Key Points
-
Introduces the current knowledge on chairside diagnostic adjuncts available for oral cancer/precancer detection.
-
Provides the proposed uses of available diagnostic adjuncts to the dental practitioner.
-
Uses illustrations to provide a guide to their use.
Abstract
A variety of devices and techniques are now available to aid the clinician in visualising clinical changes that may be found in the oral cavity. These techniques can now be applied at chairside to characterise these changes and many offer a real time result at the point of care. They may assist in a practitioner referring a case earlier to a specialist to undertake further investigations. The translational value of the research published so far has been limited as these technologies have not been adapted for routine use in primary care. This review aims to examine the utility of these adjunctive aids in clinical practice based on the current available evidence.
Similar content being viewed by others
Introduction
Over 50% of oral carcinomas seen in specialist units are advanced in stage at the time of presentation. Cancers of the oral cavity treated in advanced stages have a poor prognosis and their 5-year survival is close to 50% in most countries. The treatment is complex and costly. For those who survive the quality of life is affected with many disabilities particularly in mastication, speech and swallowing. Early detection of oral cancer when the tumour is small significantly improves survival and contributes to less morbidity following therapy. Fortunately, a proportion of oral cancers are preceded by premalignant changes (now referred to as oral potentially malignant disorders [OPMD]) and if such changes are detected before the evolution of a tumour there is the possibility to intervene to reduce the risk of cancer. However, the rates of transformation of these OPMDs vary, their risk assessment is complicated and cannot be easily performed in primary care by clinicians with limited experience in the management of these disorders.
The detection of oral cancer or these potentially malignant disorders is based on clinical visual examination and palpation of the affected site and neck. Most cancers present as new lumps or persistent ulcers and some early stages may be a red or a white patch on the oral mucosa. These signs are not specific for cancer and many benign conditions may have similar clinical presentations. For this reason primary care practitioners have difficulty in detecting these early changes. NICE guidelines1 developed in 2005 (https://www.nice.org.uk/guidance/csg6/) and revised in 2016 to assist the early detection of head and neck cancers are a useful resource. However, audits show that these signs and symptoms lack specificity and the predictive value of referring a case as suspected of oral cancer/with cancer symptoms was low, estimated at 7.9%.2 A tissue biopsy remains the gold standard for confirmation of an oral malignancy and in the UK the patient has to be referred and seen in a specialist unit for such investigations. Due to the potential limitations of the conventional visual oral examination, adjunctive aids to assist in chairside detection of lesions that are suspected of oral cancer or potentially malignant disorders have been developed and are commercially available.
Adjunctive aids
A variety of devices and techniques are now available to aid the clinician in visualising clinical changes that may be found in the oral cavity, and these could be applied at chair-side to characterise the changes. Many of these offer a real time result at the point of care. These adjunctive aids could be broadly divided into three categories as: optical imaging devices; those with high resolution microscopy (in vivo microscopy); and vital staining techniques that use various dyes to delineate any altered mucosa. The optical devices (for example, VELscope, Vizilite, Microlux and Orascoptic) generally detect changes in optical properties of the surface epithelium and submucosa based on light absorption, scattering, or fluorescence of tissue. In vivo microscopy uses specific probes that allow real-time imaging of morphometric features at a gross level that give an impression of nuclear and cellular features of the lining mucosa, for example, confocal microscopy and high-resolution microendoscopy. Staining techniques developed so far use either toluidine blue or Lugol's iodine to map out gross areas of abnormalities. Before we consider these adjunctive aids in detail, it is important to reflect on their proposed uses in the hands of a clinician.
Proposed uses of adjunctive aids
Most adjuncts aids have been developed under the premise that they will serve as diagnostic aids. However, their use can vary depending on the expertise of the user and can be catalogued under several domains of use:
-
As an aid either to detect an occult lesion or to confirm the presence of a visually detected lesion
-
To map out high risk areas when a field change is observed clinically
-
To assess the risk of a potentially malignant disorder
-
To select the optimal site of biopsy
-
For purposes of surveillance of a high risk lesion during follow-up.
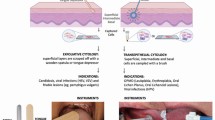
Many manufacturers state on their websites that these systems assist in the early detection of cancer and make similar claims on their promotional literature distributed to dentists and oral health professionals. However, the evidence suggests that at best what these adjuncts would achieve when used in primary care is to accelerate the referral process of a patient from primary care to a secondary care facility. In secondary care facilities these adjuncts may assist in selecting the biopsy site when cancer is suspected (Fig. 1a) and in the surveillance of OPMDs during follow up. Of the many adjunctive techniques that are on the market, this paper provides an overview of the tools that have been widely researched.
VELscope
The Visually Enhanced Lesion Scope (VELscope) is a hand held device originally developed by LED Dental Inc. (White Rock, British Columbia, Canada) jointly with the experts at British Colombia Cancer Agency.3 The VELscope emits a blue excitation light at a wave length 400–460 nm and works on the principle that at this wavelength healthy tissues show auto-fluorescence. This is due to endogenous autofluorescent substances, including collagen and fluorophores such as flavin adinine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NADH), found in healthy oral mucosa. Linkage of collagen fibres, loss of basal lamina and the reduction in flavins due to the Warburg effect in malignancies reduces the emission of autofluorescence. This results in a dark patch and is referred to as loss of fluorescence (FVL) (Fig. 1b). So far 12 studies have been published on the use of this technique for oral cancer/precancer detection mostly in secondary care facilities, of which one was conducted in the UK.4 These published studies give a wide range in the sensitivity and specificity of the VELscope for the detection of oral carcinoma and particularly for OPMDs. A meta-analysis of the reported studies by the Cochrane group5 estimated the test has a sensitivity of 0.91 (0.73–0.97) with a specificity of 0.58 (0.22–0.87). One interesting feature observed through the VELscope is that loss of fluorescence (FVL) often extends beyond the clinically visible lesion suggesting some field cancerisation.
Vizilite
Chemiluminescence has been used for many years as an adjunct in the examination of the cervical mucosa for premalignant or malignant lesions. The ViziLite system involves an oral rinse with 1% acetic acid solution for one minute to help remove surface debris and slightly desiccate the oral mucosa (Fig. 1c). This is followed by direct visual examination of the oral cavity using the chemiluminescent blue-white light stick with an average wavelength of 490 to 510 nm. Normal cells absorb the illumination and appear lightly bluish, whereas abnormal cells having higher nuclear-cytoplasmic ratio reflects the illumination and appear 'aceto-white' with brighter, sharper, more distinct margins. Thirteen studies have used Vizilite for the evaluation of white or red patches suspected of cancer or precancer and, of these, three were conducted in USA,6,7,8 one in Australia9 and one in the UK.10 All have reported an improvement in brightness and sharpness of leukoplakia lesions when compared with conventional visual examination. One disadvantage reported is that chemiluminescence preferentially detects white patches over red lesions. So in effect some high-risk OPMDs which are just red patches may be missed by sole use of the technique.
Microlux
Microlux (AdDent, Inc., Danbury, CT, USA) is a hand-held device that emits a blue-white LED-light and with a wavelength (λ)-ranging from 410 to 710 nm, with peaks at λ = 460nm and λ = 560 nm. The instrument is based on a similar principle to the earlier mentioned chemiluminescence optical devices. McIntosh et al.,11 used Microlux in a specialist clinic and reported the sensitivity of 77.8%; there was an improvement in margin detection but the device failed to detect two dysplastic lesions.
High resolution microendoscopy (HRME)
High-resolution imaging techniques, such as microendoscopy, enable clinicians to visualise in vivo morphometric features of keratinocytes (epithelial cells) as seen through a microscope. HRME images of tissue primarily reveal cell nuclei as discrete bright dots on a dark background (Fig. 2a). Morphological features used by oral pathologists to identify atypical cellular and nuclear features observed in oral epithelial dysplasia and in carcinoma are applied to these images seen via the endoscopy probe (Fig. 2b). Topical contrast agents such as acetic acid and proflavine solution enhance the appearance of cell nuclei (to be graded as enlarged or crowded), allows counting of nuclei, to observe nuclear pleomorphism, and quantify the nuclear-to-cytoplasm (N/C) ratio in real-time. Gillenwater's group have published compulsive data on the application of microendoscopy for the early detection and developed a multimodal optical imaging system (MMIS) to evaluate tissue in situ, at macroscopic and microscopic scales.12 These techniques hold promise for the development of microendoscopy techniques in the future for large scale studies.13
Vital staining with toluidine blue
The most frequently reported adjunctive test to asses oral mucosal abnormalities is the toluidine blue (TB) test. TB is an acidophilic metachromatic dye which in solution takes on a blue violet colour. Published studies have used both mouth rinsing with TB or direct staining with a swab. The dye selectively stains acidic tissue components imparting a blue colour to the mucosa. Once de-stained with acetic acid a positive test is based on the fact that malignant or dysplastic cells may retain the dye. This is explained by the presence of quantitatively more nucleic acid in abnormal tissue and the dye is also able to penetrate the dysplastic epithelium which has some loss of cohesion. These features facilitate the retention of the dye within abnormal tissues, whereas normal mucosa fails to retain the dye. A dark blue (peacock) colouration is considered positive (Fig. 1d) but some areas may demonstrate a light blue (royal blue) which could be considered as equivocal staining. There is no agreement in the literature on whether to include light blue-stained lesions as positive or not. A US group14 have shown that stained foci may contain nuclei with chromosomal abnormalities, but this finding has not been confirmed by any other groups.
False positive results occur due to dye retention in natural crevices or due to uptake by inflammatory, benign ulcerative conditions which have regeneration potential. The manufacturer recommends re application of the vital stain after 2 to 3 weeks to allow healing of benign ulcerations to reduce false positive results. However, this is not practical as patients may become anxious after a positive test result and demand further investigation that may lead to an unnecessary biopsy. False negative results occur when there is thick keratinised mucosa such as in verucous leukoplakia that prevent the penetration of the dye.
A wide range is reported in the sensitivity and specificity of the TB test for the detection of oral carcinoma and particularly for OPMDs. A meta-analysis of 14 reported studies by the Cochrane group5 estimated the test has a sensitivity of 0.84 (0.74–0.90) with specificity of 0.70 (0.59–0.79). Generally carcinomas and higher grades of dysplasias have a higher sensitivity.15 Inclusion of low grade dysplasias in the analyses lowers the sensitivity of the test result.16,17 Inclusion of equivocal test results referred to above lowers the sensitivity of the test as biopsies from these areas may prove to have a normal architecture.
Discussion
Several technologies that may assist in the detection and diagnosis of oral precancer and early cancers are currently in development.18 The objective of these technologies is to optimise the detection of lower stage malignancies (ie stage I/II) or oral epithelial dysplasia in patients, and ultimately to predict which patients with OPMDs are at the highest risk for developing a malignancy.19
With reference to earlier detection of cancer research data, the diagnostic tools to date do not support that the available adjuncts have the capacity to deliver this objective. Several systematic reviews have discussed the sensitivity and specificity of various techniques and concluded that though the sensitivity is moderately good the specificity remains poor. This leads to more false positives being referred from primary care leading to increased anxiety among patients who are suspected to have positive findings. Only few sporadic reports exist as to the detection of occult lesions using any of these adjuncts.
The second objective is to understand the utility of these test systems in the identification of the high risk groups that have a significantly increased risk of cancer due to the presence of high grade OPMDS. The results of ViziLite testing has revealed low sensitivity for the detection of high-risk lesions while TB staining identified high-risk oral premalignant lesions.20 A further study on TB identified high risk lesions with poor outcomes.14 The numbers detected in the high risk category in these studies were small and its difficult to draw meaningful conclusions as there are no long term follow-up studies.
The concept of combining technologies to assess potentially malignant disorders was adapted by Awan et al. who applied both optical and dye techniques to a large cohort of patients seen with potentially malignant disorders. They reported an improvement in the specificity of the test result by combining these tests.21
Based on a recent systematic review that reported on 25 primary studies on light-based adjunctive aids, there is limited evidence for their use in primary care as diagnostic tools.22 They may assist in a practitioner referring a case earlier to a specialist to undertake further investigations ie biopsy. The translational value of the research published so far has been limited as these technologies have not been adapted for routine use.23 Further research and development is needed to propel these adjunctive aids if they are to be used as diagnostic tools for cancer detection or for risk stratification of OPMDs.
References
NICE. Improving outcomes in head and neck cancers. Available at https://www.nice.org.uk/guidance/csg6 (accessed June 2017).
Singh P, Warnakulasuriya S . The two-week wait cancer initiative on oral cancer; the predictive value of urgent referrals to an oral medicine unit. Br Dent J 2006; 201: 717–720.
Lane P M, Gilhuly T, Whitehead P et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt 2006; 11: 10.1117/1.2193157.
Awan K H, Morgan P R, Warnakulasuriya S . Evaluation of an autofluorescence based imaging system (VELscope) in the detection of oral potentially malignant disorders and benign keratoses. Oral Oncol 2011; 47: 274–277.
Macey R, Walsh T, Brocklehurst P et al. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst Rev 2015; 5: Cd010276.
Huber M A, Bsoul S A, Terezhalmy G T . Acetic acid wash and chemiluminescent illumination as an adjunct to conventional oral soft tissue examination for the detection of dysplasia: a pilot study. Quintessence Int. 2004; 35: 378–384.
Epstein J B, Gorsky M, Lonky S, Silverman S Jr, Epstein J D, Bride M . The efficacy of oral lumenoscopy (ViziLite) in visualizing oral mucosal lesions. Spec Care Dent 2006; 26: 171–174.
Kerr A R, Sirois D A, Epstein J B . Clinical evaluation of chemiluminescent lighting: an adjunct for oral mucosal examinations. J Clin Dent 2006; 17: 59–63.
Farah C S, McCullough MJ . A pilot case control study on the efficacy of acetic acid wash and chemiluminescent illumination (ViziLite) in the visualisation of oral mucosal white lesions. Oral Oncol 2007; 43: 820–824.
Awan K H, Morgan P R, Warnakulasuriya S . Utility of chemiluminescence (ViziLite™) in the detection of oral potentially malignant disorders and benign keratoses. J Oral Pathol Med 2011; 40: 541–544.
McIntosh L, McCullough M J, Farah C S . The assessment of diffused light illumination and acetic acid rinse (Microlux/DL) in the visualisation of oral mucosal lesions. Oral Oncol 2009; 45: e227–223.
Muldoon T J, Roblyer D, Williams M D, Stepanek V M, Richards-Kortum R, Gillenwater A M . Noninvasive imaging of oral neoplasia with a high-resolution fibre-optic microendoscope. Head Neck 2012; 34: 305–312.
Pierce M C, Schwarz R A, Bhattar V S et al. Accuracy of in vivo multimodal optical imaging for detection of oral neoplasia. Cancer Prev Res (Phila) 2012; 5: 801–809.
Zhang L, Williams M, Poh C F et al. Toluidine blue staining identifies high-risk primary oral premalignant lesions with poor outcome. Cancer Res 2005; 65: 8017–8021.
Epstein J B, Silverman S Jr, Epstein J D, Lonky S A, Bride M A . Analysis of oral lesion biopsies identified and evaluated by visual examination, chemiluminescence and toluidine blue. Oral Oncol 2008; 44: 538–544.
Awan K H, Yang Y, Morgan P, Warnakulasuriya S . Utility of toluidine blue as a diagnostic adjunct in the detection of potentially malignant disorders of the oral cavity – a clinical and histological assessment. Oral Dis 2012; 18: 728–733.
Cancela-Rodríguez P, Cerero-Lapiedra R, Esparza-Gómez G, Llamas-Martínez S, Warnakulasuriya S . The use of toluidine blue in the detection of pre-malignant and malignant oral lesions. J Oral Pathol Med 2011; 40: 300–304.
Gillenwater A, Papadimitrakopoulou V, Richards-Kortum R. Oral premalignancy: new methods of detection and treatment. Curr Oncol Rep 2006; 8: 146–154.
Kerr A R, Shah S S . Standard examination and adjunctive techniques for detection of oral premalignant and malignant lesions. J Calif Dent Assoc 2013; 41: 329–341.
Chainani-Wu N, Madden E, Cox D, Sroussi H, Epstein J, Silverman S Jr . Toluidine blue aids in detection of dysplasia and carcinoma in suspicious oral lesions. Oral Dis 2015; 21: 879–885.
Awan K H, Morgan P R, Warnakulasuriya S . Assessing the accuracy of autofluorescence, chemiluminescence and toluidine blue as diagnostic tools for oral potentially malignant disorders – a clinicopathological evaluation. Clin Oral Investig 2015; 19: 267–272.
Rashid A, Warnakulasuriya S . The use of light-based (optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: a systematic review. J Oral Pathol 2015; 44: 307–328.
Warnakulasuriya S . Translational research in oral oncology – A bridge between basic science and clinical application. Transl Res Oral Oncol 2016; 1: 1–2.
Acknowledgements
I wish to thank Dr Ann Gillenwater at the University of Texas, MD Anderson Cancer Centre for providing Figure 2 for my use. I also wish to thank Drs Kamran Awan and Helen McParland for assisting me in clinical research studies in testing these devices or agents.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Warnakulasuriya, S. Diagnostic adjuncts on oral cancer and precancer: an update for practitioners. Br Dent J 223, 663–666 (2017). https://doi.org/10.1038/sj.bdj.2017.883
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2017.883
This article is cited by
-
Narrow band imaging observed oral mucosa microvasculature as a tool to detect early oral cancer: an Indian experience
European Archives of Oto-Rhino-Laryngology (2021)
-
Comparative evaluation of autofluorescence imaging and histopathological investigation for oral potentially malignant disorders in Taiwan
Clinical Oral Investigations (2019)
-
Mouth cancer: presentation, detection and referral in primary dental care
British Dental Journal (2018)