Abstract
Visceral adiposity is a risk factor for cardiovascular disorders, type 2 diabetes mellitus (T2D) and associated metabolic diseases. Sub-cutaneous fat is believed to be intrinsically different from visceral fat. To understand molecular mechanisms involved in metabolic advantages of fat transplantation, we studied a rat model of diet-induced adiposity. Adipokine genes (Adiponectin, Leptin, Resistin and Visfatin) were expressed at 10,000 to a million-fold lower in visceral fat depot as compared to peripheral (thigh/chest) fat depots. Interestingly, autologous transplantation of visceral fat to subcutaneous sites resulted in increased gene transcript abundance in the grafts by 3 weeks post-transplantation, indicating the impact of local (residence) factors influencing epigenetic memory. We show here that active transcriptional state of adipokine genes is linked with glucose mediated recruitment of enzymes that regulate histone methylation. Adipose depots have “residence memory” and autologous transplantation of visceral fat to sub-cutaneous sites offers metabolic advantage.
Similar content being viewed by others
Introduction
Obesity is now considered as a global health disorder since it is associated with low grade inflammation, type 2 diabetes and adverse metabolic outcomes1. More than 1.5 billion adults are overweight worldwide of which five hundred million are estimated to be obese, largely due to consumption of high energy food containing saturated fats and sugars combined with reduced physical activity. However, apart from the amount of body fat, it is now well known that the distribution of fat is an important predictor of metabolic abnormalities2,3. The high prevalence of cardiovascular disease and type 2 diabetes in South Asians and Indians is associated with accumulation of visceral fat4. Adipose tissue is now recognized as an endocrine organ that secretes several inflammatory and immune mediators or adipokines, including leptin, visfatin, resistin and adiponectin5,6, which play an important role in outcome of metabolic disease. Subcutaneous adipose tissue (SAT) is proposed as a “metabolic sink” for circulating non-esterified fatty acids and by increased clearance of triacylglycerol from circulation7. Therefore low subcutaneous thigh fat is associated with higher glucose and lipid levels8. Conversely high visceral adiposity is independently associated with insulin resistance9,10, type 2 diabetes mellitus11,12,13, cardiovascular disorders14,15 and inflammatory disorders16,17 through dysregulation of adipokine secretion and free fatty acid toxicity. It is evident that visceral fat represents a physiologically important reservoir of adipose tissue that is functionally distinct from the subcutaneous fat depots. In fact, there are now several lines of evidence that converge to believe the beneficial effects of subcutaneous fat on metabolism. First, a recent study on obese women demonstrates that women with a healthy metabolic profile had significantly lower visceral fat volume and less insulin resistance than those with metabolic abnormalities18. These observations support the previous notion that subcutaneous fat depots have beneficial effects on insulin resistance19,20 in overweight or obese individuals. Secondly, these beneficial effects of subcutaneous fat depots are in line with the effects of pioglitazone treatment, which improves insulin sensitivity despite increasing total body fat mass; mainly subcutaneous fat21. The third line of evidence comes from studies in transgenic ob/ob mice with forced expression of adiponectin22. Here, authors observed that transgenic animals, expressing Adiponectin, had better metabolic profiles as compared to wild type ob/ob mice and this was associated with increase in adiposity, primarily due to increases in sub-cutaneous fat depots22,23. Lastly, transplantation of exogenous visceral fat to sub-cutaneous region24 or transplantation of exogenous sub-cutaneous fat to donor visceral region3 has shown beneficial effects in reversing the metabolic outcome by lowering insulin resistance in these mice. All of these studies confirm the intrinsic differences in visceral and peripheral adipose depots25. During post-natal life, fat cells are exposed to extrinsic factors such as hormones, growth factors and supply of nutrients, including high levels of local glucose concentrations in visceral/gut region26. Based on the idea that visceral fat has deleterious effects on metabolism, surgical removal of visceral fat was proposed to be a way forward in achieving lower visceral adiposity. However, removal of visceral fat alone does not provide metabolic advantages27,28, possibly because visceral fat is highly mobilized. Although fat transplantation involving donor and recipient mice has been carried out earlier3,24, we raised the question: Does autologous transplantation of visceral fat to sub-cutaneous region offer metabolic advantage?
In the current study, we investigated this question by carrying out autologous transplantation of visceral (intra-abdominal) fat to sub-cutaneous (thigh/chest) sites and examined the effects of this fat translocation on metabolic outcome at physiological and at molecular level. Surprisingly, we find that translocation of visceral fat to sub-cutaneous sites offers metabolic advantage, while mere removal of equivalent visceral fat did not provide this advantage. We present molecular analysis of epigenetic changes that occur after autologous fat transplantation (translocation) and demonstrate that glucose epigenetically regulates the expression of adipokines in visceral and peripheral fat to confer this advantage to fat depots in peripheral sites.
Results
Body composition and adipokine expression analyses
Since metabolic outcomes seen in human populations are difficult to be reproduced in genetic knockout models, we studied a colony of Wistar rats maintained on a high fat diet for 2 consecutive generations (Supplementary figure 1). Hyperinsulinemic euglycemic clamp studies demonstrate that F2 rats are insulin resistant as compared to age and sex matched F0 controls (Supplementary figure 1D). All studies discussed henceforth in this manuscript are carried on 8 month old F2 animals. We measured gene transcript levels of the adipokines; adiponectin (Adipoq), Leptin (Lep), Resistin (Retn) and Visfatin (Nampt) by using TaqMan-based real-time quantitative PCR (qPCR) in 8 different fat depots (Figure 1A). We observe significant differences in the abundance of gene transcripts for these 4 adipokines across all the fat depots (Figure 1B). Surprisingly, the expression of the 4 adipokines (Adipoq, Lep, Retn, Nampt) in the visceral fat was 10,000 to a million-fold lower than that observed in subcutaneous (thigh or chest) regions. This observation led us to plan fat translocation studies so as to assess the fate of autologous transplanted visceral fat in peripheral (subcutaneous) sites.
Adipokine gene expression in fat depots.
(A) Adipose tissue from different sites was snap frozen and collected for gene expression analysis using TaqMan-based quantitative real-time PCR. (B) Expression of Adipoq (Adiponectin), Lep (Leptin), Retn (Resistin) and Nampt (Visfatin) were significantly lower in visceral adipose tissue (red circles) as compared to adipose tissue obtained from other (peripheral) sites / depots. Gene expression for each animal is shown in the scatter plot (N = 9).
Autologous translocation of visceral fat to subcutaneous region
Around 40% of visceral fat was surgically removed (Supplementary figure 2) and 95% of this excised fat was transplanted sub-cutaneously, either in the thigh region (Supplementary figure 2E) or chest region (Supplementary figure 2G) of the same rat. No incidence of graft rejection was recorded in any of the autologous fat transplant surgeries carried out.
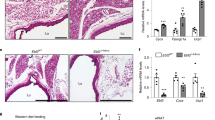
We then assessed the morphology of visceral and sub-cutaneous fat on the day of transplant (Supplementary figure 3A,B) as well as 4 or 21 days after transplantation (Supplementary figure 3C,D). We observe that adipose cells in the visceral fat depot are generally larger as compared to those in the thigh fat depot (Supplementary figure 3A,B). However, we observe that visceral fat showed changes in cell size and appear similar to cells in the thigh adipose tissue during the 21 days of translocation (Supplementary figure 3C,D). Next, we confirmed that the excised visceral fat grafts were vascularized as evidenced by presence of vasculature (Supplementary figure 3E,F) and analysis of CD31+ endothelial cells in visceral fat grafts recovered from thigh region (Supplementary figure 3G–J). We then assessed gene transcript abundance for Adiponectin, Leptin, Resistin and Visfatin (Figure 2) in visceral fat (day 0/day of transplant and day 21 post-transplant), thigh fat and visceral fat graft in the thigh (day 21 post-transplant). Interestingly, we observe that after translocation to sub-cutaneous sites, the expression profiles of the 4 adipokine genes in the graft was similar to the expression profile of residual fat in the thigh region. When visceral fat was translocated to the chest region, we also observe similar increases in gene expression profile (Supplementary figure 4). These data clearly indicate that the subcutaneous niche can impart “residence memory” to grafted visceral tissue leading to increase in the abundance of gene transcripts in the visceral fat grafted in thigh (VT) or chest (VC) regions (Figure 2).
Adipokine gene expression in Visceral fat depot matches expression levels in resident (thigh) depot following autologous transplantation.
Following 3 weeks after transplantation of visceral adipose tissue (V) to subcutaneous (thigh) region (T) the abundance of adipokine gene transcripts in the transplanted (VT) tissue was estimated by TaqMan-based quantitative PCR. We observe that gene transcript abundance in the grafts (VT) was significantly higher than that observed on the day of transplantation (Visceral;V) and not different from the abundance of gene transcript detected in the resident thigh fat.
Increase in gene expression correlates with relaxed chromatin conformation
Quantitative real time PCR is a very sensitive technique that measures the abundance of gene transcripts at a given time. However, this increase in adipokine gene transcripts seen in visceral grafts following VT or VC translocation could be either due to increase in rate of gene expression or decrease in rate of transcript degradation. It is well known that an increase in rate of gene expression is directly related to accessibility of the transcriptional machinery to gene promoter regions. We therefore carried out chromatin immunoprecipitation (ChIP) analysis using histone methylation-specific antibodies that identify active (H3K4) or inactive (H3K9) marks of lysine methylation (Figure 3). We observe that all the 4 adipokine gene promoter regions in the thigh and chest (resident) fat tissue show relatively higher H3K4 dimethylation (Figure 3A). Interestingly, it was observed that adipokine promoters in the grafted fat (VC or VT) acquired the active (H3K4me2) marks after translocation to these peripheral sites. Similar, albeit modest changes were measured in H3K9 methylation (Figure 3B) at all the 4 adipokine gene promoter regions.
Adipokine gene promoters in visceral fat acquire active marks (H3K4 methylation) following translocation of visceral fat to peripheral (thigh/chest) sites.
Chromatin immunoprecipitation (ChIP) followed by quantitative real-time PCR was carried out using promoter-specific probe-primers for each adipokine promoter region. Data are presented relative to methylation at promoter region of visceral fat (V) isolated at time of transplantation. We assessed H3K4 (A) and H3K9 (B) dimethylation as active and inactive marks of gene expression respectively. Following fat translocation to thigh (T) or chest (C) region, a significant increase in H3K4 methylation was observed for almost all the adipokine gene promoter regions.
Methylation of histone tails is regulated by 2 broad classes of enzymes; methyltransferases and histone demethylase29. LSD1 is a nuclear amine oxidase that uses oxygen as an electron acceptor to reduce methylated lysine to form lysine. LSD1 demethylates H3K4me2 and H3K4me1 leading to suppression of gene expression. SuV39h1 (also known as KMT1a) is a histone methyl transferase that is capable of methylating lysine 9 of histone 3 (H3K9) leading to chromatin compaction and transcriptional repression. Thus, recruitment of either, or both of these enzymes at gene promoter regions would indicate repression of gene expression. We carried out immunoprecipitation for LSD1 and KMT1a in visceral, thigh and chest fat depots/graft. We measured changes in recruitment of these enzymes to adipokine promoter regions. Interestingly, we observe that LSD1 and/or KMT1a were recruited at relatively much higher levels in visceral fat (Figure 4). Surprisingly, translocation to peripheral sites caused reduction in recruitment of LSD1 and/or KMT1a (Figure 4) at these adipokine promoters.
Changes in adipokine promoter methylation involve recruitment of LSD1 and KMT1a.
LSD1 and KMT1a recruitment at promoter regions is suggestive of gene repression (see text). Recruitment of LSD1 and KMT1a at adipokine gene promoter regions was assessed by carrying out chromatin immunoprecipitation for the 2 enzymes. Either LSD1 and/or KMT1a abundance at adipokine promoters was seen to decrease significantly following transplantation of visceral fat (V) to Thigh (VT) or chest (VC) region.
Translocation of fat from visceral to peripheral sites offers metabolic advantage
We then assessed the effects of autologous fat transplantation on metabolism. We observe that translocation of visceral fat to subcutaneous sites leads to increase in expression of the brown adipose tissue uncoupling protein (UCP) 130 as well as the mammalian analogs UCP2 and UCP3. This indicates improved thermogenesis/heat energy metabolism due to fat transplantation. Expression of RBP4 however did not change in the transplanted tissue at 1 month after the surgery (Supplementary figure 5).
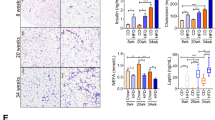
We measured circulating concentrations of adiponectin and leptin, which are known to play an important role in the outcome of metabolic disease. It is known that Adiponectin prevents lipopolysaccharide (LPS/endotoxin) induced liver injury and that obesity increases liver sensitivity to low doses of endotoxin, quickly developing steatohepatitis. Our data indicates that animals receiving subcutaneous transplant have improved levels of circulating adiponectin along with lower levels of serum endotoxin (Figure 5A,C). Improvement in metabolic state was suggestive from decline in circulating leptin concentrations after transplantation (Figure 5B).
Autologous transplantation of fat offers metabolic advantage.
To confirm any metabolic advantages offered as a result of autologous fat transplantation procedure, we measured circulating concentrations of (A) adiponectin, (B) leptin and (C) endotoxin in serum of rats before (day 0) or after (day 21) surgery. Hyperinsulinemic euglycemic clamps were carried out (D,E) to confirm that translocation of visceral fat to sub-cutaneous sites offered improved insulin sensitivity in these rats. P<0.001, N = 5.
We then measured sensitivity to insulin in these animals at 1 month after transplantation by using hyperinsulinemic-euglycemic clamps. Under steady state of euglycemia, glucose infusion rate (GIR) equals glucose uptake by all the tissues in the body and clamp studies are therefore considered as an ideal measure of insulin sensitivity. GIR was significantly different (p < 0.001) between pre-and post-transplant rats (Figure 5D,E). Post-transplant animals showed 2.5-fold increase in GIR confirming that animals receiving the subcutaneous fat transplant improved their glucose utilization during hyerinsulinemic-euglycemic clamp. (Figure 5D,E). Tolerance tests carried out (Supplementary figure 7) also confirm insulin sensitivity in these rats.
Glucose regulates epigenetic changes in adipose tissue
Our data demonstrates that adipokine gene transcription in visceral adipose tissue was epigenetically repressed (Figures 3, 4). It therefore became intriguing to identify extrinsic factors that lead to repression of gene expression. One of the major extrinsic factors known to be present at high concentrations in the gut region, as compared to peripheral sites, is glucose26. There is now increasing evidence to believe that this fundamental energy source can influence chromatin conformation31,32,33, gene expression34,35 as well as other important processes36 including beta cell regeneration/proliferation37.
We measured glucose concentrations at pre-hepatic and peripheral sites at 30 minutes after oral glucose load. Glucose concentrations in the peripheral region are significantly lower (p≤0.001) than those in the gut region, before the first pass through liver (Figure 6A,B). To assess effects of glucose on gene expression, we cultured visceral fat explants for 72 hours in media containing lower concentrations of glucose (1 mg/ml). We observe that exposure to lower glucose concentration induced 200 to 2000-fold increase in transcript abundance of adiponectin, leptin, resistin and visfatin genes (Figure 6C). Similarly, subcutaneous adipose tissue biopsy slices cultured in high glucose concentrations demonstrated a 400- to 1500-fold suppression in gene expression (Supplementary figure 6). These studies demonstrate that glucose alone can induce changes in gene expression, thereby mimicking the results obtained via subcutaneous transplantation/translocation. Although these observations confirm the mechanism of regulation of gene expression, we investigated if these effects of glucose conferred a “global” repression in gene expression. We carried out TaqMan low density arrays using microfluidics cards that were custom printed for selected genes involved in glucose-insulin metabolism. We observe that although adipokines (Figure 2, Suppl. figure 4) and other key genes involved in glucose-insulin metabolism were regulated to match the level of gene expression in resident tissue (Figure 7A), several other genes remained unaltered (Figure 7B). We identify these genes as glucose responsive (Figure 7A) or glucose non-respnsive (Figure 7B) genes and are presently carrying out further studies to identify the their biological processes, cellular components and molecular functions.
Glucose alone can induce increase in adipokine gene expression.
Blood glucose measurements were simultaneously carried out at 30 minutes after oral glucose was administered (A). We observe that local concentration of glucose in portal circulation are higher before first pass through liver, N = 6♂,7♀(B). Rats generally eat throughout the day/night and visceral adipose tissue is therefore exposed to high concentrations of glucose as compared to peripheral adipose tissue. To test the role of glucose, we harvested visceral fat from adult rats and exposed it to low glucose conditions for 72 hours (C). Following exposure to low glucose conditions, adiponectin, leptin, resistin and visfatin mRNA increased by at least 200-fold as compared to the abundance of these transcripts in the harvested (day 0) visceral tissue (N = 4 rats).
Analysis of glucose-regulated genes.
Taqman low density array (TLDA) analysis of gene expression in visceral (V) and transplanted fat (VT) in comparison to thigh fat (T) was carried out for several genes that are known to be involved in the metabolic syndrome. Two distinct groups of genes were observed; (A) glucose-responsive genes (N = 9; 14 genes): These were influenced as a result of transplantation to peripheral site, while other (B) glucose non-responsive or site independent genes (N = 9, 18 genes) did not show significant change in gene expression. (Adipor1: Adiponectin receptor 1, Adipor2: Adiponectin receptor 2, Hand1: Heart and neural crest derivatives expressed 1, Npy: neuropeptide Y, Ahcy: adenosylhomocysteinase, Dhfr: Dihydrofolate reductase, Sgpl1: sphingosine phosphate lyase 1, Irs1: Insulin receptor substrate 1, Faah: Fatty acid amide hydrolase, Cubn: Cubilin, Crp: C-reactive protein, Lpl: Lipoprotein lipase, Lrp2: Low density lipoprotein receptor-related protein 2, Prkaa2: protein kinase, AMP-activated, alpha 2 catalytic subunit, Mal2a: mal, T-cell differentiation protein 2, Mtr: 5-methyltetrahydrofolate-homocysteine methyltransferase, Nfkb: Nuclear factor kappa-B, Calca: Calcitonin related polypeptide alfa, Hmbs: hydroxymethylbilane synthase, Fto: Fat mass and obesity associated, Pparg: peroxisome proliferator-activated receptor gamma, Srebf1: sterol regulatory element binding transcription factor 1, Agrp: Agouti related protein homolog, Irs2: Insulin receptor substrate 2, Tcn2: transcobalamin 2, Rela: v-rel reticuloendotheliosis viral oncogene homolog A, FoxP1: Forkhead box P1, Prkag2: protein kinase, AMP-activated, gamma 2 non-catalytic subunit, Cnr1: cannabinoid receptor 1 (brain), Fabp4: fatty acid binding protein 4, adipocyte, Atp2a1: Ca++ transporting, cardiac muscle, fast twitch 1, Bmpr2: Bone morphogenetic protein receptor 2)
Discussion
Distribution of body fat is considered as an important predictor of metabolic abnormalities in obese individuals. Adipose tissue is now considered as an endocrine organ that secretes several inflammatory and immune mediators including adiponectin, leptin, resistin and visfatin. Subcutaneous thigh fat is inversely associated with glucose and lipid concentrations, while increased visceral adiposity is independently associated with insulin resistance, T2D, cardiovascular disorders, inflammatory disorders and certain forms of cancers through dysregulation of adipokine secretion. The site-specific differences in visceral and peripheral fat depots is therefore important in understanding the role of visceral adiposity as a causal factor for metabolic disease. Leptin, an adipose tissue derived protein, is known to be important in regulating energy intake, energy expenditure, appetite and metabolism. Circulating concentrations of leptin are proportional to body fat. Adiponectin is a 24 kDa polypeptide, which mediates important metabolic processes such as glucose regulation and fatty acid catabolism. Exclusively secreted from the adipose tissue into the blood stream, its serum levels are inversely correlated with body fat percentage in adults.
The aim of this investigation was to assess the fate of surgically transplanted visceral fat following autologous transplantation. We demonstrate that gene expression profiles of visceral adipose tissue are influenced following translocation to peripheral sites. These changes in gene expression are retained even at several weeks after transplantation and involve modifications in chromatin conformation. It is increasingly appreciated that methyl groups at the histone tails can be modified as a result of demethylation. Indeed, until recently, it was believed that methyl groups could not be removed from histones. However, recent studies characterize lysine-specific demethylase 1 (LSD1) and Jumonji C (JmjC) as H3 demethylases38,39,40. LSD1 is a nuclear amine oxidase that uses oxygen as an electron acceptor to reduce methylated lysine to lysine41,42. LSD1 demethylates H3K4me2 and H3K4me1 and is consistent with its role in removal of the active methylation marks. LSD1 is found in co-repressor complexes and promotes suppression of gene expression41,42. KMT1A (SuV39h1) has a conserved Set [Su(var)3–9, Enhancer-of zeste, Trithorax] catalytic domain that belongs to the histone methyl-transferase family and is capable of methylating lysine 9 of histone H3 (H3K9) to mediate transcriptional repression. Our data demonstrates that differences in adipokine gene expression observed amongst all fat depots are epigenetically regulated via recruitment of LSD1 and KMT1a at adipokine promoter regions. We also demonstrate that recruitment of enzymes involved in histone methylation is mediated via glucose and that these effects are reversible as in vitro exposure to low glucose concentrations reverses the repression in gene expression (Figure 6). Although we have not assessed DNA methylation in this study, other studies suggest positive correlation between glucose concentrations and DNA methylation34,35.
Brown adipose tissue (BAT) is also known to play an active role in thermogenesis. BAT employs the uncoupling protein (UCP), which is a membranous mitochondrial ion carrier30. UCP, (also called “thermogenin”) mediates the passive re-entry of protons in mitochondrial matrix, producing heat and a concomitant decrease in the ATP yield of oxidative phosphorylation. As a consequence, oxidative metabolism in the BAT proceeds at a maximum rate, enabling its mitochondria to produce more heat. Norepinephrine, released from sympathetic nerves and acting via beta-adrenoceptors and cAMP, is the main positive regulator of both UCP synthesis and activity. BAT thermogenesis is thus known to play a critical role in thermoregulation and in overall energy balance. Although we have not directly measured heat production / oxygen consumption in these animals post-translocation, our present findings direct us to focus future studies on addressing these questions. Our findings using in vivo translocation as well as in vitro fat explants represent a paradigm shift in understanding the relationship between epigenetic changes and hyperglycemic memory in the context of metabolic disease. It becomes interesting to study the epigenetic effects of elevated glucose concentrations on chromatin conformation, transcription regulation and gene expression. In this regard, we are involved in assessing clinical samples from 2 major studies concerning individuals that progressed to type 1 diabetes (INIT-II) and those who progress to insulin-requiring type 2 diabetes in the FIELD Study cohort. Future studies involving gene ontology analysis will help in understanding the role of hyperglycemia on epigenetic suppression of gene expression with reference to metabolic disease in human populations. Although further studies need to be carried out to understand the complex networks of glucose-regulated and -independent genes, the present study demonstrates for the first time the role of “residence factors”, specifically glucose, in epigenetic regulation of adipokine gene promoters to offer a metabolic benefit following autologous fat transplantation.
Methods
Animals and surgical procedures
Wistar rats (Supplementary figure 1) were taken for autologous fat transplantation studies as per the institutional approved ethics protocol # EAF2007/B-100. Surgeries were carried out under isofluorane anesthesia in a sterile operating theater fitted with HEPA filters. In pilot studies, visceral adipose tissue was estimated at ∼ 8 grams in each animal. Around 40% of visceral fat was removed and 95% of this excised fat was implanted subcutaneously in the thigh or in the chest region. Remaining 5% of the excised fat was frozen for analysis. Animals were euthanized on day 4, day 21 or 1 month after surgery (n = 8/time point) and transplanted adipose tissues and residual fat depots were retrieved. All surgical and animal procedures are carried out as per the institutional guidelines for use of laboratory animals and as approved by the Ethics Committee of NCCS. Animals were analyzed for assessment of abdominal fat by MRI scan (Siemens 1.5 Tesla) before and after the surgery. Whole body fat DXA measurements are made using dual energy X-ray absorptiometry (pDEXA® Sabre ORTHOMETRIX, INC.).
Histology and immunochemistry
The residual fat tissue samples and grafted visceral fat was analyzed by conventional histochemical analysis. Tissues were fixed in 10% formalin, embedded in paraffin and sectioned for hematoxilin and eosin (H&E) staining. Representative sections from the same tissue blocks were immunostained for anti-rat CD31 antibody (AbCam, USA) and nuclei (visualized by Hoechst 33342) before observation using LSM 510 Meta confocal microscope (Carl Zeiss GmbH, Munich).
Assays
TaqMan assays
Adipose tissue samples were snap frozen upon removal using and stored at −80°C until further processing. All samples were thawed on ice and homogenized using conventional battery operated handheld homogenizer. Tissue lysates were solubilized in 1 ml of Tri reagent (Sigma, St. Louis, MO) and re-suspended to ensure that all tissue lysates are solubilized. An oil layer (upper phase) observed at this time was removed and samples were then processed as per the manufacturer's instructions. Total RNA was quantified on ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) and reverse transcribed to obtain cDNA using ‘High Capacity cDNA Reverse Transcription Kit’ (Applied Biosystems, Foster City, CA). Ten nanograms equivalent of cDNA was taken for gene transcript analysis using validated Assay-on-demand TaqMan PCR probe-primers (Life Technologies, Carlsbad, CA). Fold-changes were calculated after normalization for 18S rRNA using 2−ΔΔ Ct method as described earlier43. Gene transcript analysis was then carried out using 7500 FAST or 7900 HT real-time PCR system (Life Technologies, Carlsbad, CA). For simultaneous assessment of an array of genes using TaqMan chemistry, we custom printed microfluidics cards (TLDAs; TaqMan Low Density Arrays) using an inventoried set of gene expression assays from Applied Biosystems (Life Technologies, Carlsbad, CA).
Chromatin Immunoprecipitaion (ChIP) assays
Residual adipose tissues and/or grafts were taken for ChIP using a slight modification of existing protocols44,45. Briefly, adipose tissue was finely chopped, cross-linked using 1% formaldehyde (Sigma, St. Louis, MO) and sonicated to generate 300 bp to 1.2 kb DNA fragments. Quality and quantity of chromatin was assessed and immunoprecipitation using 2 μg of specific dimethyl antibodies for H3K4 and H3K9 (Millipore, Billerica, MA) was carried out. Precipitation cocktail include protein A/G plus beads (Pierce, Pittsburgh, PA), sonicated salmon sperm DNA (Amersham Biosciences Pittsburgh, PA) and BSA (Sigma, St. Louis, MO). Rabbit IgG (Upstate, Millipore, Billerica, MA) is used as isotype control. Chromatin was eluted using 2%SDS, 0.1 M NaHCO3 and 10 mM DTT. Cross-links are reversed by incubating the eluted chromatin in 4 M NaCl for 4 hours at 65°C. This is followed by proteinase-K digestion. DNA is then extracted using phenol- chloroform- isoamyl alcohol followed by precipitation and two washes of 70% ethanol and finally dissolved in nuclease-free water. The online Ensembl database was used (http://www.ensembl.org) to select 1 kb region upstream of 1st exon for designing promoter specific primers and using online primer designing tool (http://www.idtdna.com/Scitools/Applications/Primerquest/) (Supplementary table 1). These primers were used to carry out real time PCR on input, immunoprecipitated and isotype control DNA. All real time PCR results were normalized to GAPDH promoter (Active/positive control)/intergenic region and carried out in duplicate and presented as fold-chage as compared to Gapdh promoter.
Hyperinsulinemic-euglycemic clamps and tolerance tests
All clamp studies were carried out as described earlier46,47. Briefly, rats are fasted for 10 hours and catheterized after being anesthetized with isoflurane. The left common carotid artery and the right jugular vein are catheterized for sampling. The free catheter end from carotid artery is used to collect 50 µl blood per 10 min. The free catheter end from the jugular vein is used to infuse insulin and glucose. The insulin clamp begins at 0 min with a primed-continuous infusion of human insulin 300 mU/kg bolus followed by 4 mU/kg/min. Normoglycemia (80–110 mg/dl) is maintained during clamps by measuring blood glucose every 5–10 min starting at 0 min and infusing 10% dextrose as necessary. Blood samples were taken every 10 min for 120 min and processed to determine insulin. For insulin tolerance test, rats were fasted overnight and injected intraperitoneally with 1 unit/kg (Actrapid; Novo Nordisk, Denmark). Glucose concentrations were measured before insulin injections and at every 30 minutes for 2 hours. For glucose tolerance test, overnight fasted rats were injected with 2 g/kg glucose (intraperitoneally) and glucose concentrations were measured before and at every 30 minutes after glucose injection for next 2 hours.
Blood Sampling and Chromogenic LAL (Limulus Amoebocyte Lysate) assay
Fasting blood samples were collected in sterile tubes by retro-orbital bleeding under isofluorane anaesthesia. The LAL assay (Lonza Biosciences-QCL 1000 kit) is used for detection of gram negative bacterial endotoxin with a sensitivity range between 0.1 EU/ml to 1.0 EU/ml. All samples were collected in double autoclaved nuclease-free 1.7 ml tubes to avoid microbiological or endotoxin contamination.
Serum leptin and adiponectin assay
Serum samples were processed for assessing leptin and adiponectin using ELISAs (Millipore Corporation, Billerica, MA) as per the manufacturer's protocol.
In vitro culture of adipose tissue explants
Visceral adipose tissue explants were prepared by slicing visceral adipose tissue with a sterile surgical scalpel blade. Slices were usually around 0.5 to 1 mm in thickness. Explants were washed twice with fresh DME/HF12 media before culture in tissue-culture treated 24-well plates and processed for RNA isolation and cDNA preparation at 72 hours of exposure to low glucose conditions. Explants that were cultured in high glucose concentrations (5 mg/ml) were taken for comparison. Osmolarity was maintained using non-metabolizable glucose supplementation and was identical in both media.
Blood glucose measurements
Animals were placed under isofluorane anesthesia and canulated to assess pre-portal and peripheral circulation. Glucose load of 2 g/Kg body weight was administered orally and blood was collected in plasma-EDTA tubes at 30 minutes after glucose load.
References
Alvehus, M. B. J., Sjöström, M., Goedecke, J., Olsson, T. The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 18, 879–883. (2010).
Santosa, S. & Jensen, M. D. Why are we shaped differently and why does it matter? Am J Physiol Endocrinol Metab 295, E531–535 (2008).
Tran, T. T., Yamamoto, Y., Gesta, S. & Kahn, C. R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab 7, 410–420 (2008).
Banerji, M. A., Faridi, N., Atluri, R., Chaiken, R. L. & Lebovitz, H. E. Body composition, visceral fat, leptin and insulin resistance in Asian Indian men. J Clin Endocrinol Metab 84, 137-144 (1999).
Hutley, L. & Prins, J. B. Fat as an endocrine organ: relationship to the metabolic syndrome. Am J Med Sci 330, 280–289 (2005).
Scherer, P. E. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55, 1537–1545 (2006).
Frayn, K. N. Adipose tissue as a buffer for daily lipid flux. Diabetologia 45, 1201–1210 (2002).
Snijder, M. B. et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 48, 301–308 (2005).
Umegaki, H., Haimoto, H., Ishikawa, J. & Kario, K. Visceral fat contribution of insulin resistance in elderly people. J Am Geriatr Soc 56, 1373–1375 (2008).
Cefalu, W. T. et al. Contribution of visceral fat mass to the insulin resistance of aging. Metabolism 44, 954–959 (1995).
Anan, F. et al. Visceral fat accumulation is a significant risk factor for white matter lesions in Japanese type 2 diabetic patients. Eur J Clin Invest 39, 368–374 (2009).
Anjana, M. et al. Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care 27, 2948–2953 (2004).
Gastaldelli, A. et al. Metabolic effects of visceral fat accumulation in type 2 diabetes. J Clin Endocrinol Metab 87, 5098–5103 (2002).
Matsuzawa, Y., Nakamura, T., Shimomura, I. & Kotani, K. Visceral fat accumulation and cardiovascular disease. Obes Res 3, 645S–647S (1995).
Mahabadi, A. A. et al. Association of pericardial fat, intrathoracic fat and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 30, 850–856 (2009).
Fontana, L., Eagon, J. C., Trujillo, M. E., Scherer, P. E. & Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 56, 1010–1013 (2007).
Zhang, H., Chen, X., Aravindakshan, J. & Sairam, M. R. Changes in adiponectin and inflammatory genes in response to hormonal imbalances in female mice and exacerbation of depot selective visceral adiposity by high-fat diet: implications for insulin resistance. Endocrinology 148, 5667–5679 (2007).
Hayes, L. et al. Do obese but metabolically normal women differ in intra-abdominal fat and physical activity levels from those with the expected metabolic abnormalities? A cross-sectional study. BMC Public Health 10, 723.
Misra, A. et al. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res 5, 93–99 (1997).
Snijder, M. B. et al. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes Res 11, 104–111 (2003).
Miyazaki, Y. et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 87, 2784–2791 (2002).
Kim, J. Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117, 2621–2637 (2007).
Kim, H. J. et al. Depot-specific regulation of perilipin by rosiglitazone in a diabetic animal model. Metabolism 56, 676–685 (2007).
Gavrilova, O. et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 105, 271–278 (2000).
Gesta, S. et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A 103, 6676–6681 (2006).
Abumrad, N. N., Cherrington, A. D., Williams, P. E., Lacy, W. W. & Rabin, D. Absorption and disposition of a glucose load in the conscious dog. Am J Physiol 242, E398–406 (1982).
Fabbrini, E. et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 139, 448–455.
Montoya, T., Monereo, S., Olivar, J., Iglesias, P. & Diaz, P. Effects of orlistat on visceral fat after liposuction. Dermatol Surg 35, 469–474 (2009).
Culhane, J. C. & Cole, P. A. LSD1 and the chemistry of histone demethylation. Curr Opin Chem Biol 11, 561–568 (2007).
Tran, T. T. & Kahn, C. R. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol 6, 195–213.
Tonna, S., El-Osta, A., Cooper, M. E. & Tikellis, C. Metabolic memory and diabetic nephropathy: potential role for epigenetic mechanisms. Nat Rev Nephrol 6, 332–341.
Brasacchio, D. et al. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes 58, 1229–1236 (2009).
El-Osta, A. et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205, 2409–2417 (2008).
Bouchard, L. et al. Leptin gene epigenetic adaptation to impaired glucose metabolism during pregnancy. Diabetes Care 33, 2436–2441.
Yang, B. T. et al. Insulin promoter DNA methylation correlates negatively with insulin gene expression and positively with HbA(1c) levels in human pancreatic islets. Diabetologia 54, 360–367.
Ferrer, J. Glucose as a mitogenic hormone. Cell Metab 13, 357–358.
Porat, S. et al. Control of Pancreatic beta Cell Regeneration by Glucose Metabolism. Cell Metab 13, 440–449.
Metzger, E. et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437, 436–439 (2005).
Tsukada, Y. et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature 439, 811–816 (2006).
Wissmann, M. et al. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol 9, 347–353 (2007).
Shi, Y. J. et al. Regulation of LSD1 histone demethylase activity by its associated factors. Mol Cell 19, 857–864 (2005).
Shi, Y. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 (2004).
Joglekar, M. V., Wei, C. & Hardikar, A. A. Quantitative estimation of multiple miRNAs and mRNAs from a single cell. Cold Spring Harb Protoc 2010, pdb prot5478.
Hauser, C., Schuettengruber, B., Bartl, S., Lagger, G. & Seiser, C. Activation of the mouse histone deacetylase 1 gene by cooperative histone phosphorylation and acetylation. Mol Cell Biol 22, 7820–7830 (2002).
Xu, C. R. et al. Chromatin "prepattern" and histone modifiers in a fate choice for liver and pancreas. Science 332, 963–966.
Ayala, J. E., Bracy, D. P., McGuinness, O. P. & Wasserman, D. H. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55, 390–397 (2006).
Ayala, J. E. et al. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3, 525–534.
Acknowledgements
Authors are thankful to Dr. G.C.Mishra, Director, NCCS and Dr. Sandhya Sitaswad, Scientist NCCS, for providing relevant facilities, Dr Ramanmurthy and Dr. Kishori Apte, for providing support related to the animal studies, Dr. Avinash Pradhan, Pathology Unit, K.E.M, Pune for assistance in adipose tissue sectioning and Prof. Sanjeev Galande, IISER, Pune for providing LSD1 antibody. AAH acknowledges the Victorian State Government's Department of Innovation, Industry and Regional Development's Operational Infastructure Support Program. AAH specifically acknowledges the suggestions and invaluable advice provided by Prof. John Prins, Professor of Endocrinology, University of Queensland, during interactions of work presented in this manuscript at a meeting in Hyderabad, India. ASP was supported through a fellowship from Lady Tata Memorial Trust, Mumbai, Inda. MDW is supported by Australian Post-graduate Award, The University of Melbourne, Australia. Authors acknowledge Dr. MV Joglekar, St. Vincent's Institute, Australia for comments and assistance in writing and finalizing this manuscript.
Author information
Authors and Affiliations
Contributions
AAH identified the research question and planned the study. SNS carried out most experiments and wrote the first draft. ASP and SK carried clamp studies and DXA measurements, AR and MG carried out MRI. SNS, MDW and AAH carried out in vitro assessments. RRB, MSK, YS, MP and CSY contributed to the development of this model of insulin resistant rats by providing scientific inputs, suggestions as well as infrastructural resources. This research was supported by an intramural project grant to AAH (NCCS/DBT2007-TJ201), from the National Center for Cell Science, Department of Biotechnology, Government of India and partly by Barbara Walker Fellowship to AAH.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary figures and table
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Satoor, S., Puranik, A., Kumar, S. et al. Location, location, location: Beneficial effects of autologous fat transplantation. Sci Rep 1, 81 (2011). https://doi.org/10.1038/srep00081
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00081
This article is cited by
-
Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat
Nature Communications (2018)
-
Muscle-specific downregulation of GR levels inhibits adipogenesis in porcine intramuscular adipocyte tissue
Scientific Reports (2017)
-
Diet-induced variability of the resistin gene (Retn) transcript level and methylation profile in rats
BMC Genetics (2015)
-
Gene expression profiling reveals distinct features of various porcine adipose tissues
Lipids in Health and Disease (2013)
-
An atlas of DNA methylomes in porcine adipose and muscle tissues
Nature Communications (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.