent studies have suggested that CD4 T cells simultaneously secreting IFN-γ, TNF-α and IL-2, which have been termed as multifunctional Th1 cells, are associated with control of chronic bacterial and viral infections4,5,6. The presence of multifunctional Th1 cells in the lung has also been reported to correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice7. In humans, some reports have suggested that multifunctional M.tuberculosis-specific CD4 T cells play an important role in protective immunity against tuberculosis8,9. However, Caccamo et al reported that multifunctional CD4T cells correlate with active M. tuberculosis infection, instead of correlating with protective immunity10. Similarly, studies in cynomolgus macaque tuberculosis models revealed that M. tuberculosis-specific multifunctional T cells are better correlates of antigen load (i.e., disease status), than of protection11. While these paradoxical findings may be due to the different populations studied and the different protocols used7,9,10,11,12, they clearly indicate further investigations are warranted to understand the role of multifunctional Th1 cells in human tuberculosis.
To characterize multifunctional CD4 T cell responses associated with M. tuberculosis infection, we compared PMA/ionomycin stimulated and M. tuberculosis-specific multifunctional CD4 T cell responses among TB patients, patients with latent tuberculosis infection (LTBI) and healthy control individuals (HC). We also evaluated the relationship between sputum smear positivity and multifunctional CD4 T cell response. Furthermore, we assessed the severity of pulmonary tuberculosis by high-resolution computed tomography (HRCT) score13,14 and analyzed its association with multifunctional CD4 T cells.
Results
Patients with TB present decreased ex vivo PMA/ionomycin-stimulated multifunctional CD4 T cell responses compared to LTBI and HC
We first compared expression of IFN-γ, IL-2 and TNF-α in CD4 T cells from patients with active TB, LTBI subjects and HC, after short-term ex vivo stimulation with PMA/ionomycin. Responding CD4 T cells were classified as triple (3+), double (2+) or single (1+) cytokine-producing populations according to expression profile of IFN-γ, IL-2 and TNF-α (Figure 1A). As shown in Figure 1B, while there are no significant differences between HC and LTBI, patients with TB had decreased percentages of IFN-γ+IL-2+TNF-α+, IFN-γ+IL-2+ and IFN-γ+TNF-α+ producing CD4 T cells, but increased TNF-α single positive CD4 T cells compared with HC and LTBI. However, the relative proportion of 3+ (IFN-γ+IL-2+TNF-α+) CD4 T cells within total population of cytokine-producing CD4 T cells is not significantly decreased in patients with TB (Figure 1C), due to simultaneously decrease of 2+ and 1+ cytokine-producing CD4 T cell in patients with TB (Figure 1B). Therefore, the frequency of 3+ CD4 T cells within total CD4 T cells, but not the relative proportion of 3+ CD4 T cell within cytokine-producing CD4 T cells, is associated with TB disease outcome.
Patients with TB present decreased PMA/ionomycin stimulated multifunctional CD4 T cell responses, compared with LTBI and HC.
(A) Whole blood from HC, LTBI and TB patients was stimulated for 6 hrs with PMA/ionomycin and cells were analyzed by flow cytometry for intracellular expression of IFN-γ, IL-2 and TNF-α in CD4 (CD3+CD8−) T cells. (A) Representative dot plots show the gating procedure for analyzing cytokine production of CD4 (CD3+CD8−) T cells. (B) Summary cumulative data show the percentage of CD4 T cells with different patterns of cytokine production in HC (n = 90), LTBI (n = 108) and TB (n = 131). Data were expressed as mean ±SEM. ** p<0.01, ***p<0.001. (C) The pie charts summarize the fractions of single (1+, green), double (2+, blue) and Triple (3+, red) CD4 T cell producers of IFN-γ, IL-2 and TNF-α in HC, LTBI and TB.
M. tuberculosis-specific multifunctional CD4 T cell responses are increased in patients with TB compared to LTBI and HC
In contrast to PMA/ionomycin-stimulated multifunctional CD4 T cell responses, patients with TB had significantly increased percentages of M. tuberculosis-specific 3+ and 2+ CD4 T cell responses, when compared with LTBI and HC (Figure 2 A&B). There are no significant differences between any of the single-positive CD4 T cell responses among the three different populations (Figure 2B). Accordingly, the relative proportions of 3+ and 2+ CD4 T cells were not significantly increased in TB (Figure 2C).
Patients with TB demonstrate increased multifunctional CD4 T cell responses to TB antigens compared with LTBI and HC.
(A) PBMCs from HC, LTBI and TB donors were stimulated for 16 h with M. tuberculosis lysate and analyzed by flow cytometry for intracellular expression of IFN-γ, IL-2 and TNF-α in CD4 T cells. (A) Representative dot plots showed the gating procedure for analyzing cytokine production of CD4 (CD3+CD8-) T cells. (B) Summary of cumulative data showing the percentages of CD4 T cells with different patterns of cytokine production in HC (n = 66), LTBI (n = 30) and TB (n = 61). Data are expressed as mean ±SEM. *p<0.05, ** p<0.01, ***p<0.001. (C) The pie charts summarizing the fractions of single (1+, green), double (2+, blue) and triple (3+, red) CD4 T cell producers of IFN-γ, IL-2 and TNF-α in HC, LTBI and TB.
PMA/ionomycin-stimulated IFN-γ+IL-2+TNF-α+ CD4 T cells are decreased in culture and smear-positive TB patients compared to smear-negative ones and correlates with the sputum smear Acid-Fast bacilli
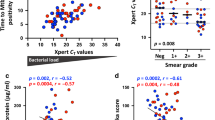
It has been reported that sputum smear positivity in patients with TB correlated with disease severity and was associated with response to anti-tuberculosis treatment14,15,16,17. We found that patients with sputum-culture negative TB had significantly increased PMA/ionomycin-stimulated 3+ cytokine-producing CD4 T cells compared with patients with culture-positive TB (Figure 3A). There were no significant differences in any other PMA/ionomycin-stimulated 2+ or 1+ cytokine combinations of CD4 T cells found between the two groups of TB patients (data not shown). Notably, a significant negative correlation between percentage of 3+ CD4 T cells and sputum smear grade at diagnosis within smear-positive TB patients was found (r = −0.438, p = 0.0136) (Figure 3B). In contrast, culture-negative TB patients had lower proportions of M. tuberculosis-specific 3+, 2+ (IFN-γ+ IL-2+ and IFN-γ+ TNF-α+) and 1+ (IFN-γ+) CD4 T cells than culture-positive TB patients, although these differences did not attain statistical significance (Figure 4).
PMA/ionomycin stimulated 3+ cytokine-producing CD4 T cell responses are associated with culture and sputum smear-positivity.
Whole blood of TB patients was stimulated with PMA/ionomycin and IFN-γ, IL-2, TNF-α cytokine production was analyzed as described in Figure 1. (A) The percentage of 3+ cytokine-producing CD4 T cells in TB patients with negative culture for M. tuberculosis [culture(-), n = 93] is higher compared with those with positive cultures [culture(+), n = 37]. Each dot represents results for one patient. P value is indicated. (B) Correlation analysis between the percentage of 3+ cytokine-producing CD4 T cells and sputum smear grade in culture (+) TB patients (n = 32).
Antigen-specific multifunctional CD4 T cell responses were not different between M. tuberculosis culture (-) (n = 38) and culture (+) (n = 25) TB patients.
PBMCs from TB patients were stimulated with M. tuberculosis lysate for 16 h and analyzed for IFN-γ, IL-2, TNF-α cytokine production as described in Figure 2. The percentages of CD4 T cells with different patterns of cytokine production were compared between the two groups.
Correlation between PMA/ionomycin-stimulated IFN-γ+IL-2+TNF-α+ CD4 T cell and HRCT score of pulmonary TB patients
HRCT scores had been generated from 74 patients with TB who completed HRCT exams. Among them, fifteen were sputum smear-positive TB patients, while the rest of patients were smear-negative. Correlation analysis revealed that only PMA/ionomycin-stimulated 3+ CD4 T cells, but not any other subset of CD4 T cells, significantly correlated with total HRCT score for all 74 patients with TB, although the correlation efficient (Pearson's r) was low (Figure 5A). When the correlation analysis was performed within the subgroup of smear-positive TB patients, a higher correlation efficient was observed (Figure 5B). Again, there was no significant correlation between M. tuberculosis-specific multifunctional CD4 T cell response and HRCT score (data not shown).
The percentages of PMA/ionomycin stimulated 3+ cytokine-producing CD4 T cells correlate with HRCT score in patients with TB.
Correlation analyses demonstrate significance between the percentage of 3+ cytokine-producing CD4 T cells and HRCT score of pulmonary disease in (A) TB patients (n = 74) regardless the positivity of sputum smear and (B) in smear-positive TB patients (n = 15).
PMA/ionomycin stimulated IFN-γ+IL-2+TNF-α+ CD4 T cells are correlated with Th17 cells in TB patients
It has been reported that Th17 cells and IL-17 play important roles in protective immunity against TB18,19,20. Suppressed Th17 responses are associated with M. tuberculosis infection, development and severity of TB diseases in humans21,22. Therefore, it is important to understand the relationship between multifunctional CD4 T cells and theTh17 response. As shown in Figure 6, PMA/ionomycin-stimulated 3+ cytokine-producing CD4 T cells correlate well with the frequency of Th17 cells regardless of donors' disease status. Given our knowledge of the roles played by Th17 cells in TB, these data suggest that there may be similar or overlapping roles for PMA/ionomycin stimulated 3+ CD4 T cells.
Discussion
CD4 T cells simultaneously expressing IFN-γ,TNF-α and IL-2 (termed multifunctional Th1 cells), have been reported to play a critical role in controlling chronic bacterial and viral infections4,5,6. However, the inconsistent or controversial results generated so far have made it difficult to define the role of these cells in tuberculosis7,8,9,10,11. To better understand the role of multifunctional Th1 cells in TB, we analyze the association between multifunctional Th1 cells and severity of disease in TB patients, as defined by both sputum smear-grade and HRCT score of pulmonary tuberculosis at diagnosis13,14. Consistent with most previous studies7,9,10,11, our data showed that the percentage of M. tuberculosis-specific multifunctional CD4 T cells are significantly higher in TB patients than in LTBI and HC. However, we did not find significant differences in multifunctional CD4 T cell responses between smear-positive and smear negative TB patients, which is inconsistent with two recent reports that M. tuberculosis-specific multifunctional Th1 responses are associated with sputum smear grading9,23. In addition, there is no significant correlation between antigen-specific multifunctional Th1 response and severity of pulmonary tuberculosis defined by HRCT score and sputum smear grading. Taken together, these results did not establish a direct association between the levels of antigen-specific multifunctional Th1 cell response and mycobacterial antigen load. This inconsistency could be due to the differences in study populations and experimental protocols, for example, M. tuberculosis specific multifunctional Th1 cells are memory responses to both BCG vaccination and M. tuberculosis infection in our population. Alternatively, there are likely other mechanisms regulating multifunctional Th1 response during M. tuberculosis infection that may interfere with this correlation24. Whatever the exact reason, our data argue against the notion that antigen-specific multifunctional Th1 responses in peripheral blood can serve as correlates of protective immunity against TB25.
Interestingly, we found that PMA/ionomycin-stimulated multifunctional Th1 cells are significantly decreased in patients with TB, compared to LTBI and HC. In addition, patient with sputum-culture negative TB had significantly increased PMA/ionomycin-stimulated 3+ cytokine-producing CD4 T cells compared with patients with culture-positive TB. Moreover, PMA/ionomycin-stimulated 3+ cytokine-producing CD4 T cell percentages significantly inversely correlated with both smear grade and HRCT score. Previous studies have indicated that sputum smear grading at the time of diagnosis not only correlates with response to anti-tuberculosis treatment, but also with disease severity by chest radiograph scoring13,15,17. It is also reported that smear grading is predicative of relapse and recurrence of TB disease15. Since PMA/ionomycin-stimulated multifunctional Th1 cells were detected in whole blood with short-term PMA/ionomycin stimulation, presumably only antigen-experienced T cells can be detected. Considering the fact that the frequency of M. tuberculosis-specific multifunctional Th1 is low (less than 0.1%); it is probable that majority of PMA/ionomycin stimulated multifunctional T cells are not M. tuberculosis-specific, but specific for other microbes to which the individual has been exposed. Therefore, it is intriguing to find that the frequency of PMA/ionomycin stimulated multifunctional Th1 cells is decreased in patients with TB and correlates with disease severity. It is well known that antigen-experienced T cells, regardless of their specificity, are attracted to sites of infection during tuberculosis26,27. A previous report also demonstrated that, even in healthy individuals, there are significantly higher frequencies of antigen-specific CD4+ T cells against influenza, S. pneumoniae and M. tuberculosis in bronchoaveolar fluid (BAL) than in peripheral blood25. This is particularly true in the case of multifunctional Th1 cells, as there are significantly higher proportions of multifunctional Th1 cells in BAL than in peripheral blood25. As demonstrated in HBV infection, the hepatic inflammation initiated by HBV recruits not only HBV-specific T cells to the site of infection, but also non-HBV specific antigen experienced T cells are recruited as well and these T cells outnumber the HBV-specific ones28. Therefore, it is highly possible that antigen-experienced multifunctional Th1 cells as detected by short-term PMA/ionomycin stimulation, which include both M. tuberculosis specific and non-M. tuberculosis specific, are recruited to the site of diseases and result in their decrease in peripheral blood. The finding that frequency of PMA/ionomycin stimulated multifunctional Th1 cells inversely correlates with severity of pulmonary TB suggests that more multifunctional Th1 cells may infiltrate into the site of infection in patients with more severe diseases, which is probably due to higher inflammatory cytokines/chemokines, such as IL-6, IL-1β and IL-11, produced in the lung29. Further studies are warranted to investigate their role in the pathogenesis of TB. This is important since interactions between T cells responding to concurrent mycobacterial and influenza infections have been reported to affect effector responses to pathogens30.
We have reported previously that Th17 response is decreased in patients with TB when compare to LTBI22. In addition, patients with severe TB have significantly lower Th17 response than those with mild TB22. Interestingly, we found in this study that PMA/ionomycin-stimulated 3+ cytokine-producing CD4 T cells significantly correlated with Th17 responses in HC, LTBI and TB patients. Given the fact that multifunctional Th1 cells can secret IL-1724 and that Th17 cells can also produce other Th1 cytokines including TNF-α, IFN-γ and IL-231,32,33, it is probable that multifunctional Th1 cells, at least some of 3+ cytokine-producing CD4 T cells, simultaneously secrete IL-17. Further investigation is warranted to clarify these possibilities since it may provide new insight for clinical implementation of multifunctional Th1 and Th17 cells in TB.
In summary, we found significant differences in the profiles of M. tuberculosis-specific and polyclonal non Ag-specific multifunctional Th1 responses among individuals with TB, LTBI and HC. These findings may be applied for clinical diagnosis of active TB from LTBI. Further investigations on dynamic changes of lung multifunctional Th1 cells, both M.tuberculosis-specific and non-specific, during development and resolution of pulmonary TB, as well as their relationships to severity of disease will improve our understanding of their roles in TB.
Methods
Patients and samples
A total of 329 participants including healthy control individuals (HC, n = 90), individuals with LTBI (LTBI, n = 108) and pulmonary TB patients (TB, n = 131) were recruited for this study from the city of Shenzhen, China. All study participants gave written, informed consent for the study, which was approved by Human Research Ethics Committee of Shenzhen Third People's Hospital. All subjects were ≥18 years of age and were sero-negative for HIV. The sex ratios (male/female) of each groups are HC (43/47), LTBI (60/48) and TB (86/45). The ages of each group (median [interquartile range]) were: HC, 28.0 (25.0–32.0); LTBI, 28.0 (25.0–36.0); PTB, 32.0 (25.0–43.5). A previously established M. tuberculosis–specific IFN-γ enzyme-linked immunospot (ELISPOT) assay was used to differentiate individuals with LTBI from true healthy controls34. Diagnosis of pulmonary tuberculosis (TB) was based on signs and symptoms, roentgenographic findings (chest X-ray and/or HRCT) consistent with TB, sputum bacterium examination and response to anti-tuberculosis treatment. The number of bacilli in sputum smears was counted according to World Health Organization Guidelines with minor modifications35. Briefly, at least 3 sequential sputum samples were collected from each patient before starting anti-tuberculosis chemotherapy and Acid-Fast Bacilli (AFB) were identified by Ziehl-Neelsen staining. Of 131 TB patients, 37 had sputum positive culture for M. tuberculosis. Among them, 32 had sputum positive smear microscopy for M. tuberculosis and were divided into 4 groups as per grading of the sputum AFB smear: group I (sputum1+, n = 13): 1 to 9 AFB per 100 fields; group II (sputum 2+, n = 11): 10 to 99 AFB per 100fields; group III (sputum 3+,n = 6): 1 to 10 AFB per field in 50 fields; and group IV (sputum 4+, n = 2): More than 10 AFB per field in 20 fields). Sputum samples were classified according to the highest number of AFB per specimen. Peripheral blood samples were obtained from all subjects. Clinical samples from patients with TB were obtained within 7 days prior to initiating standard anti-TB treatment.
Intracellular cytokine staining and flow cytometric analysis
Fresh heparinized whole blood (250 μl/well) was incubated with phorbol myristate acetate (PMA; 50 ng/ml; Sigma-Aldrich, USA) and ionomycin (1 µg/ml; Sigma-Aldrich) for 2 hs at 37°C. Blood incubated with no stimulation served as negative controls. Brefeldin A (10 μg/ml; Sigma-Aldrich) was then added and the incubation continued for an additional 4 h. Following the 6 h incubation, 2 mM EDTA was added for 10 min, followed by red cell lysis. Cells were washed again in PBS, then stained with surface mAbs against CD3 and CD8 , fixed with FACS Lysing Solution(BD Bioscience, San Jose, USA), permeabilized with permeabilizing solution (BD Bioscience) and intracellularly stained with mAbs against IFN-γ, IL-2, TNF-α and IL-17 (all from BD Bioscience). Cells were then washed and fixed in 1% paraformaldehyde. At least 0.2 million events were acquired using a FACS Canto (BD Biosciences, San Jose, CA), for analysis using FACS Diva software (BD Biosciences).
To measure antigen-specific cytokine production, peripheral blood mononuclear cells (PBMCs) were isolated from heparinized fresh whole blood as described previously22. PBMCs were cultured in complete RPMI 1640 medium at a final concentration of 1×106/mL in the absence or presence of heat killed M. tuberculosis lysate (10 μg/ml) and soluble anti-CD28 antibody (0.1 mg/ml ,eBiscience, San Diego, USA). Cells were plated in a 24-well flat-bottom plate and incubated at 37°C in a 5% CO2 incubator. After 4 hrs culture, Brefeldin A (10 μg/ml; Sigma, The Netherlands) was added and the cell cultures were continued for an additional 12 hrs. Cell incubated with anti-CD28 alone served as a negative control. After 16 hrs culture, cells were stained with surface and intracellular cytokine antibodies for flow cytometric analysis as described above.
HRCT examination and scoring
HRCT were performed at 10 mm section interval (120 kV, 50–450 mAs), (1 mm slice thickness, 1.5 s scanning time) with a window level between −550 and 40 Hounsfield Units (HU) and window width between 300 and 1600 HU using the Toshiba Aquilion 64 CT Scanner (Toshiba, Tokyo, Japan). HRCT scans were analyzed two independent chest radiologists and final conclusions on the findings were reached by means of consensus. The arbitrary scores were based on the percentage of lung parenchyma abnormality as previously described14.
Data analysis
Statistical testing was performed using GraphPad Prism v4.0a software. For analysis of intracellular cytokine staining (ICS) assay data, differences between the three participant groups were first assessed using the Kruskal–Wallis test, followed by the Dunn's posttest. If those data were found to be significant (p < 0.05), pairwise comparisons were analyzed using the Mann–Whitney U test. The difference between smear-positive and smear-negative groups was compared by unpaired t-test. Correlations were assessed using Spearman's rank correlation.
References
Organization, W. H. Global Tuberculosis Control. World Health Organization, Geneva, Switzerland. (2010).
Cooper, A. M. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 27, 393–422 (2009).
Jouanguy, E. et al. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet 21, 370–8 (1999).
Darrah, P. A. et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13, 843–50 (2007).
Ciuffreda, D. et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol 38, 2665–77 (2008).
Kannanganat, S. et al. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J Virol 81, 12071–6 (2007).
Forbes, E. K. et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 181, 4955–64 (2008).
Kalsdorf, B. et al. HIV-1 infection impairs the bronchoalveolar T-cell response to mycobacteria. Am J Respir Crit Care Med 180, 1262–70 (2009).
Day, C. L. et al. Functional Capacity of Mycobacterium tuberculosis-Specific T Cell Responses in Humans Is Associated with Mycobacterial Load. J Immunol 187, 2222–32 (2011).
Caccamo, N. et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol 40, 2211–20 (2010).
Mattila, J. T., Diedrich, C. R., Lin, P. L., Phuah, J. & Flynn, J. L. Simian immunodeficiency virus-induced changes in T cell cytokine responses in cynomolgus macaques with latent Mycobacterium tuberculosis infection are associated with timing of reactivation. J Immunol 186, 3527–37 (2011).
Sutherland, J. S., Adetifa, I. M., Hill, P. C., Adegbola, R. A. & Ota, M. O. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol 39, 723–9 (2009).
Lee, J. J., Chong, P. Y., Lin, C. B., Hsu, A. H. & Lee, C. C. High resolution chest CT in patients with pulmonary tuberculosis: characteristic findings before and after antituberculous therapy. Eur J Radiol 67, 100–4 (2008).
Ors, F. et al. High-resolution CT findings in patients with pulmonary tuberculosis: correlation with the degree of smear positivity. J Thorac Imaging 22, 154–9 (2007).
Hesseling, A. C. et al. Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int J Tuberc Lung Dis 14, 560–70 (2010).
Gopi, P. G. et al. Association of conversion & cure with initial smear grading among new smear positive pulmonary tuberculosis patients treated with Category I regimen. Indian J Med Res 123, 807–14 (2006).
El-Sony, A., Enarson, D., Khamis, A., Baraka, O. & Bjune, G. Relation of grading of sputum smears with clinical features of tuberculosis patients in routine practice in Sudan. Int J Tuberc Lung Dis 6, 91–7 (2002).
Wozniak, T. M., Saunders, B. M., Ryan, A. A. & Britton, W. J. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect Immun 78, 4187–94 (2010).
Okamoto Yoshida, Y. et al. Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol 184, 4414–22 (2010).
Qiao, D. et al. ESAT-6- and CFP-10-specific Th1, Th22 and Th17 cells in tuberculous pleurisy may contribute to the local immune response against Mycobacterium tuberculosis infection. Scand J Immunol 73, 330–7 (2011).
Babu, S., Bhat, S. Q., Kumar, N. P., Kumaraswami, V. & Nutman, T. B. Regulatory T cells modulate Th17 responses in patients with positive tuberculin skin test results. J Infect Dis 201, 20–31 (2010).
Chen, X. et al. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med 181, 734–42 (2010).
Young, J. M., Adetifa, I. M., Ota, M. O. & Sutherland, J. S. Expanded polyfunctional T cell response to mycobacterial antigens in TB disease and contraction post-treatment. PLoS One 5, e11237 (2010).
Metenou, S. et al. Filarial infection suppresses malaria-specific multifunctional Th1 and Th17 responses in malaria and filarial coinfections. J Immunol 186, 4725–33 (2011).
Jambo, K. C. et al. Bronchoalveolar CD4+ T cell responses to respiratory antigens are impaired in HIV-infected adults. Thorax 66, 375–82 (2011).
Manca, F. et al. Limited clonal heterogeneity of antigen-specific T cells localizing in the pleural space during mycobacterial infection. Infect Immun 59, 503–13 (1991).
Dieli, F. et al. Sequestration of T lymphocytes to body fluids in tuberculosis: reversal of anergy following chemotherapy. J Infect Dis 180, 225–8 (1999).
Maini, M. K. et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 191, 1269–80 (2000).
Lyadova, I. V. et al. In mice, tuberculosis progression is associated with intensive inflammatory response and the accumulation of Gr-1 cells in the lungs. PLoS One 5, e10469 (2011).
Co, D. O. et al. Interactions between T cells responding to concurrent mycobacterial and influenza infections. J Immunol 177, 8456–65 (2006).
Volpe, E. et al. Multiparametric analysis of cytokine-driven human Th17 differentiation reveals a differential regulation of IL-17 and IL-22 production. Blood 114, 3610–4 (2009).
Zhu, J. & Paul, W. E. Heterogeneity and plasticity of T helper cells. Cell Res 20, 4–12 (2010).
Kryczek, I. et al. Phenotype, distribution, generation and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114, 1141–9 (2009).
Chen, X. et al. Diagnosis of active tuberculosis in China using an in-house gamma interferon enzyme-linked immunospot assay. Clin Vaccine Immunol 16, 879–84 (2009).
de Kantor, I. et al. Laboratory Services in Tuberculosis Control. World Health Organization, Geneva. (1998).
Acknowledgements
We thank Dr. Tao Chen, Dr. Mutong Fang, Dr. Qunyi Deng, Dr. Zhi Liu and Mrs. Meihong Gu for recruiting and following-up donors in this study. Financial support for this work is provided by Twelve -Fifth Mega-Scientific Project on “prevention and treatment of AIDS, viral hepatitis and other infectious diseases” (2008ZX10003-005), Natural Science Foundation of China Grant (30872258, 81172732, 81171535).
Author information
Authors and Affiliations
Contributions
ZQ, MZ, YZ, performed experiments; FZ and PL, evaluated HRCT score; HL and MG, wrote the manuscript; BZ and XC, designed the study and wrote the manuscript, are the guarantors of the entire manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Qiu, Z., Zhang, M., Zhu, Y. et al. Multifunctional CD4 T Cell Responses in Patients with Active Tuberculosis. Sci Rep 2, 216 (2012). https://doi.org/10.1038/srep00216
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00216
This article is cited by
-
Combined Detection of IFN-γ and Lymphocyte Subsets with Activation Indicators in the Clinical Application of Mycobacterium Tuberculosis Infection at Different Times
Current Microbiology (2023)
-
Multivariate profiling of African green monkey and rhesus macaque T lymphocytes
Scientific Reports (2019)
-
Th1 cytokines, true functional signatures for protective immunity against TB?
Cellular & Molecular Immunology (2018)
-
Host resistance to pulmonary Mycobacterium tuberculosis infection requires CD153 expression
Nature Microbiology (2018)
-
Comparison of immunogenicity and vaccine efficacy between heat-shock proteins, HSP70 and GrpE, in the DnaK operon of Mycobacterium tuberculosis
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.