Key Points
-
Summarises the development of the plaque biofilm.
-
Highlights that periodontal disease is largely driven by the presence of plaque.
-
Discusses the role of bacteria in the aetiology of periodontal disease.
-
Suggests future treatment strategies for periodontal disease by improving the host's ability to prevent colonisation or by eliminating the microbial species.
Abstract
An appreciation of dental plaque and the host response provides an essential basis from which to understand the disease process and treatment rationale. This information will help the reader to understand not only the way that plaque may have an impact on oral tissues but also why regular effective cleaning may improve periodontal health and why some individuals appear to have a greater susceptibility to periodontitis than others, either intrinsically or in relation to various systemic factors.
Similar content being viewed by others
Biofilms
Dental plaque is a bacterial biofilm which causes chronic gingivitis and periodontitis. Conceptually, one may regard periodontal disease as a host-microbial interaction in which both host and bacterial factors determine the outcome, such that changes in the balance between host and bacterial factors can result in a change from health to disease. The balance may be changed, for example, by a reduction in the host resistance, an increase in the plaque biofilm or an increase in bacterial virulence. The clinical manifestation of periodontal disease is further modified by local and/or systemic factors.boxed-text
Development of the plaque biofilm
The plaque biofilm may be defined clinically as bacterial deposits which cannot be easily rinsed away. It may form on teeth, mucosa or other solid surfaces. These deposits can be readily visualised with vegetable or synthetic dyes in disclosing solutions, and can become calcified to form calculus.
The dental biofilm is an organised bacterial community which forms when a solid structure is placed in an aqueous environment. In the oral cavity, the solid surfaces are either teeth or restorative materials – the metal, ceramics or acrylic in appliances. Dental biofilms differ from biofilms on mucosal surfaces as they form on non-shedding surfaces; stable communities can therefore become established.
In the initial stages of biofilm formation, adsorption of macromolecules (salivary mucins and proteins) results in the formation of an acquired pellicle. Bacteria can readily adhere to these surfaces through adhesins (specific surface receptors). Once attached, the bacteria actively grow and synthesise outer membrane components, which facilitate bacterial adherence. The bacterial mass increases in size due to continued growth of those microbes already adhering to the biofilm, and by the adherence of new microbes. The synthesis of extracellular polymers further facilitates adherence of bacterial species that are unable to adhere directly to pellicle.
The superficial layer is loose and irregular in appearance and is itself bordered by a fluid layer. As the thickness of the biofilm increases, diffusion of nutrients in and out becomes progressively more difficult. Oxygen gradients form as a result of rapid utilisation by superficial bacterial layers and poor diffusion of oxygen through the biofilm matrix. Anaerobic conditions eventually develop. Supragingival plaque obtains nutrients from dietary products dissolved in saliva, whereas microbes in the depths of the periodontal pockets obtain nutrients from the periodontal tissues, gingival crevicular fluid, blood supply or other microorganisms.
The primary colonisation consists of aerobic and facultative anaerobes such as Gram-positive cocci (e.g. streptococci). Gram-positive rods appear, increase in number and eventually outnumber the cocci. Gram-positive filaments, such as Actinomyces spp., may later predominate. There are specific surface receptors on the Gram-positive cocci and rods that allow the adherence of Gram-negative bacteria, which otherwise lack the ability to attach directly to pellicle. As time progresses there is a shift in the microflora from Gram-positive to Gram-negative organisms, and an increase in heterogeneity of the microbial species.
Stable bacterial communities are established with nutrients being exchanged between different microbes and also the production of bacteriocins (which kill specific bacteria). The local environment may protect growing plaques, for example in stagnation areas where the microbes are effectively housed away from the self-cleansing actions within the oral cavity. Specific bacterial communities are then established in different sites, according to the local environments, with differences existing between the shallow gingival crevice compared with a deep periodontal pocket, a flat enamel surface compared with a fissure. These communities are more resistant to antibiotics and effectively require much higher doses to exert a microbicidal effect as a result of the complex inter-relationships within these bacterial communities.
Role of bacteria in periodontal disease
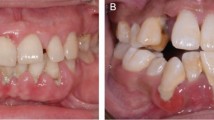
Although there is evidence that bacterial plaque/biofilms play a major role in the aetiology of periodontal disease, it is not clear whether the bacteria initiate disease non-specifically or specifically (Fig. 1). There are now three hypotheses about the role of bacteria in periodontal disease, which largely ignore, however, the role of host factors (see the 'Host responses' section below).
Non-specific plaque hypothesis
Periodontal disease is due to bacterial accumulation, irrespective of its composition.
This implies that no one specific bacterial species is any more significant than another in its ability to cause periodontal disease. The implication from the non-specific plaque hypothesis is that all patients must maintain a high standard of oral hygiene to prevent periodontal disease, as all bacteria are perceived as playing a role. Although the amount of plaque present may correlate well with disease severity in cross-sectional studies, it correlates poorly in longitudinal studies. This hypothesis does not consider variations in the dental biofilm that may affect its pathogenicity or, most importantly, host determinants.
Specific plaque hypothesis
Periodontal disease is the result of an infection with a single specific pathogen.
This may help to explain why there are many patients who have considerable plaque deposits but only a minority suffer from severe destructive periodontitis. The implications from the specific pathogen hypothesis are that one need only worry about the bacterial pathogen responsible for periodontal disease, and therefore need only employ procedures that lead to the elimination of this species and not all other bacterial species. Treatment could specifically target the identified pathogen as one would do for any other monospecific infection such as tuberculosis or syphilis.
If one assumes that the 'real' pathogen is a strict anaerobe, it may be unnecessary to eliminate all plaque deposits and sufficient to either eliminate the pathogen or promote the development of a new community where anaerobes are unable to survive. This may be achieved by simply disrupting the biofilm and could explain the success that is achieved by root surface debridement. Special-risk patients might be identified by the presence of the specific pathogen in the oral cavity. This might lead to treatments targeted at specific bacteria, employing antibiotic chemotherapy once the antibiotic sensitivities are known, or newer therapeutic measures including vaccinations, or the use of peptides to prevent bacterial adherence and the ensuing colonisation. However, to date no one pathogen has been specifically linked to chronic gingivitis or periodontitis. The increased relative risk of aggressive periodontitis in adolescent Moroccans who have Aggregatibacter actinomycetemcomitans, particularly the JP2 clone, is the most compelling evidence for a role of a single species in periodontal disease.
Multiple pathogen hypothesis
Periodontal disease is the result of infection with a relatively small number of interacting bacterial species.
One major difficulty lies in identifying the possible combinations of pathogens that are important. It should be appreciated that this current list of periodontopathogens may be superseded once the results from bacterial culture and isolation using molecular techniques are combined and reinterpreted. One could, nevertheless, arbitrarily determine antibiotic sensitivities of the top ten periodontopathogens and then employ these antibiotics to eliminate the organisms.
Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Treponema denticola, Fusobacterium nucleatum and Campylobacter spp. are present in diseased sites and have been implicated in disease progression. However, this in itself does not prove that they are responsible for tissue damage, and indeed one may argue that these organisms are more likely to be found in deeper pockets and at the sites with more inflammation simply because they thrive in such ecological niches.
Difficulties in establishing the aetiology of periodontal disease
One may wonder why it is so difficult to identify the cause of such a relatively common disease. There are many problems in trying to determine which organisms are important in the pathogenesis of periodontal disease:
-
Over 600 species may be identified from the periodontal pockets of different individuals; there may be between 30 and 100 species for any single site
-
The habitat determines what can grow in periodontal pockets and so the bacteria one finds may be proliferating as a consequence of this environment
-
Many species are difficult to grow or cannot be grown. Molecular techniques recognising DNA sequences enable us to identify even more species, but without viable microbes antibiotic sensitivities cannot be determined
-
Difficulties in taking a representative sample from the pocket. There are inevitably a number of different species, and therefore there is a strong possibility that contaminating species will complicate analysis and interpretation
-
If there are subtle shifts in the bacterial communities at active phases of disease, the time of sampling may be critical
-
The current classification of periodontal diseases based on clinical features may not allow us to distinguish between different types of destructive periodontal disease. Each disease grouping in our current classification may represent highly heterogeneous groups
-
Different sites in the mouth may break down as a result of different pathogens, and sites may show activity due to one pathogen at one time and another pathogen at a later time
-
It is difficult to distinguish between opportunistic species secondarily proliferating as a result of the disease rather than as the cause of the disease
-
If there are multiple pathogens it is extremely difficult to evaluate the possible pairs or mixtures of species that may be involved in the disease process. Pathogens such as P. gingivalis may be found in low numbers in healthy individuals free of destructive periodontal diseases. Different strains may be virulent or avirulent
-
The individual variation in immune responses to pathogens may be the most important factor.
Host responses
One should remember that both chronic gingivitis and periodontitis are chronic inflammatory lesions and therefore display inflammation as well as attempts at healing. One can detect the cardinal signs of inflammation and various degrees of repair. Not all signs have to be present in order to establish that inflammation is present; for example, pain and loss of function is neither necessarily present nor common in these conditions.
Innate immunity
The host immune response may be conveniently divided into innate and adaptive immunity (Fig. 2). Both innate and adaptive immunity operate together and not in isolation, complementing each other to maintain health and prevent disease.
Innate immune mechanisms include a number of relatively non-specific mechanisms, including the barrier effect of an intact epithelium. Oral mucosa is bathed in saliva, which contains a number of protective factors. Bacteria can be recognised by non-clonal receptors, otherwise known as pattern recognition receptors. These receptors recognise substances such as lipopolysaccharide (LPS) from Gram-negative bacteria and peptidoglycan from Gram-positive bacteria. Innate responses are relatively non-specific and there is therefore greater potential for bystander damage to tissues.
Neutrophils appear to be crucial for the maintenance of periodontal health, as disease severity is increased in neutropenia, agranulocytosis and where cellular function is impaired, such as leukocyte adhesion deficiency, lazy leukocyte disease and Papillon-Lefèvre, Chediak-Higashi and Down's syndromes, as well as diabetes mellitus.
Adaptive immunity
The adaptive immune response is characterised by specificity, memory and the capacity to distinguish self from non-self. Once recognition of microbial antigens has taken place by the appropriate receptor on macrophages or dendritic cells, then cytokines are released which activate T and B cells, thereby engaging cell-mediated and humoral immune responses. The two arms of immunity therefore function together; the earlier responses being predominantly innate, subsequently helping to focus adaptive immune responses. In humoral or cell-mediated immunity, specificity of the responses is thought to limit bystander damage by focusing the adaptive or specific immune system.
Secretory IgA (sIgA) protects mucosal surfaces, mainly by preventing bacterial adherence. If bacteria cannot adhere to this epithelial surface then they are less likely to be able to cause infection. sIgA is different from serum IgA in that it consists of dimers of IgA held together by a joining chain (or J-chain) and protected from proteolytic breakdown with secretory component.
Gingival crevicular fluid originates from gingival capillaries and contains some immune cells. The fluid flow is continuous into the gingival crevice but increases during gingival inflammation. Antibodies such as IgG, IgA and IgM, complement components, as well as enzymes are found in it. These soluble proteins and cellular components then enable innate and adaptive responses to occur at the site of bacterial challenge.
Histopathology of periodontal disease
The histopathology of periodontal disease may be divided into initial, early, established and advanced lesions based on studies of experimental gingivitis in humans and periodontitis in animals (Table 1). Although one should be careful about how this evidence is interpreted, it nevertheless provides a useful framework from which to construct an understanding of disease pathogenesis.
It is worth noting that an established lesion can persist for many years and the change to an advanced lesion marks the transition from a chronic and successful defence reaction to a destructive immunopathological mechanism (periodontitis). The factors responsible for this progression are unknown. There are two principal schools of thought:
-
1
Host immune response may be involved
-
2
Some specific microorganism in bacterial plaque, or change in virulence, may be responsible for the development of the advanced lesion.
The biggest problem is establishing which factors are responsible for the transition from reversible gingivitis to destructive periodontitis. There is no real evidence to suggest that destructive periodontitis necessarily develops from pre-existing gingivitis. Some preceding gingival inflammation may lead to periodontitis but this is not always the case.
The 'advanced' lesion is clinically recognised as periodontitis, with the classical features of pocket formation, ulceration of the pocket's epithelium, destruction of the collagenous periodontal ligament, and bone resorption. These changes lead to mobility and eventually to tooth loss. There is an extension of the inflammatory infiltrate apically and laterally, with reduction of the collagen content and a dense accumulation of lymphocytes, plasma cells and macrophages. Peripherally there is reparative fibrosis. There is a breakdown of the epithelial barrier between plaque and periodontal connective tissue, which might be associated with a significant change in the immune response and permit direct access of plaque antigens and metabolites. At this stage there is irreversible loss of attachment, and loss of periodontal ligament and bone with progressive increase in pocket formation. High concentrations of IgG, IgA, IgM and complement as well as polymorphs can be found in gingival crevicular fluid. However:
-
Antibodies are inconsistently invoked in periodontitis and do not appear to protect from disease
-
Antibody titres may rise during treatment but this is more likely a consequence of instrumentation effectively inoculating antigens
-
Antibody titres may reflect more the intrinsic mitogenicity or immunogenicity of plaque antigens rather than the importance of any one microbial organism in the pathogenesis of periodontal disease.
The mechanisms involved in mediating tissue damage are more easily described as hypersensitivity-like responses and include antibody-mediated, cellular cytotoxicity, and IgEmediated hypersensitivity-like reactions.
Mechanisms of tissue destruction
Bacteria can cause damage directly and indirectly. The various mechanisms are described in Table 2. Cytotoxic cellular immune responses to self, and pro-inflammatory responses involving release of interleukin 1β (IL-1β), tumour necrosis factor α (TNF-α) and interleukin6 (IL-6) could lead to tissue destruction (Table 3).
Conclusion and potential future treatment strategies
Periodontal disease represents a complex interaction between host and microbes. In future one could employ strategies which improve the host's ability to prevent bacterial colonisation or eliminate the important microbial species. For example, attention has been focused on eliciting immune responses to adhesins and proteases of Porphyromonas gingivalis. Alternatively, preformed anti-adhesin anti-bodies could be produced and applied to dental surfaces to prevent attachment and bacterial colonisation.
If one believes that the presence of plasma cells is linked to the ensuing tissue destruction in periodontitis, then one could tip the balance in favour of diminishing the humoral response by altering the pro-inflammatory/anti-inflammatory antibody responses, (Th1/Th2 CD4 T helper cell populations) so that plasma cell differentiation is much reduced or absent.
Suppressing exaggerated host responses, restoring balance or regulation of dysregulated cytokine networks, or tipping the Th1-type response to a Th2 response (anti-inflammatory but with a protective humoral response) could lead to prevention of disease progression. Intervention, for example in the form of receptor-mediated antagonism, could help regulate the complex cytokine networks, thereby limiting bystander damage at foci of inflammation. However, there are so many potential antigens that could be affecting the outcome it is unlikely that such immunomodulation could be of clinical benefit to patients in the near future.
There is far more heterogeneity in human CD4 T cell populations in terms of their cytokine expression than has previously been thought, making it difficult to identify T cell populations as either Th1 or Th2 subsets. Given this difficulty, the Th1/Th2 paradigm has been developed to include other T cell subsets: Th17 cells and regulatory T cells (Treg). Th17 cells can produce pro-inflammatory IL-17 and, under the influence of other cytokines such as IL-12, can develop into Th1 cells. There is also developmental plasticity between Treg cells, such that in the appropriate cytokine milieu (IL-6 and IL-21) they can switch to Th17 cells. This functional plasticity offers potentially different therapeutic approaches, in which Th17 and/or Treg cells are the immunological targets rather than Th1 or Th2 subsets or the elusive microbial antigens.
Notes
This series represents chapters 2, 9 and 10 from the BDJ Book A clinical guide to periodontology, 3rd ed, edited by Richard Palmer, Mark Ide and Peter Floyd. All other chapters are published in the complete clinical guide available from the BDJ Books online shop.
References
Further reading: Reviews
Buduneli N, Kinane D F . Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol 2011; 38: 85–105.
Karimbux N Y, Saraiya V M, Elangovan S et al. Interleukin-1 gene polymorphisms and chronic periodontitis in adult whites: a systematic review and meta-analysis. J Periodontol 2012; 83: 1407–1419.
Marsh P D, Devine D A . How is the development of dental biofilms influenced by the host? J Clin Periodontol 2011; 38: 28–35.
Nussbaum G, Shapira L . How has neutrophil research improved our understanding of periodontal pathogenesis? J Clin Periodontol 2011; 38: 49–59.
Palmer R M, Wilson R F, Hasan A S, Scott D A . Mechanisms of action of environmental factors – tobacco smoking. J Clin Periodontol 2005; 32: 180–195.
Preshaw P M, Taylor J J . How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol 2011; 38: 60–84.
Wade W G . Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J Clin Periodontol 2011; 38: 7–16.
Further reading: Background/historical literature
Moore W E C, Moore L H, Ranney R R, Smibert R M, Burmeister J A, Schenkein H A . The microflora of periodontal sites showing active destructive progression. J Clin Periodontol 1991; 18: 729–739.
Page R C, Shroeder H E . Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest 1976; 33: 235–249.
Seymour G J, Gemmell E, Reinhardt R A, Eastcott J, Taubman M A . Immunopathogenesis of chronic inflammatory periodontal disease: cellular and molecular mechanisms. J Periodontol Res 1993; 28 (6 pt2): 478–486.
Socransky S S, Haffajee A D . The bacterial etiology of destructive periodontal diseases: current concepts. J Periodontol 1992; 63: 322–331.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Hasan, A., Palmer, R. A clinical guide to periodontology: Pathology of periodontal disease. Br Dent J 216, 457–461 (2014). https://doi.org/10.1038/sj.bdj.2014.299
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2014.299
This article is cited by
-
Gender differences in oral health among prisoners: a cross-sectional study from Taiwan
BMC Oral Health (2023)
-
Combination effect of laser diode for photodynamic therapy with doxycycline on a wistar rat model of periodontitis
BMC Oral Health (2021)
-
A built-in adjuvant-engineered mucosal vaccine against dysbiotic periodontal diseases
Mucosal Immunology (2019)