Key Points
-
Highlights that dental pulp stem cells are mesenchymal stem cells capable of forming bone, nerves and connective tissue.
-
Highlights that dental pulp stem cells have potential applications in dentistry as well as wider implications in regenerative medicine.
-
Suggests that dentists should encourage the collection and storage of dental pulp stem cells by their patients to ensure that these clinically valuable cells are not discarded as medical waste.
-
Highlights that dental pulp stem cells can be collected and cryogenically stored for later use from exfoliated deciduous teeth and healthy extracted adult teeth.
Abstract
The mesenchymal stem cells (dental pulp stem cells; DPSC) found inside teeth represent a significant future source of stem cells for regenerative medicine procedures. This review describes the ontogeny of DPSC; the laboratory processing and collection of DPSC; the immuno-cytochemical characterisation of DPSC; the differentiation between adult DPSC and DPSC obtained from exfoliated deciduous teeth (SHED) and their potential use in regenerative medicine procedures in the future both in dental and general medical applications.
Similar content being viewed by others
Introduction
'Every tooth in a man's head is more valuable than a diamond,' Miguel de Cervantes, Don Quixote, 1605.
Inside every tooth there are dental pulp mesenchymal stem cells which have the potential to treat a wide range of diseases when utilised in modern regenerative medicine protocols. This review describes the origin of these cells, the identification, harvesting and storage technology, the current clinical applications and the future clinical potential in regenerative medicine. Dental pulp mesenchymal stem cells have the ability to treat stomatognathic disorders such as periodontal disease and also a wide range of connective tissue, bone and neuronal diseases.
Ontogeny of dental pulp stem cells
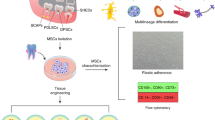
The development of teeth involves interactions between oral ectodermal epithelial cells which form the enamel, papilla and dental follicle and mesenchymal stem cells which form dentin, pulp, cementum and the periodontal ligament.1 Five subtypes of mesenchymal stem cells have been described, these are: dental pulp stem cells (DPSC), periodontal ligament stem cells (PDLSC), stem cells from apical papilla (SCAP), dental follicle stem cells (DFSC) and gingival mesenchymal stem cells (GMSC).2 Teeth are therefore an excellent source of stem cells for therapeutic procedures in the future and can be easily harvested following tooth extraction or natural shedding of deciduous teeth.3 Dental pulp stem cells (DPSC) were first isolated from third molars and were found to have high clonogenicity and the ability to produce densely calcified colonies.4 DPSC have also been confirmed as mesenchymal stem cells by demonstrating their ability to form adipocytes, osteoblasts, odontoblasts, chondrocytes, neural ectodermal cells and myoblasts.5 From a developmental view point the dental pulp is derived from ectomesenchyme arising in the periphery of the neural tube which migrates to the oral region where the cells differentiate into mesenchymal cells.6
In normal physiology the dental pulp cells maintain and repair the periodontal tissue and respond to damage. Deep caries result in the dental pulp cells migrating to the damaged area and the creation of odontoblasts and dentin in an attempt to repair the damaged tooth.7,8 These observations led to the proposal that dental pulp stem cells could be active during reparative dentinogenesis.9
Laboratory processing of dental pulp stem cells
There are many approaches to dental pulp stem cell collection and processing, the key is to ensure the quality and safety of the end product. The following describes the process used in the WideCells Institute of Stem Cell Technology: once exfoliated, or extracted, teeth are sent to the processing laboratory within 72 hours of exfoliation or extraction. The tooth is transported in sterile phosphate buffered saline with calcium and magnesium inside a validated and monitored collection kit which keeps the tooth between 4 and 26 °C. On arrival at the laboratory the tooth is opened in a Grade A clean room environment using a medical circular saw, the pulp exposed to 10% DMSO and the whole tooth is then frozen in a controlled rate freezer and stored in the vapour phase of liquid nitrogen.10 When the dental pulp stem cells are required then the tooth is thawed rapidly in a 37 °C waterbath and then processed using either one of two standard techniques: the explant method and the enzymatic digestion method. In the explant method the dental pulp is dissected from the tooth in a Grade A clean room environment and the cells are then grown in vitro from these tissue fragments.11,12,13 In the enzymatic method the dental pulp tissue is digested in collagenase and dispase, in a Grade A clean room environment, and the resultant cells then grown in vitro.14,15 Both of these processing technologies yield good numbers of viable DPSC and future research will no doubt optimise these technologies to develop a gold standard.
Immunocytochemical identification of dental pulp mesenchymal stem cells
The International Society for Cellular Therapy (ISCT) state that mesenchymal stem cells express the following surface antigens: CD105 (endoglin: a putative novel endothelial cell specification gene), CD73 (5' ectonucleotidase: an enzyme which metabolises nucleotides to nucleosides) and CD90/Thy-1 (glycosylphosphatidylinositol-anchored glycoprotein) and a negative for CD11b, CD14, CD19, CD34, CD45, CD79a surface antigens and HLA-DR. These are assessed by the use of flow cytometry.
Other workers propose that mesenchymal stem cells express STRO-1 (stromal precursor antigen 1), VCAM-1 (vascular cell adhesion molecule 1), SH2 (Src homology 2), SH3/SH4, CD271, GD2 (ganglioside 2), and SSEA-4 (stage-specific embryonic antigen-4).16,17,18,19,20 Some workers even suggest that DPSC may have a different immunophenotype to those traditionally thought to be MSC.21,22 This variation in surface antigen expression may reflect the proliferative potential of DPSC.23 STRO-1 positive DPSC have been shown to have odonto-osteogenic characteristics whereas CD34+, CD117+ and CD45- DPSC have a greater capacity for cell renewal and osteogenic differentiation.24 Other authors have referred to DPSC MSC expressing CD29+, CD44+ and CD73+.25,26,27 The expression of transcription factor genes Oct-4 and Nanog have also been used to identify DPSC MSC.28 The identification of DPSC mesenchymal stem cells is clearly a developing science which will no doubt be refined in the future to clearly describe each sub-population of DPSC.
The fact that DPSC have low expression of Class II HLA-DR (MHC)29 molecules means that they are immunologically privileged and it may be possible to transplant these cells from one person to another without the need for tissue matching. This raises the possibility of a public DPSC bank, perhaps in collaboration with key dental hospitals, to provide DPSC to anyone in need. Such donated DPSC could be extremely useful when using artificial bone to provide new bone for dental implants where the artificial bone could be used along with donated DPSC to enhance bone formation.30
Sources and characteristics of dental pulp stem cells (DPSC) and stem cells from human exfoliated deciduous teeth (SHED)
DPSC have been isolated from exfoliated deciduous teeth (SHED: stem cells from human exfoliated deciduous teeth), from permanent secondary dentition, from teeth extracted due to impaction or periodontitis and from inflamed pulp tissue.31 SHED cells have been shown to have a high proliferative rate and are capable of producing osteoblasts, adipocytes, neuronal cells and odontoblasts.32 Some workers suggest that SHED cells have a greater proliferative capacity than DPSC obtained from adult third molars, incisors or supernumerary teeth on the basis that SHED cells represent a more immature type of stem cell.33,34 This is a similar hypothesis to that put forward for the observed differences between cord blood stem cells and adult haemopoietic stem cells such as bone marrow. It has been shown that the properties of DPSC are directly related to the physical age of the tooth from which they are obtained.34 It is interesting to note that in terms of cell cycle 69.8% of SHED cells were found to be in the S and G2 stage, but only 56% of the DPSC were in those phases indicating increased proliferative capacity in SHED cells.35 The surface antigen expression of SHED cells also differs from that seen in DPSC. This is reflected in the fact that proliferation related and extracellular matrix (ECM) formation genes, for example genes encoding transforming growth factor (TGF) and fibroblast growth factor 2 (FGF), are expressed in SHED cells. Genes coding for collagen I and collagen III and pluripotency markers, such as Pou5f1, Oct3/Oct4, Sox2, and Nanog are also expressed higher in SHED cells.36 The expression of Nestin (a marker of neuroepithelial stem cells)37 is reduced in SHED resulting in their reduced ability to form neurospheres in comparison to DPSC.37
Permanent teeth, impacted third molars and supernumerary teeth are an excellent source of DPSC which have the following mesenchymal stem cell surface antigens: CD90+, CD146+, CD105+ and CD45−; and also express Oct4 and Nanog38,39,40 but lower expression than that seen in SHED cells.
Both DPSC and SHED cells are an excellent source of mesenchymal stem cells for regenerative medicine procedures,41 in addition, SHED cells have recently been proposed as potential immuno-modulators in the treatment of autoimmune encephalomyelitis and other autoimmune pathologies of the central nervous system.42
Clinical applications of DPSC and SHED cells in regenerative medicine
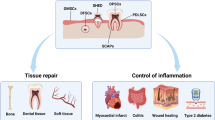
The basic cell biology described above illustrates the enormous potential of DPSC and SHED cells in regenerative medicine procedures in orthopaedics, oral and maxillofacial applications. Studies using canine DPSC have shown that the cells are capable of bone formation when grafted into the jaw.43 In human studies DPSC have been transplanted along with some sort of scaffold or porous biomaterial to enable the cells to develop to facilitate bone formation.44,45 There is clearly potential in the use of DPSC in bone formation and such an approach is the subject of current research in our WideCells ISCT using our artificial bone product Indus as the scaffold for DPSC.
The DPSC mesenchymal stem cells which are found in teeth have been shown to be capable of repairing periodontal tissue, diabetic critical limb ischaemic tissue, bone damage caused by osteonecrosis, skin lesions caused by burns, liver, neuronal tissue, skeletal muscle tissue and blood vessels.46,47,48,49,50 For these reasons DPSC are now considered to be one of the best future sources of mesenchymal stem cells for use in regenerative medicine51 including treatment for diseases of oro-facial, neurological, corneal, cardiovascular, hepatic, pancreatic and renal tissues and in muscular dystrophy.52 Medical laser activation of DPSC has been shown to enhance osteogenic differentiation53 and this is a subject of ongoing research in the WideCells ISCT.
Treatment of periodontal disease using DPSC
Periodontal disease (PD) is estimated to be present in 90% of the population of the world, making it the most common known chronic infectious disease.54 PD is also the most common cause of tooth loss in adults.55 The current standard treatment of PD often involves the use of autologous bone grafts, allografts or alloplastic materials but these types of intervention, at best, result in tissue repair rather than regeneration.56 These types of intervention for PD may also be unsuitable for many patients.57 It is possible to add differentiation factors or anti-inflammatory molecules to these treatments, which do not have a cellular base, but the short half-life of these molecules results in generally poor clinical ourcomes.58,59,60 Xenografts of human third molar DPSC into immunodeficient athymic mice have shown that the donor human cells can produce adipocytes and collagen forming cells with the potential to produce material similar to periodontal tissue cement.56 It appears that the expression of STRO-1, CD146, and CD44 along with the presence of stromal cell-derived factor-1 (SCD-1) is important in the development of periodontal tissue regeneration.60,61 SHED cells have been shown to be capable of stimulating bone formation making them a possible route to treatment for disease requiring craniofacial bone regeneration.62 It has also been shown that the treatment of DPSC with valproic acid can improve mineralised matrix formation and increase the expression of bone glycoproteins.63 It has been proposed that in the future the use of epigenetic regulators such as HDAC inhibitors64 could be useful in regenerative medicine procedures63 using DPSC. Researchers support the concept of the use of DPSC and related stem cells as a source of stem cells in the treatment of periodontal disease.65
Neural regeneration using DPSC
Neural crest stem cells have been isolated from dental pulp,66 periodontal ligaments67 and salivary glands.68 Animal studies have indicated that DPSC could be useful in the regeneration of neural tissue.69 The neural crest ontogeny of DPSC is also reflected in their ability to produce neurotrophic factors which promote neuronal survival and axonal guidance.70,71 DPSC have been used as a graft into hemisected spinal cords in animal models resulting in an increased number of surviving motor neurons70 illustrating the promise of this technology in future clinical trials. DPSC have also been shown to promote neuritogenesis when co-cultured with rat retinal cells, this is thought to be related to the ability of DPSC to induce the expression of neurotrophins such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3).71 The ultimate application in ophthalmology is to develop cell based regenerative technology which could repair or replace a damaged retina and therefore restore sight. In this context DPSC have been shown to be able to express markers found on mature photoreceptors such as BDNF and rhodopsin. This raises the possibility that DPSC could differentiate into functional photoreceptors which could in turn be used to restore sight in patients suffering from a damaged retina.72 The current opinion, supported by the literature, is that DPSC are an excellent potential source of stem cells for neural tissue engineering and for use in neural induction protocols.73
Conclusion
Dental pulp stem cells can be collected, processed and cryogenically stored each time a deciduous tooth is exfoliated or a healthy adult tooth is extracted. This represents a considerable source of mesenchymal stem cells which at present are being discarded as medical waste. These mesenchymal stem cells have enormous potential in future stem cell-based regenerative medicine procedures. Patients, dentists and physicians need to work together to ensure that this valuable resource is not wasted and that every possible dental pulp stem cell is available to use in the future.
References
Sedgley C M . Botero T M . Dental stem cells and their sources. Dental Clin North Am 2012; 56: 549–561.
Chen F-M, Sun H-H, Lu H, Yu Q . Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 2012; 33: 6320–6344.
Racz G Z, Kadar K, Foldes A et al. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. J Physiol Pharmacol 2014; 65: 327–339.
Gronthos S, Brahim J, Li W et al. Stem cell properties of human dental pulp stem cells. J Dent Res 2014; 81: 531–535.
Zhang N, Li J-H, Wang J A, Zhang H K . The effect of bone marrow mesenchymal stem cell transplantation on diabetic cardiomyopathy. Zhonghua Xin XueGuan Bing Za Zhi, 2008; 36: 1115–1119.
Chang C-C, Chang K-C, Tsai S-J, Chang H-H, Lin C-P . Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J Formosan Med Assoc 2014; 113: 956–965.
Dimitrova-Nakov S. Baudry A. Harichane Y. Kellermann O. Goldberg M. Pulp stem cells: implication in reparative dentin formation. J Endodont 2014; 40: S13–S18.
Tziafas D, Smith A J, Lesot H . Designing new treatment strategies in vital pulp therapy. J Dent 2000; 28: 77–92.
About I . Dentin–pulp regeneration: the primordial role of the microenvironment and its modification by traumatic injury and bioactive materials. Endodont Top 2013; 28: 61–89.
Lee H S, Jeon M, Kim S O, Kim S H et al. Characteristics of stem cells from human exfoliated deciduous teeth (SHED) from intact cryopreserved deciduous teeth. Cryobiol 2015; 71: 374–383.
Roozafzoon R, Lashay A, Vasei M et al. Dental pulp stem cells differentiation into retinal ganglion-like cells in a three dimensional network. Biochem Biophys Res Comm 2015; 457: 154–160.
Spath L, Rotilio V, Alessandrini M et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J Cell Mol Med 2010; 14: 1635–1644.
Hilkens P, Gervois P, Fanton Y et al. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tiss Res 2013; 353: 65–78.
Sun H-H, Chen B, Zhu Q-L et al. Investigation of dental pulp stem cells isolated from discarded human teeth extracted due to aggressive periodontitis. Biomat 2014; 35: 9, 459–9: 472.
Paschalidis T, Bakopoulou A, Papa P et al. Dental pulp stem cells' secretome enhances pulp repair processes and compensates TEGDMA induced cytotoxicity. Dent. Mat. 2014; 30: e405–e418.
Gang E J, Bosnakovski D, Figueiredo C A, Visser J W, Perlingeiro R C R . SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood 2007; 109: 1743–1751.
Gronthos S, Zannettino A C W, Hay S J et al., Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 2003; 116: 1827–1835.
Haynesworth S E, Barer M A, Caplan A I . Cell surfaceantigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone 1992; 13: 69–80.
Martinez C, Hofmann T J, Marino R, Dominici M, Horwitz E M . Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSC. Blood 2007; 109: 4245–4248.
Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers G L . Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Haematol 2002; 30: 783–791.
Espagnolle N, Guilloton F, Deschaseaux F, Gadelorge M, Sens´eb´e L, Bourin P . CD146 expression on mesenchymal stem cells is associated with their vascular smooth muscle commitment. J Cell Molecul Med 2014; 18: 104–114.
Sonoyama W, Liu Y, Fang D et al. Mesenchymal stem cell mediated functional tooth regeneration in Swine. PLoS ONE. 2006; 1, article e79.
Kawashima N . Characterisation of dental pulp stem cells: a new horizon for tissue regeneration? Arch. Oral Biol. 2012; 57: 1439–1458.
Yang K-L . Chen M-F . Liao C-H . Pang C-Y . Lin P-Y . A simple and efficient method for generating Nurr1-positive neuronal stem cells from human wisdom teeth (tNSC) and the potential of tNSC for stroke therapy. Cytotherapy, 2009; 11: 606–617.
Pivori-Unas A, Surovas A, Borutinskait V et al. Proteomic analysis of stromal cells derived from the dental pulp of human exfoliated deciduous teeth. Stem Cells Devel 2010; 19: 1081–1093.
Jo Y-Y, Lee H J, Kook S Y et al., Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Engineer 2007; 13: 767–773.
Mafi P, Hindocha S, Mafi R, Griffin M, Khan W . Adult mesenchymal stem cells and cell surface characterization – a systematic review of the literature. Open Orthopaed J 2011; 5: 253–260.
Navabazam A R, Nodoshan F S, Sheikhha M H, Miresmacili S M, Solcimani M, Fesahat F . Characterisation of mesenchymal stem cells from human dental pulp, preapical follicle and periodontal ligament. Iran J Reproduct Med 2013; 11: 235–242.
Bernardi L, Luisi S B, Fernandes R et al. The isolation of stem cells from human deciduous teeth pulp is related to the physiological process of resorption. J Endod 2011; 37: 973–979.
Maioli M, Basoli V, Santaniello S et al. Osteogenesis from Dental Pulp Derived Stem Cells: A Novel Conditioned Medium Including Melatonin within a Mixture of Hyaluronic, Butyric, and Retinoic Acids. Stem Cells Int 2016; DOI: 10.1155/2016/2056416.
Alongi D J, Yamaza T, Song Y et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med 2010; 5: 617–631.
Miura M, Gronthos S, Zhao M et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Nat Acad Sci USA 2003; 100: 5807–5812.
Sakai V T . Zhang Z . Dong, Z et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res 2010; 89: 791–796.
Nakamura S, Yamada Y, Katagiri W . Sugito T, Ito K, Ueda M . Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endodont 2009; 35: 1536–1542.
Such´anek J, Visek B, Soukup T et al. Stem cells from human exfoliated deciduous teeth—isolation, long term cultivation and phenotypical analysis. Acta Medica 2010; 53: 93–99.
Govindasamy V, Abdullah A N, Ronald V S et al. Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. J Endodont 2010; 36: 1504–1515.
Dahlstrand J, Lardelli M, Lendahl U . Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Dev Brain Res 1995; 84: 109–129.
Tirino V . Paino F. De Rosa A. Papaccio G. Identification, isolation, characterization, and banking of human dental pulp stem cells. pp. 443–463. In Singh S (ed) Somatic Stem Cells. London: Springer, 2012.
D'Aquino R, De Rosa A, Lanza V et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Europ Cells Mater 2009: 18: 75–83.
Huang A H-C, Chen Y-K, Lin L-M, Shieh T-Y, Chan A W-S . Isolation and characterisation of dental pulp stem cells from a supernumerary tooth. J Oral Path Med 2008; 37: 571–574.
Akpinar G, Kasap M, Aksoy A, Duruksu G, Gacar G. Karaoz E . Phenotypic and Proteomic Characteristics of Human Dental Pulp Derived Mesenchymal Stem Cells from a Natal, an Exfoliated Deciduous, and an Impacted Third Molar Tooth. Stem Cells Int 2014; 2014: 1–19.
Rossato C, Brandao W N, Castro S B R et al. Stem cells from human-exfoliated deciduous teeth reduce tissue-infiltrating inflammatory cells improving clinical signs in experimental autoimmune encephalomyelitis. Biologicals 2017; 49: 62–68.
Yamada Y, Ito K, Nakamura S, Ueda M, Nagasaka T . Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transplant 2011; 20: 1003–1013.
Ravindran S. Huang C-C. George A. Extra cellular matrix of dental pulp stem cells: applications in pulp tissue engineering using somatic MSC. Front Physiol 2014; 4: 395–400.
Zhang W, Ahluwalia I P, Literman R, Kaplan D L, Yelick P C. Human dental pulp progenitor cell behaviour on aqueous and hexafluoroisopropanol based silk scaffolds. J Biomed Mat Res Part A 2011; 97: 414–422.
Lu D. Chen B. Liang, Z et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabet Res Clin Prac 2011; 92: 26–36.
Rasulov M F. Vasilchenkov A V. Onishchenko N A et al. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med 2005; 139: 141–144.
Kadar K. Kiraly M, Porcsalmy B et al. Differentiation potential of stem cells from human dental origin-promise for tissue engineering. J Physiol Pharmacol 2009; 60: 167–175.
Clarke D L, Johansson C B, Wilbertz J et al. Generalized potential of adult neural stem cells. Science 2000; 288: 1660–1663.
Galli R, Borello U, Gritti A et al. Skeletal myogenic potential of human and mouse neural stem cells. Nature Neurosci 2000; 3: 986–991.
Tatullo M, Marrelli M, Shakesheff K M, White L J . Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tiss Eng Regen Med 2014; 9: 1205–1216.
Bolteho J, Cavacas M A, Machado V et al. Dental stem cells: recent progresses in tissue engineering and regenerative medicine. Ann. Med. 2017; 49: 644–651.
Pinheiro C C G, de Pinho M C, Aranha A C C et al. Low laser Therapy: a strategy to promote the osteogenic differentiation of deciduous dental pulp stem cells from Cleft Lip and Palate patients. Tissue Eng Part A 2017; 24: 569–575.
Basegmez C, Berber L, Yalcin F . Clinical and biochemical efficacy of minocycline in nonsurgical periodontal therapy: A randomized controlled pilot study. J Clin Pharmacol 2011; 51: 915–922.
Pihlstrom B L, Michalowicz B S, Johnson N W . Periodontal diseases. Lancet 2005; 366: 1809–1820.
Kinaia B M, Chogle S M A, Kinaia A M, Goodis H E . Regenerative therapy: A periodontal-endodontic perspective. Dent Clin North Amer 2012; 56: 537–547.
Gopal K K. Lankupalli A M. Stem cell therapy: A new hope for dentists. J Clin Diag Res 2012; 6: 142–144.
Saito A, Saito E, Handa R, Honma Y, Kawanami M. Influence of residual bone on recombinant human bone morphogenetic protein-2-induced periodontal regeneration in experimental periodontitis in dogs. J Periodont 2009; 80: 961–968.
Sikiri´c P . Antiinflammatory effect of BPC 157 on experimental periodontitis in rats. J Physiol Pharmacol 2009; 60: 115–122.
Du L, Yang P, Ge S . Stromal cell-derived factor-1 significantly induces proliferation, migration, and collagen type i expression in a human periodontal ligament stem cell subpopulation. J Periodont 2012; 83: 379–388.
Rettori E, De Laurentiis A, Zorrilla Zubilete M, Rettori V, Elverdin J C . Anti-inflammatory effect of the endocannabinoid anandamide in experimental periodontitis and stress in the rat. Neuro Immuno Mod 2012; 19: 293–303.
Mooney D J . Vandenburgh H . Cell delivery mechanisms for tissue repair. Cell Stem Cell 2008; 2: 205–213.
Paino F, La Noce M, Tirino I et al. Histone deacetylase inhibition with valproic acid downregulates osteocalcin gene expression in human dental pulp stem cells and osteoblasts: Evidence for HDAC2 involvement. Stem Cells 2014; 32: 279–289.
Liu L, Ling J, Wei X, Wu L, Xiao Y . Stem cell regulatory gene expression in human adult dental pulp and periodontal ligament cells undergoing odontogenic/osteogenic differentiation. J Endodont 2009; 35: 1368–1376.
Hu L. Liu, Y, Wang S . Stem cell based tooth and periodontal regeneration. Oral Dis 2017; DOI: 10.1111/odi.12703.
Sasaki R, Aoki S, Yamato M et al. Tubulation with dental pulp cells promotes facial nerve regeneration in rats. Tiss Eng Part A 2008; 14: 1141–1147.
Widera D, Grimm W-D, Moebius J M et al. Highly efficient neural differentiation of human somatic stem cells, isolated by minimally invasive periodontal surgery. Stem Cells Devel 2007; 16: 447–460.
Takahashi M, Suzawa T, Yamada A et al. Identification of gene expression profile of neural crest-derived cells isolated from submandibular glands of adult mice. Biochem Biophys Res Comm 2014; 446: 481–486.
Leong W K, Henshall T L, Arthur A et al. Human adult dental pulp stem cells enhance post-stroke functional recovery through non-neural replacement mechanisms. Stem Cells Trans Med 2012; 1: 177–187.
Nosrat I V, Smith C A, Mullally P, Olson L, Nosrat A . Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; Implications for tissue engineering and repair in the nervous system. Europ J Neurosci 2004; 19, 2388–2398.
Mead B, Logan A, Berry M, Leadbeater W, Scheven B A . Intra-vitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Invest Ophthal Vis Sci 2013; 54: 7544–7556.
Bray A F, Cevallos R R, Gazarian K . Lamas M . Human dental pulp stem cells respond to cues from the rat retina and differentiate to express the retinal neuronal marker rhodopsin. Neurosci 2014; 280: 142–155.
Mortada I. Mortada R. Bazzal M. Dental Pulp Stem Cells and Neurogenesis. Adv Exp Med Biol 2017; DOI: 10.1007/5584_2017_71.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hollands, P., Aboyeji, D. & Orcharton, M. Dental pulp stem cells in regenerative medicine. Br Dent J 224, 747–750 (2018). https://doi.org/10.1038/sj.bdj.2018.348
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2018.348
This article is cited by
-
Stem Cell-Based Tissue Engineering Approaches for Diabetic Foot Ulcer: a Review from Mechanism to Clinical Trial
Stem Cell Reviews and Reports (2024)
-
Effects of phytosomal curcumin treatment on modulation of immunomodulatory and pulp regeneration genes in dental pulp mesenchymal stem cells
Odontology (2022)
-
Methacrylated Hyaluronic Acid–Based Hydrogels Maintain Stemness in Human Dental Pulp Stem Cells
Regenerative Engineering and Translational Medicine (2020)
-
An update on human periapical cyst-mesenchymal stem cells and their potential applications in regenerative medicine
Molecular Biology Reports (2020)
-
Pharmacological Notch pathway inhibition leads to cell cycle arrest and stimulates ascl1 and neurogenin2 genes expression in dental pulp stem cells-derived neurospheres
Biotechnology Letters (2019)