Abstract
We compare the prey capture glues produced by orb-weaving spiders (viscid glue) and their evolutionary descendents, the cobweb-weaving spiders (gumfoot glue). These glues are produced in homologous glands but exhibit contrasting structure, properties and response to changing humidity. Individual glue droplet stretching measurements indicate that the gumfoot glue behaves like a viscoelastic liquid in contrast to the viscid glue, which behaves like a viscoelastic solid. Moreover, the gumfoot glue is largely humidity-resistant – elasticity and adhesion are constant across variation in humidity and there is weak volume-dependence. Viscid glue, however, is highly humidity-sensitive. The glue expands an order of magnitude and demonstrates a monotonous reduction in elasticity under increased humidity, while glue adhesion optimizes at intermediate levels of humidity. We suggest that observed differences are due to different ‘tackifiers’ used in these systems. These results shall inspire future efforts in fabricating stimuli-resistant and stimuli-sensitive materials.
Similar content being viewed by others
Introduction
Smart materials and devices that can change dimension, properties and function in response to external stimuli are a current focus of research in both materials and biological sciences. On the other hand, materials that resist particular stimuli are also actively pursued for their own unique applications. Nature contains a myriad of biomaterials that respond differently to external stimuli, ranging across both extremes and that are often the source of inspiration for developing next-generation materials. The opening and closing of pine cones1, the rapid and reversible stiffening of connective tissue in echinoderms2, the coiling and uncoiling of wheat awns3 and the reversible color change in the feathers of tree swallows4 are just a few examples of functionally responsive biomaterials, while self-cleaning lotus leaves5, water-repelling Australian sands6 and hydrophobic water-strider legs7 are examples of biomaterials whose function depends upon a lack of responsiveness to key stimuli. These materials and phenomena are just a few examples of the critical role that responsiveness to external stimuli per se plays in the functional adaptation of biological systems. However, evolution itself provides a powerful tool to move biomimetic research beyond simply exploiting individual materials in nature toward understanding the key elements that control environmental responsiveness of biomaterials.
Spider major ampullate (dragline) silk dramatically increases softness and extensibility under high humidity8. Relatively less is understood about the humidity-responsiveness of spider prey capture glues. Both orb web and cobweb spiders use adhesive silk threads to capture prey that are coated with glue from evolutionarily homologous aggregate glands. However, cobweb spiders evolved from an ancient orb web ancestor in the early Cretaceous9. The two lineages of spiders now employ silk glues in completely different webs with very different roles to play in capturing prey, providing an “evolutionary experiment” for investigating changes in the properties and humidity-responses of biological glues during transitions in ecological function. The viscid capture spirals spun by orb web spiders are intricate composites of a core pair of viscoelastic flagelliform axial silk fibers covered by micron-size glue droplets10. In contrast, the adhesive capture threads spun by cobweb spiders, gumfoot silk, consist of much larger glue droplets covering two pairs of stiffer major ampullate silk fibers11. Viscid silk glue is a complex assembly of glycoproteins12 that behave like viscoelastic solids13 and an aqueous solution of low molecular weight hygroscopic salts that regulate water content in the drop14 and keep the glycoproteins soft and tacky to maintain the stickiness in variable humidity environments15. Viscid silk functions primarily to retain insects, while the web as a whole dissipates their flight energy16. Although the mechanical behaviour of gumfoot silk glue remains unknown, the major ampullate dragline silk upon which the glue is laid is orders of magnitude stiffer than orb web spider flagelliform silk11 and the glue itself contains two novel peptides with metal-binding properties17. Gumfoot threads also target walking, rather than flying, insects18. Individual gumfoot threads act as spring-loaded traps, with the tension in the cobweb literally pulling small pedestrian insects off of the ground19.
Here, by probing individual glue droplets we show that the gumfoot silk glue droplets behave like viscoelastic liquids in contrast to the viscoelastic solid behavior of viscid silk glue droplets. The viscid silk glue droplets exhibit maximum stickiness at intermediate humidity (40% – 60% R.H). At low R.H., the droplets are very stiff and dense, fail to establish proper contact and hence adhere less. At higher R.H., lubrication caused by water and low elasticity reduces the adhesion, even though the droplets are softer and spread much better on the surface. Excess water disrupts hydrogen bonding, reduces electrostatic interactions (glycoproteins are negatively charged) and over lubricates, all reducing adhesion. On the other hand, the adhesion of gumfoot silk glue droplets is humidity-resistant. The behaviors of these two glues are in stark contrast to other bioadhesives, such as the monotonous increase in adhesion with humidity of gecko toes20 and of the cribellar silk21 produced by orb-weaving Uloboridae spiders. The evolutionary transition in humidity responsiveness of spider silk glue likely reflects functional adaptations to the silks' new and divergent roles in the webs spun by the orb web spiders and their evolutionary descendents, the cobweb spiders.
Results
Differences in the structure and the humidity-responses
Viscid silk glue and gumfoot silk glue differ in structures and properties. Viscid silk glue droplets are heterogeneous with a dense polymeric core surrounded by a sparse, translucent mixture of glycoproteins and an aqueous solution of salts (Figure 1a). In contrast, gumfoot silk glue droplets appear largely homogeneous with no visible dense core (Figure 1b). These glues also respond very differently to humidity. Viscid silk glue droplets swell by close to an order of magnitude compared to their desiccated volumes (Figure 2), while gumfoot silk glue droplets instead coalesce together to form bigger droplets such that the total increase in volume is much less than in viscid silk glue (Figures 1c, 1d & 2). The ‘flow’ and coalescing of gumfoot silk droplets is probably due to the absence of a dense central core, which is hypothesized to act as an anchor for the viscid silk glue droplets thereby keeping them firmly attached to the axial silk fibers22. The absence of the core likely explains why glue droplets can be easily removed from the gumfoot silk by adhering onto an adhesive surface, unlike viscid silk glue which is firmly attached to the axial silk fibers and hence returns to the viscid silk after exhibiting the ‘suspension bridge’ mechanism22. The glycoproteins in viscid silk glue behave like a crosslinked network and exhibit viscoelastic solid-like behavior13. While, gumfoot silk glue contains water-soluble adhesive peptides17 and GABamide23; the presence or absence of high-molecular weight branched adhesive polymers (proteins) is not known yet.
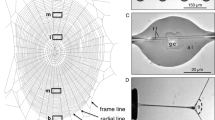
Gumfoot silk glue vs. viscid silk glue (a) and (b) show individual viscid silk thread and gumfoot silk thread spun by Larinioides cornutus and Latrodectus hesperus , respectively.
Capture threads were laid on clean cover slips for both the cases. The difference in the wetting kinetics of the coating peptides and the high-molecular-weight adhesive polymers (probably glycoproteins) gives the appearance of a ‘diffuse core’ in the gumfoot silk glue droplets. The glue droplets homogenize with time which disperses the core. Also, this core is not observed in pictures of suspended gumfoot silk threads. Scale bar is 20 µm for both the cases. (c) and (d) show a gumfoot silk thread at 0% R.H. and 90% R.H., respectively. It was observed that when a gumfoot silk thread is humidified, the glue droplets flow and coalesce to form bigger droplets.
Water uptake of the glues.
Change in volume of the viscid silk glue (squares) and gumfoot silk glue (circles) as the silk threads are exposed to a high-humidity environment. Insets a and b show gumfoot silk glue while c and d show viscid silk glue at 0% R.H. and 100% R.H., respectively. Similar to figure 1c and d, inset b shows fewer but bigger glue drops than inset a. Scale bar is 100 µm for all the figures. The uptake of water in viscid silk glue drops is due to the presence of low molecular weight hygroscopic compounds present in the glue14. It was experimentally determined that there is no hysteresis in water uptake with humidity cycling (data not shown). In the case of the gumfoot silk glue, however, the order of changing humidity plays a role. While going up in humidity for the first time, the glue drops on gumfoot silk coalesce to form bigger drops and a slight change in total glue volume is observed (circles). Reducing the humidity subsequently restores the original glue volume but obviously not the original number of glue drops. Subsequent humidity cycles are completely reversible in terms of both glue volume and number of glue drops.
Dependence of adhesion on humidity
Capture-thread glue drops swell (to different extents depending on which glue) when exposed to high humidity (Figure 2). This absorbed water dilutes the glue drops, thus improving their wettability. The effect of humidity on adhesion of these glues is investigated by equilibrating threads at different levels of humidity before performing the individual glue droplet measurements. Capture thread, equilibrated at the desired humidity, is immobilized on a glass substrate and a conical glass probe of 10 µm base diameter is brought into contact with its glue droplets. The whole assembly is observed through an optical microscope and is enclosed in a humidity-controlled chamber. The probe is then retracted at constant speeds while the force is recorded as a function of distance (Figure 3). To account for the change in modulus (softness) with humidity and to objectively compare the two glues, the normal pre-force for bringing the probe in contact with an individual drop was kept constant for every value of humidity for both glues. After reaching the critical ‘pull-off’ force, the tip releases contact. The critical pull-off forces depend on the rate at which the droplets are stretched. The force-displacement behavior for individual drops during their stretching is shown in Figure 3.
Effect of humidity on the stretching behavior of the glues.
Force-displacement behavior when glue drops of viscid silk (gumfoot silk), equilibrated at 15% R.H. a(b), 40% R.H. c (d) and 90 % R.H. e (f), were stretched at 1 µm/s (inverted triangles), 10 µm/s(upright triangles), 50 µm/s(squares) and 100 µm/s (circles).
The effect of humidity on the adhesive behavior of these glue droplets can be understood by comparing the load-displacement behavior at the same stretching rate (50µm/s) at different values of R.H. (Figures 4a and b). For the viscid silk, the glue drops become softer with increasing R.H. (the initial elastic modulus decreases with increase in R.H.). Because the adhesion between the probe and the glue droplets is used to stretch the droplet, the extension of the glue droplets at break is also dependent on humidity. In contrast, humidity does not have any significant effect on the adhesion of gumfoot silk glue (Figure 4b). The pull-off forces for gumfoot silk glue depend only on the rate of stretching and are independent of the surrounding humidity. Also, despite the larger sizes of the gumfoot silk glue droplets, their extension-to-break values are much lower than that of the viscid silk glue droplets. This might be due to gumfoot silk glue's reduced uptake of water compared to viscid silk glue. When the glue ‘pull-off’ forces for both silks were compared with the capillary effect-induced forces of two model viscous liquids (unentangled PDMS, γ = 20mN/m and a solution similar to the aqueous coating of the viscid silk glue, γ = 40mN/m, respectively), both silk glue forces were two orders of magnitude larger than either liquid, even at 90% R.H stretched at 1 µm/sec (Figure 4c). Furthermore, the glue pull-off forces depend on the rate of stretching while the liquid viscous forces are rate-independent. This implies that gumfoot silk glue exhibits viscoelasticity, which, like viscid silk glue, indicates the presence of physical or chemical crosslinks likely caused by high-molecular-weight adhesive polymers. Also, rate-dependent pull-off forces demonstrate that the viscid silk glue does not lose its viscoelastic character even when diluted and swollen by up to an order of magnitude (i.e. at 90% R.H.).
Comparison between viscid silk glue and gumfoot silk glue.
(a) and (b) Force-displacement behavior when individual glue drops of viscid silk and gumfoot silk, equilibrated at 15% R.H. (circles), 40% R.H. (squares) and 90 % R.H. (upright triangles), are stretched at 50 µm/s, respectively (data from Figure 2). (c) Comparison of the pull-off force obtained from Figure 4a and b with the capillary forces exerted by unentangled PDMS (γ ∼ 20 mN/m) and an aqueous solution of composition similar to the viscous coat used by modern orb-weaving spiders to coat their capture threads (γ ∼ 40 mN/m). VSS glue denotes viscid spiral silk glue whereas GFS glue denotes gumfoot silk glue. GFS glue is represented by box and whiskers outlined by red (15% R.H.), blue (40% R.H.) and green (90% R.H.), whereas, for VSS glue, boxes and whiskers are outlined with black and boxes are filled with the color. PDMS is represented by box filled with purple whereas aqueous solution is represented by box and whiskers outlined with purple. d) Comparison of energy of adhesion between viscid silk glue, gumfoot silk glue and the Uglue values obtained using the energy model (supplementary information). Values are obtained by multiplying the area under the force-displacement curve obtained from individual glue drop stretching measurements by 42 (number of glue drops in contact with a 2 mm glass substrate used for the peeling experiments). Even though gumfoot silk does not have 42 droplets per 2 mm length and thread peeling measurements were not performed with it, values plotted are obtained by multiplying the area under the force-displacement curve by 42, to compare it with viscid silk glue and the Uglue values obtained using the energy model. Values are plotted as box and whiskers from 5 measurements each. VSS glue is represented by box and whiskers outlined with black and filled with red (1µm/s), blue (10µm/s), green (50 µm/s) and purple (100 µm/s). GFS glue, depending on the rate of stretching, is outlined by one of the above colors. Uglue values are represented by blank boxes outlined with black.
The area under the load-displacement curve represents the energy required to separate the tip from the glue (referred to as adhesive energy). Figure 4d compares the adhesive energies as a function of rate and humidity for both glues. The adhesive energy is higher for faster stretching rates due to viscous dissipative forces. Interestingly, gumfoot silk glue does not adhere as strongly as the viscid silk glue at any humidity. For gumfoot silk, the adhesive energy remains unaffected by the level of R.H., just like its adhesive forces. For viscid silk glue, at the same stretching rates, both adhesive energy and adhesive forces are maximized at intermediate levels of humidity.
Surprisingly, whole viscid silk thread adhesion (Figure S1a), just like single droplet viscid silk glue adhesion, maximizes at intermediate levels of humidity too (Figure S1b). This pattern is somewhat counter-intuitive since viscid silk threads demonstrate monotonic increase in softness and extensibility with increasing relative humidity (Figure S1c), which should promote adhesion. Since the gumfoot silk thread releases its glue upon coming into contact with a surface, whole-thread adhesion measurements were not performed with it.
Physical or Chemical Crosslinking
Viscid silk glue drops act like a viscoelastic solid, which helps the spider in retaining trapped prey long enough to be subdued13. The viscoelastic solid nature of these glue drops could be due to either physical or chemical crosslinking. If it is physical crosslinking, like hydrogen bonding or electrostatic interactions, then the glue drops should behave as a liquid at long times at high R.H.. If it is chemical crosslinking on the other hand, the glue drops should behave as a solid at long times, irrespective of the humidity. To test these predictions, load-relaxation measurements were performed in which the glue droplets, equilibrated at desired humidity, were stretched by a constant distance and the load was allowed to relax (Figure 5). For the viscid silk glue the magnitude of the load plateau decreases as the humidity increases. This implies a reduction in the crosslinking density and hence, the elasticity, of viscid silk glue droplets (Figures 5a, c, e and 6a). Although this suggests the presence of physical crosslinking, chemical crosslinking cannot be completely ruled out because the relative magnitude of the load plateau at 90% R.H. versus the capillary pull-off forces (measured above) is not known due to limited resolution of the force measurements. The swelling of the viscid glue droplets at high humidity while maintaining their shapes (Figure 2), as well as the presence of amino acid sequences similar to elastin and flagelliform spider silk in one of the glycoproteins in the silk produced by Nephila clavipes24, suggest chemical crosslinking as well.
Effect of humidity on crosslinkers.
Load-relaxation behavior of individual glue drops of viscid silk (gumfoot silk) equilibrated at 15% R.H. a (b), 40% R.H. c (d) and 90% R.H. e (f) stretched by a constant length at rates of 1 µm/s (inverted triangles), 10 µm/s (upright triangles), 50 µm/s (squares) and 100 µm/s (circles). Values are plotted as mean ± s.d. from 5 measurements each. When viscid silk glue is stretched at 100 µm/s at 15% R.H., it releases contact with the tip before stretching 100 µm (Figure 3a), hence load relaxation measurements could not be performed at these conditions. (Figure 5a)
Effect on glue elasticity.
Plateau values, indicative of the amount of elasticity in the glue, reduce with increasing humidity in the case of viscid silk glue (a) but remain constant for gumfoot silk glue (b). Plateau values for gumfoot silk glue are plotted using a fitting function since the values were lower than the resolution of the equipment (1µN).
Gumfoot silk glue droplets, on the other hand, behave like a viscoelastic liquid at all levels of humidity (Figures 5b, d, f and 6b). Any load plateau is lower than the resolution of the equipment, which suggests the presence of very little, if any, crosslinking. The easy separation of the glue droplets from gumfoot silk to any substrate to which they adhere contrasts with the formation of a ‘suspension bridge’ and eventual release of the viscid silk glue droplets. In addition, gumfoot silk glue ‘flows’ and coalesces at long times such that the drops lose their shape, as opposed to the viscid silk glue droplets which stay intact. All of these observations support the viscoelastic liquid nature of the gumfoot silk glue.
Discussion
Both orb web and cobweb spiders depend upon liquid glue droplets for their silk to adhere to insect prey. Both types of spiders use the same sets of glands to produce the adhesive. Aggregate glands evolved initially in orb spiders to coat their elastic capture spirals and then were co-opted during the evolutionary origin of cobwebs to coat the base of gumfoot capture threads. Despite close evolutionary homology, the two bio-adhesives are remarkably different, especially in how they interact with water. For the viscid silk glue in orb webs, the change in the adhesion energy of the glue droplets as a function of humidity is controlled by several competing processes. The hygroscopic salt plays an intrinsic role not only in sequestering water but also in solvating the glycoproteins15. The increase in water content increases the spreading of the glue droplet. This spreading of the glue enlarges its contact area with the surface. In addition, the long-time plateau in the force relaxation measurements also decreases with increase in humidity. This indicates that the effective crosslink density also decreases with increase in water content. The complexity of the problem is further evident if we consider that the glycoprotein is negatively charged such that changes in concentration of water also change the electrostatic forces and thus the adsorption of the glycoproteins on the glass substrate.
To simplify the problem and to understand the underlying mechanism, we have designed a polymer model consisting of high molecular weight polyethylene oxide (PEO) dissolved in water. Measurements, similar to those conducted on individual glue droplets were performed on PEO/water solutions of different concentrations. For the same pull-off rates, the energy of adhesion (i.e. area under the load-displacement curve) increased with higher water concentration, reached a maximum and then reduced with further increase in humidity (Figure 7a,b).
Polymer model to understand the humidity effect.
(a) Pull-off energy plotted as a function of concentration of the PEO/water solution at a pull-off rate of 1mm/sec. (b) Energy calculated as area under the load-displacement curve during pull-off plotted as a function of the pull-off rate for concentrations of 13.7% (circle), 17.7% (upright triangles), 35.5% (squares) and 52.2 % (inverted triangles) of the PEO/Water solutions. (c) A schematic of the state of the glue drops at different values of R.H. Chemical crosslinking (red) remains unaffected with changes in humidity while the viscosity and elasticity reduce with increasing humidity. Lubricating action becomes predominant at higher values of humidity.
This trend captures the results obtained for the viscid silk glue drops produced by spiders. The optimum concentration of water for adhesion can be explained by two competitive mechanisms. The total work done in pulling the probe out of the PEO solutions is similar to the empirical equations used to describe the rate-dependent work done in peeling viscoelastic adhesives ∼ Go (1 + f(R,T))25. Go is related to the thermodynamic work of adhesion and f(R,T) is a term that reflects the energy expended in irreversible processes that include elastic and viscous forces. At lower concentrations of water, the viscosity and elasticity are very high, which tends to increase the contribution of the irreversible work of adhesion. However, the spreading rates are very slow at these lower concentrations thereby reducing the area of contact. At high concentrations of water, the spreading rates are fast, but the viscous and elastic forces are lower. In addition, the effectiveness of the interfacial contact of the glycoproteins is reduced at high water concentration due to lubrication. The interplay between these two competing effects leads to an optimum stickiness at intermediate humidity. Figure 7c shows a schematic of the state of the glue drop at different values of humidity. The chemical crosslinking (red squares) remains unaffected whilst the water content of the drop increases at higher values of R.H., which reduces the glue drop's viscosity and elasticity and also lubricates its interface with the glass substrate. At intermediate humidity (40% – 60% R.H.), these parameters are optimized such that the adhesion is maximized. Considering that the salts are the predominant hygroscopic component of viscid silk glue, the optimal R.H. that maximizes adhesion should largely depend on the concentration of salts in the viscous coat. Shifts in salt concentration would therefore provide an easy mechanism for evolution to act on the adhesion of spider silk glue, particularly across species whose habitats vary in ambient humidity.
Significantly less is understood about the chemical composition of the gumfoot silk. The weak effect of humidity on the droplet size, the ability of the droplets to flow, coalesce and separate easily from the gumfoot thread and the display of a viscoelastic liquid-like behavior unaffected by humidity, are all behaviors in stark contrast to the viscid silk glue. We hypothesize that the polymers (probably glycoproteins) in the glue are not crosslinked, which results in the absence of the central dense core seen in viscid drops, easy separation of the glue from the gumfoot silk and the flow and coalescing of these glue droplets. Also, while viscid silk glue maintains ‘fluidity’ due to the water absorbed by the hygroscopic salts15, gumfoot silk glue instead maintains fluidity due to the presence of the low-molecular-weight water-soluble coating peptides (Spider Coating Peptides17). These differences explain why gumfoot silk glue's adhesion and elasticity are resistant to changes in humidity. Water swells the glue slightly, which causes enough increase in fluidity to make these droplets flow, but not significantly enough to cause a change in their structure or adhesion. This humidity-resistant strategy works very well for these spiders since widow spiders must maintain adhesiveness in their glue across a wide range of environments, some of which are quite arid. The inability of viscid silk glue to adhere at low humidity is the reason why the individual glue drop measurements with viscid silk were performed at 15% R.H. and not 0% R.H. At 0% R.H, the stiffness of the viscid silk glue did not allow the microscopic glass probe to penetrate inside it at the pre-force range used for these measurements. Figure S1d shows the difference in glue adhesion at 0% R.H. and 15% R.H.).
A second possible adaptive explanation for the evolutionary shift in humidity responsiveness of spider glues during the origin of cobwebs relates not to microhabitats but instead to the structures of the webs themselves – humidity resistance could prevent ‘local’ supercontraction in the cobweb. The entire capture spiral of an orb web is encased in its highly hydroscopic glue and water therefore infiltrates the flagelliform silk core, causing it to supercontract. This is an essential feature that helps to make the silk soft, highly extensible and resilient. In contrast, cobweb silk glue is laid upon only a small portion of the gumfoot capture thread, which is composed of dry major ampullate silk threads. This silk can shrink as much as 50% of its length when wetted and generate stresses in excess of 100 MPa (11). If whole webs supercontract then the stresses generated in individual threads can be equalized, thereby maintaining the structure and function of the web (Boutry and Blackledge, unpublished). However, this would not be the case if gumfoot glue drops were hydroscopic and highly responsive to humidity because they coat only the bottom portion of a gumfoot thread. If just this region supercontracted then it could cause the separation of the gumfoot thread from the surface because the stress exceeds the strength of the piriform disk attaching it to the substrate (results not shown). Local supercontraction of individual threads would also alter the tensions of threads in the web and likely attenuate the vibration-transmission efficiency due to the softening of the gumfoot thread.
Nature exhibits many intriguing strategies that take advantage of water, the most common liquid on earth. Gecko toes when exposed to high humidity adhere better to surfaces20. Tree swallows appear yellower when wet4. Spiders have used a combination of synergistic materials to promote or maintain high adhesion to capture prey. Here, we have shown that cobweb-weavers, using a cocktail of short peptides and long adhesive polymers (likely glycoproteins), maintain the adhesion of their prey capture glue over a large variation in humidity. The combination of the humidity-resistant glue and strength of the dry major ampullate silk fiber is necessary for catching pedestrian insects. On the other hand, the glue produced by orb-weavers is highly responsive to water. Hygroscopic salts present in the viscid glue of orb spiders make it highly humidity-sensitive. Humidity swells these glue droplets and promotes the spreading of viscoelastic glycoproteins present therein to increase the adhesive contact with the substrate. Viscid silk threads take advantage of the synergistic combination of this glue and the underlying flagelliform silk fibers in catching prey flying into the web at high velocity. The understanding of how nature takes advantage of these strategies to enhance the survival and proliferation of their species provides a plethora of ideas for designing synthetic adhesives that work in presence of water or humidity.
Methods
Single drop pull-off and load-relaxation measurements
Viscid silk threads from orb webs spun by the furrowed orb-weaver Larinioides cornutus and gumfoot silk from the cobwebs spun by the western black widow Latrodectus hesperus were equilibrated at the desired humidity and immobilized on a glass slide. Measurements of adhesion were performed using an MTS Nano Bionix that measured force to ±1µN. The glass slide was fixed firmly on the lower clamp while a clean conical glass probe (base diameter = 10 µm) was fixed on the upper clamp. To measure adhesion at different values of R.H., the conical probe was lowered at 1 µm/sec onto the droplet till the force registered was 3 µN (the whole assembly was observed with an optical microscope). After 60 seconds, the probe was pulled away from the silk at known rates. The stretching behavior of the glue drop was observed using an optical microscope simultaneously with recording the load-displacement response every 0.01 seconds.
For the load-relaxation measurements, the conical probe was lowered at 1 µm/sec onto the droplet till the probe went the same depth into the droplet for all different values of R.H. After 60 seconds, the probe was pulled away from the silk at known rates such that the drop in contact is stretched by a constant length for and the load was allowed to relax after this. All single drop measurements were conducted close to 25°C.
Thread pull-off measurements
Individual fibers of capture spiral silk were first collected from the webs spun by Larinioides cornutus and adhered to cardboard mounts across 16 mm gaps. After mounting the sample in the Nano Bionix and letting it equilibrate at the desired humidity, we pressed the silk thread onto a 2-mm-wide piece of glass mounted on a small tack. The glass was replaced regularly so that every run was performed on a clean surface. The sample was first lowered until it initially contacted the glass and then pressed until the force registered 80 µN, to ensure firm contact. Finally, the silk was pulled away from the substrate at known rates. The stickiness was measured directly as the force registered when the silk released from the substrate.
Stress-Strain measurements
Individual fibers of capture spiral silk were first collected from the webs spun by Larinioides cornutus and adhered to cardboard mounts across 16 mm gaps. After mounting the sample in the Nano Bionix and letting it equilibrate at the desired humidity, the threads were stretched such that the rate of stretching is similar to what it experiences during thread pull-off measurements. For the individual drop force measurements and whole thread measurements, the samples were held at each humidity values for around 5 hours, before starting the measurements (The chamber for these measurements has an inlet through which a mix of nitrogen and water vapor enters). The reason for choosing 5 hours is because the rate of volume change of glue droplets after 5 hours becomes negligible.
Drop volume measurements
Individual silk threads from the webs spun by Larinioides cornutus and Latrodectus hesperus were mounted on a cardboard holder across a 16 mm gap and were equilibrated in a desiccator (0% R.H.) for 24 hours after which they were placed in a humidifier (100% R.H) and images were taken at t = 0, 30, 60, 90, 120 and 150 minutes using an optical microscope at 100× magnification. The chamber used to humidify the samples is just like a desiccator, except that, instead of the P2O5 pellets, there is water.
Polyethylene oxide (PEO) measurements
PEO/Water solutions of concentrations 13.7%, 17.7%, 37.5% and 52.2% were prepared. Aluminum pans filled with these solutions were placed on the lower clamp of the Nanobionix whilst a glass probe of diameter 30 µm, placed firmly on the top grip was lowered onto the solution at a rate of 0.1 mm/sec till the force registered during penetration reached 10 µN after which the probe was allowed to relax for 60 seconds and then pulled back at controlled rates.
References
Dawson, C., Vincent, J. F. V. and Rocca, A. How pine cones open. Nature 390, 668 (1997).
Capadona, J. R. et al. Stimuli-responsive polymer nanocomposites inspired by sea cucumber dermis. Science 319, 1370–1374 (2008).
Elbaum, R. et al. The role of wheat awns in the seed dispersal unit. Science 316, 884–886 (2007).
Eliason, C. M. and Shawkey, M. D. Rapid, reversible response of iridescent feather color to ambient humidity. Optics Express 18, 21284–21292 (2010).
Cheng et al. Effects of micro- and nano-structures on the self-cleaning behavior of lotus leaves. Nanotechnology 17, 1359–1362 (2006).
Franco et al. Hydrophobic properties and chemical characterization of natural water repellent materials in Australian sands. J. Hydrol. 231, 47–58 (2000).
Gao, X. and Jiang L. . Water-repellent legs of water striders Nature . 432, 36 (2004).
Vollrath, F. and Edmonds, D. T. Modulation of the mechanical properties of spider silk by coating with water. Nature 340, 305–307 (1989).
Blackledge et al. Reconstructing web evolution and spider diversification in the molecular era. Proc. Nat. Acad. Sc. 106, 5229–5234 (2009).
Denny, M. The physical properties of spider's silk and their role in the design of orb-webs. J. Exp. Biol. 65, 483–506 (1976).
Blackledge, T. A., Summers, A. P. and Hayashi, C. Y. Gumfooted lines in black widow cobwebs and the mechanical properties of spider capture silk. Zoology 108, 41–46 (2005).
Vollrath, F. and Tillinghast, E. K. Glycoprotein glue beneath a spider web's aqueous coat. Naturwissenschaften 78, 557–559 (1991).
Sahni, V. et al. (2010) Viscoelastic solids explain spider web stickiness. Nat. Commun 1:19 doi: 10.1038/ncomms1019.
Vollrath, F. et al. Compounds in the droplets of the orb spiders' viscid spiral. Nature 345, 526–528 (1990).
Sahni, V., Blackledge, T. A. and Dhinojwala, A. Spiders Use ‘Salty Silk’ To Capture Prey. Unpublished
Blackledge, T. A., Eliason, C. M. Functionally independent components of prey capture are architecturally constrained in spider orb webs. Biology Letters 3, 456–458 (2007).
Hu et al. Analysis of aqueous glue coating proteins on the silk fibers of the cob weaver, Latrodectus hesperus . Biochemistry 46, 3294–3303 (2007).
Eberhard, W. G., Agnarsson, I. and Levi, H. W. 2008 Web forms and phylogeny of theridiid spiders (Araneae: Theridiidae). Systematics and Biodiversity 6, 415–475 (2008).
Argintean, S., Chen, J., Kim, M. and Moore, A. M. F. Resilient silk captures prey in black widow cobwebs. Appl. Phys. A-Mat. Sc. & Process. 82, 235–241 (2006).
Puthoff, J. B. et al. Changes in material properties explain effect of humidity on gecko adhesion . J. Exp. Biol. 213, 3699–3704 (2010).
Hawthorn, A. C. and Opell, B. D. van der Waals and hygroscopic forces of adhesion generated by spider capture threads. J. Exp. Biol. 206, 3905–3911 (2003).
Opell, B. D. and Hendricks, M. L. The role of granules within viscous capture threads of orb-weaving spiders. J. Exp. Biol. 213, 339–346 (2010).
Tillinghast, E. K. The chemical fractionation of the orb web of Argiope spiders. Insect. Biochem. 14, 115–120 (1984).
Choresh, O., Bayarmagnai, B. and Lewis, R. V. Spider web glue: two proteins expressed from opposite strands of the same DNA sequence. Biomacromolecules 10, 2852–2856 (2009).
Gent, A. N. Adhesion and strength of viscoelastic solids. Is there a relationship between adhesion and bulk Properties? Langmuir 12, 4492–4496 (1996).
Acknowledgements
We express sincere gratitude to Andrew Sensenig and Cecilia Boutry for providing assistance with spiders. Chelsea Golias helped with the viscid silk glue drop volume data. This work is supported by the National Science Foundation. We also acknowledge the financial support of Austen Bioinnovation Institute in Akron (ABIA).
Author information
Authors and Affiliations
Contributions
V.S. performed the experiments. V.S. and A.D. analyzed the data. V.S., T.A.B. and A.D. wrote the paper. V.S., A.D. and T.A.B. discussed the results and commented on the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Sahni, V., Blackledge, T. & Dhinojwala, A. Changes in the Adhesive Properties of Spider Aggregate Glue During the Evolution of Cobwebs. Sci Rep 1, 41 (2011). https://doi.org/10.1038/srep00041
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00041
This article is cited by
-
Deluded zombies: induced behavioral modification in a cobweb spider does not increase the survival of its parasitoid wasp
Behavioral Ecology and Sociobiology (2024)
-
Silkworm and spider silk electrospinning: a review
Environmental Chemistry Letters (2021)
-
Spidroin profiling of cribellate spiders provides insight into the evolution of spider prey capture strategies
Scientific Reports (2020)
-
Functional trade-offs in cribellate silk mediated by spinning behavior
Scientific Reports (2019)
-
Orb weaver glycoprotein is a smart biological material, capable of repeated adhesion cycles
The Science of Nature (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.