Key Points
-
Citrox® is a bioflavonoid-containing product derived from citrus fruit, available in two formulations that have been used in a range of cleansers and disinfectants.
-
Citrox® showed substantial antimicrobial activity against a range of oral bacteria and Candida species.
-
Citrox® bioflavonoid preparations may be useful antimicrobial agents in future mouthwash and oral care products.
Abstract
Background Citrox® is a formulation of soluble bioflavonoids obtained from citrus fruits. The non-toxic and antimicrobial properties of natural bioflavonoids are well documented, and consequently there has been interest in the therapeutic application of these substances.
Objective To determine the antimicrobial activity of two Citrox® formulations (BC30 and MDC30) with different bioflavonoid combinations against a range of oral microorganisms.
Methods The antimicrobial activity of both formulations was tested against 14 bacterial species and six Candida species. The two Citrox® formulations (dilution range 0.007–8% v/v) were firstly evaluated by determining the in vitro Minimal Inhibitory Concentration (MIC) against planktonic microorganisms in a broth microdilution assay. Secondly, the ability of the same serial dilutions to inhibit microbial growth was assessed in a modified microtitre biofilm assay.
Results Both Citrox® formulations exhibited antimicrobial activity. The BC30 formulation demonstrated greater activity than MDC30 and significantly inhibited growth of all bacterial species and most candidal species tested at a concentration of 1% (v/v) in both the broth and the biofilm assay.
Conclusion Bioflavonoid preparations of Citrox® have a broad-spectrum of antimicrobial activity against oral microorganisms, and as such have the potential to be used within therapeutic preparations for the control of the oral microflora.
Similar content being viewed by others
Introduction
A variety of antimicrobial mouthwashes are available commercially and these have been shown to be clinically beneficial in the management of oral disease.1,2,3,4 However, for a variety of reasons the search for novel and more effective antimicrobial agents continues. In addition, adverse effects associated with some available preparations, in particular unpleasant taste and staining of the teeth, have reduced patient acceptability and compliance.5 Furthermore, the presence of alcohol in some mouthwashes has been shown to cause mucosal irritation in certain patients, particularly those with mucositis, and is felt to be inappropriate by some because of the association of denatured alcohol with the development of oral cancer.4,6,7,8 Finally, the long term use of antimicrobial agents has raised concern regarding the potential for an undesirable shift in the composition, site colonisation and emergence of resistance within the complex oral microflora.9,10
In recent years an association between members of the oral microflora and the development of some forms of systemic diseases has been reported. There is increasing evidence that poor control of the oral flora and severe periodontal disease may be important factors in the onset and progress of coronary heart disease and diabetes.11 In addition, it is well documented that the composition of the oral flora in hospitalised and debilitated patients undergoes an early microbial shift to one predominated by Gram-negative bacteria. It is now recognised that plaque can therefore act as a reservoir for potential pathogens, including highly resistant microorganisms, for infection at other body sites.12,13,14,15 Specifically, it has been demonstrated that the oropharyngeal microflora has a role in ventilator-associated pneumonia, and the use of therapeutic preparations containing either chlorhexidine or essential oils can reduce the incidence of this significant infection.16 As the number of immunocompromised individuals in the population continues to increase, so too does the incidence of mucosal infections in the mouth, in particular oral candidosis. Given the concern over the emergence of resistance of yeasts to systemic antifungal agents, there is a clinical requirement for new and effective topical anticandidal strategies.17 It is against this background that the need for alternative antimicrobial agents with improved antimicrobial profiles and fewer adverse effects becomes greater.
There has been considerable interest in the use of 'natural' antimicrobial agents. Natural antimicrobial agents can be defined as bioactive compounds derived from biological sources. Although traditional antibiotics strictly fall into this definition, there is concern regarding the prophylactic use of antibiotics due to the high potential for promotion of microbial resistance.18 However, there is a range of alternative non-microbial 'natural agents' that have antimicrobial activity and the effectiveness of plant extracts, such as bacteriocins, defence peptides and phenolics has been demonstrated.19,20,21 Polyphenolic plant derivatives, which are part of a plant's natural defence mechanisms against viral and bacterial pathogens, have been the main focus of investigation.
In addition to greater antimicrobial activity and improved safety profiles, there is also a perception that natural agents may be more acceptable to patients. Although the scientific evidence is unclear, there is a view that natural agents may be less likely to promote the development of resistance.18 Many of the 'natural' antimicrobials have the added advantage that they may be used in aqueous solution, removing the need for inclusion of alcohol in therapeutic preparation.
Citrox® is an antimicrobial whose components are based on soluble bioflavonoids derived from citrus fruits. Bioflavonoids are hydroylated phenolic structures synthesised by plants and have previously been shown to have activity against bacteria, fungi and viruses.22,23,24,25 Citrox® BC and Citrox® MDC formulations both contain bioflavonoids with the former comprising of a blend of bioflavonoids with small amounts of malic and citric acids, designed to be primarily anti-bacterial. Citrox BC is present in OralClens® mouthrinse and toothpaste, while MDC is currently used in a range of sanitising products for surface disinfection. The aim of the present study was to determine the antimicrobial activity of these two Citrox® formulations against a variety of bacterial species encountered in the mouth, including those implicated in periodontal disease, and a range of candidal species. The formulations would be assessed against the test strains in planktonic state and within in vitro-generated biofilms.
Methods
Preparation of microorganisms
A total of eight bacterial species and six fungal species (Table 1) were used to evaluate the Citrox® formulations. All bacteria were initially cultured on Fastidious Anaerobic Agar (FAA) supplemented with 5% defibrinated sheep blood (TCS Biosciences Ltd., Buckingham, UK) and then, before use in experiments, in Brain-Heart Infusion (BHI) broth. Candida species were cultured on Sabouraud's dextrose agar (SDA) and subsequently in liquid Sabouraud's medium. All media, unless otherwise stated, was obtained from Lab M™ (International Diagnostics Group plc, Bury, UK). Candida isolates and the two streptococcal species were maintained at 37°C under aerobic conditions, whereas the remaining six species of bacteria were grown in an anaerobic environment (10% v/v CO2, 20% v/v H2, 70% v/v N2) at 37°C.
Planktonic assay
The two formulations of Citrox® (BC30 and MDC30) were first assessed with regard to their antimicrobial activity against planktonic suspensions of the test species. In these experiments an overnight culture of each strain was prepared in the appropriate liquid medium to a turbidity level equal to a MacFarland standard 3.0. Serial dilutions were then made of the two Citrox® formulations using either BHI or liquid Sabouraud's medium as the diluent. A 100 μl volume of each Citrox® dilution was added to an equal volume of the microbial suspension, giving a range of Citrox® concentration between 0.007% and 8% (v/v). Controls included bacterial suspensions containing no Citrox® and un-inoculated broth.
A 200 μl volume of the mixed preparations was incubated in 96-well microtitre plates for 24 hours at 37°C, under the appropriate atmospheric conditions. After incubation, the relative amount of each microbial species was estimated by measuring the turbidity of the well by spectrophotometric absorbance at 544 nm. Absorbance readings were standardised using 'microbial-free' Citrox® dilutions. As recommended by Espinel-Ingroff and Cantón,26 the minimal inhibitory concentration (MIC) value was recorded as the lowest concentration of Citrox® that showed ≥80% reduction in absorbance compared to the control.
Biofilm assay
The concentrations of both Citrox® formulations required to inhibit the growth of microbial biofilms were determined. Suspensions of each organism (MacFarland standard 3.0) were incubated for 24 hours at 37°C in the appropriate broth and atmospheric conditions, without agitation so as to allow the formation of biofilms. The medium was then removed by gentle aspiration and the biofilm washed with 200 μL of phosphate buffered saline (PBS) to remove planktonic cells. Fresh medium containing Citrox® at concentrations ranging between 0.007–8% v/v was then added to the biofilm. Each antimicrobial concentration was prepared in triplicate and a control broth containing no Citrox® was also used. Biofilms were then incubated for a further 24 hours without movement under the same conditions as before. The medium was subsequently removed by gentle aspiration and the biofilm again washed with PBS. Fresh broth was added and the biofilms disrupted by pipetting and agitation. The turbidity of the resuspended biofilm was then observed by measuring the absorbance at a wavelength of 620 nm. A second absorbance reading (at 620 nm) was taken after a further incubation over 6 hours. The relative growth of the microorganisms was then determined by the change in absorbance over this 6 hours period. The mean value was calculated from triplicate results and the MIC recorded as the lowest concentration of Citrox® that demonstrated a ≥80% reduction in absorbance compared to the control.
Results
Planktonic assay
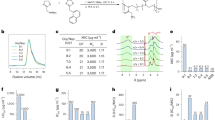
The MICs of each of the two Citrox® formulations against the 14 microorganisms are shown in Table 2 and Figure 1. The planktonic growth of all of the microorganisms studied was inhibited by Citrox® BC30. The growth of most of the species did not appear to be significantly inhibited by Citrox® MDC30, even when it was present at the highest concentration 8% (v/v) used in this study. The MIC for each microorganism was lower with BC30 than MDC30, with the exception of Porphyromonas gingivalis for which the MIC of both formulations was the same at 2% (v/v). Overall, this suggested that BC30 was more effective than MDC30 at inhibiting microbial growth. Furthermore, as can be seen in Figure 1, BC30 inhibited the growth of each microorganism at concentrations of between 1-2% (v/v).
Biofilm assay
The MIC values recorded in biofilm assay for both formulations are shown in Table 3 and Figure 2. In general the BC30 formulation was more effective at inhibiting the growth of microorganisms. One notable exception was that MDC30 appeared to be more effective against C. albicans and C. dubliniensis biofilms compared to BC30, for which there was no apparent growth inhibition even with the highest concentration of 8% (v/v).
Discussion
There is a need for novel antimicrobial agents with improved activity and safety profiles. A range of substances extracted from plants have shown promise in this respect. Peel extracts from Citrus sudachi have been found to have antimicrobial activity against bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and Helicobacter pylori.23 Other bioactive plant extracts, containing flavonoids and phenols as major components, have proven effective against MRSA and a range of Gram-negative bacteria.27,28 From the oral perspective, naringin, a flavonoid extract from grapefruit, has shown promise in the growth inhibition of A. actinomycetemcomitans and P. gingivalis in planktonic phase.29 Polyphenols extracted from hop extracts and cranberry have shown activity against streptococcal species associated with dental caries30,31,32 and a citrus extract combined with lemon juice and lemon grass has been used successfully to treat oral candidosis.33
The results of the present study show for the first time the impact of a citrus fruit bioflavonoid based product (Citrox®) on the growth of a range of oral microorganisms. Both formulations of Citrox® tested showed substantial antimicrobial activity, with BC30 exhibiting biofilm MIC values below 0.5% for all bacterial strains tested. Although the MIC values are not directly comparable, the antimicrobial activity and range compare favourably with those reported for chlorhexidine, an essential oil mouthwash and a herbal mouthwash containing grapefruit seed extract when tested against a similar range of oral bacteria.34 In general the BC30 formulation demonstrated higher activity against both bacteria and yeasts. Interestingly, the two species of Candida demonstrating a high MIC with the BC30 preparation (C. albicans and C. dubliniensis) were significantly more susceptible to the MDC30 formulation. For these two species MDC30 demonstrated greater activity than that previously reported for chlorhexidine.35 Citrox® MDC contains the same bioflavonoids as BC30 but, in addition, incorporates citric acid and choline ascorbate.
The antimicrobial activity of the bioflavonoid preparations when tested against bacterial and fungal biofilms is significant and of direct clinical relevance. Biofilms can be defined as microbial cells attached to a surface and organised into structured communities embedded within a matrix of extracellular material that has been produced by the biofilm cells.36 Bacterial dental plaque is perhaps the most widely studied biofilm due to its association with caries and periodontal disease and there is increasing interest in the nature of biofilms formed by Candida spp.37 It is widely recognised that both the biofilm structure and the phenotype of cells within a biofilm can afford protection against both host defence processes and administered antimicrobial agents.36,38 Enhanced resistance of a biofilm to an antimicrobial has been related to failure of the agent to diffuse the biofilm, sequestering of the agent within the biofilm matrix, and the presence of persister cells within the biofilm that have a low activity status that promotes their survival in the presence of an antimicrobial. Studies have shown that antimicrobial activity is elevated up to 500-fold in biofilms when compared with equivalent planktonic studies.39 Potentially useful antimicrobial agents must therefore demonstrate activity against bacteria in both planktonic and biofilm states.35 In this context, it is of interest that chlorhexidine at its working concentration of 0.2% does not appear to effect a total kill in a biofilm and that the essential oil-containing preparations would appear to have greater activity against microorganisms in biofilm state than planktonic phase.35,40
Bacterial biofilm susceptibility to Citrox BC30 was exhibited at concentrations below 1%. However, elevated MICs were recorded against biofilms generated by the two closely related yeast species of C. albicans and C. dubliniensis. As in the planktonic assay, the MICs of the MDC30 preparations were correspondingly low for these species. Further investigation is required to explain the mechanisms behind this phenomenon.
In summary, the present study has demonstrated that working concentrations (1-2% v/v) of Citrox® BC30 are effective at inhibiting the growth of a range of bacteria and Candida when cultured in either broth suspension or as a biofilm. In addition, Citrox™ MDC demonstrated activity against microorganisms exhibiting high MICs with BC30. The findings support the further investigation of both formulations of Citrox® as potentially significant antimicrobial agents in future mouthwash preparations and other oral care products.
References
Addy M, Moran J M . Evaluation of oral hygiene products: science is true; don't be misled by the facts. Periodontol 2000 1997; 15: 40–51.
Pitten F A, Splieth C, Kramer A . Prophylactic and therapeutic application of antimicrobial agents in the oral cavity. Pharmazie 2000; 55: 635–639.
Barnett M L. The role of therapeutic antimicrobial mouthrinses in clinical practice: control of supragingival plaque and gingivitis. J Am Dent Assoc 2003; 134: 699–704.
Silverman S Jr, Wilder R. Antimicrobial mouthrinse as part of a comprehensive oral care regimen. Safety and compliance factors. J Am Dent Assoc 2006; 137(Suppl): 22S–26S.
Foote R L, Loprinzi C L, Frank A R et al. Randomized trial of a chlorhexidine mouthwash for alleviation of radiation-induced mucositis. J Clin Oncol 1994; 12: 2630–2633.
Winn D M, Blot W J, McLaughlin J K et al. Mouthwash use and oral conditions in the risk of oral and pharyngeal cancer. Cancer Res 1991; 51: 3044–3047.
Elmore J G, Horwitz R I . Oral cancer and mouthwash use: evaluation of the epidemiologic evidence. Otolaryngol Head Neck Surg 1995; 113: 253–261.
Sreenivasan P, Gaffar A . Antiplaque biocides and bacterial resistance: a review. J Clin Periodontol 2002; 29: 965–974.
Sullivan A, Edlund C, Nord C E . Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 2001; 1: 101–114.
Shapiro S, Giertsen E, Guggenheim B . An in vitro oral biofilm model for comparing the efficacy of antimicrobial mouth rinses. Caries Res 2002; 36: 93–100.
Rautemaa R, Lauhio A, Cullinan M P, Seymour G J . Oral infections and systemic disease--an emerging problem in medicine. Clin Microbiol Infect 2007; 13: 1041–1047.
Johanson W G, Pierce A K, Sanford J P . Changing pharyngeal bacterial flora of hospitalized patients. Emergence of gram negative bacilli. N Engl J Med 1969; 281: 1137–1140.
Valenti W M, Trudell R G, Bentley D W . Factors predisposing to oropharyngeal colonization with gram-negative bacilli in the aged. N Engl J Med 1978; 298: 1108–1111.
Dantas S R, Moretti-Branchini M L. Impact of antibiotic-resistant pathogens colonizing the respiratory secretions of patients in an extended-care area of the emergency department. Infect Control Hosp Epidemiol 2003; 24: 351–355.
El-Solh A A, Pietrantoni C, Bhat A et al. Colonization of dental plaques: a reservoir of respiratory pathogens for hospital-acquired pneumonia in institutionalized elders. Chest 2004; 126: 1575–1582.
Chan E Y, Ruest A, Meade M O, Cook D J . Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ 2007; 334: 889.
Belazi M, Velegraki A, Koussidou-Eremondi T et al. Oral Candida isolates in patients undergoing radiotherapy for head and neck cancer: prevalence, azole susceptibility profiles and response to antifungal treatment. Oral Microbiol Immunol 2004; 19: 347–351.
Sang Y, Blecha F . Antimicrobial peptides and bacteriocins: alternatives to traditional antibiotics. Anim Health Res Rev 2008; 9: 227–235.
Hamill P, Brown K, Jenssen H, Hancock R E . Novel anti-infectives: is host defence the answer? Curr Opin Biotechnol 2008; 19: 628–636.
Alviano D S, Alviano C S . Plant extracts: search for new alternatives to treat microbial diseases. Curr Pharm Biotechnol 2009; 10: 106–121.
Jenssen H, Hancock R E . Antimicrobial properties of lactoferrin. Biochimie 2009; 91: 19–29.
Aqil F, Ahmad I, Owais M . Evaluation of anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and synergy of some bioactive plant extracts. Biotechnol J 2006; 1: 1093–1102.
Nakagawa H, Takaishi Y, Tanaka N, Tsuchiya K, Shibata H, Higuti T . Chemical constituents from the peels of Citrus sudachi. J Nat Prod 2006; 69: 1177–1179.
Oliveira A C, Shinobu C S, Longhini R, Franco S L, Svidzinski T I . Antifungal activity of propolis extract against yeasts isolated from onychomycosis lesions. Mem Inst Oswaldo Cruz 2006; 101: 493–497.
Tait S, Salvati A L, Desideri N, Fiore L . Antiviral activity of substituted homoisoflavonoids on enteroviruses. Antiviral Res 2006; 72: 252–255.
Espinel-Ingroff A, Cantón E . Antifungal Susceptibility Testing of Yeasts. In Schwalbe R, Steele-Moore L, Goodwin A C (eds) Antimicrobial Susceptibility Testing Protocols. pp 173–207. Boca Raton: CRC Press, 2007.
Aqil F, Khan M S, Owais M, Ahmad I . Effect of certain bioactive plant extracts on clinical isolates of beta-lactamase producing methicillin resistant Staphylococcus aureus. J Basic Microbiol 2005; 45: 106–114.
Ahmad I, Aqil F . In vitro efficacy of bioactive extracts of 15 medicinal plants against ESbetaL-producing multidrug-resistant enteric bacteria. Microbiol Res 2007; 162: 264–275.
Tsui V W, Wong R W, Rabie A B . The inhibitory effects of naringin on the growth of periodontal pathogens in vitro. Phytother Res 2008; 22: 401–406.
Shinada K, Tagashira M, Watanabe H et al. Hop bract polyphenols reduced three-day dental plaque regrowth. J Dent Res 2007; 86: 848–851.
Smullen J, Koutsou G A, Foster H A, Zumbé A, Storey D M . The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res 2007; 41: 342–349.
Bodet C, Grenier D, Chandad F, Ofek I, Steinberg D, Weiss E I . Potential oral health benefits of cranberry. Crit Rev Food Sci Nutr 2008; 48: 672–680.
Wright S C, Maree J E, Sibanyoni M . Treatment of oral thrush in HIV/AIDS patients with lemon juice and lemon grass (Cymbopogon citratus) and gentian violet. Phytomedicine 2009; 16: 118–124.
Haffajee A D, Yaskell T, Socransky S S . Antimicrobial effectiveness of an herbal mouthrinse compared with an essential oil and a chlorhexidine mouthrinse. J Am Dent Assoc 2008; 139: 606–611.
Meiller T F, Kelley J I, Jabra-Rizk M A, DePaola L G, Baqui A, Falkler W A Jr. In vitro studies of the efficacy of antimicrobials against fungi. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 91: 663–670.
Donlan R M, Costerton J W . Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002; 15: 167–193.
Seneviratne C J, Jin L, Samaranayake L P . Biofilm lifestyle of Candida: a mini review. Oral Dis 2008; 14: 582–590.
Mukherjee P K, Chandra J . Candida biofilm resistance. Drug Resist Updat 2004; 7: 301–309.
Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M . Microbial biofilms. Ann Rev Microbiol 1995; 49: 711–745.
Filoche S K, Soma K, Sissons C H . Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol Immunol 2005; 20: 221–225.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed paper
Rights and permissions
About this article
Cite this article
Hooper, S., Lewis, M., Wilson, M. et al. Antimicrobial activity of Citrox® bioflavonoid preparations against oral microorganisms. Br Dent J 210, E22 (2011). https://doi.org/10.1038/sj.bdj.2010.1224
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2010.1224
This article is cited by
-
Effectiveness of mouthwashes on reducing SARS-CoV-2 viral load in oral cavity: a systematic review and meta-analysis
BMC Oral Health (2023)
-
Effects of bioflavonoid-containing mouth rinses on optical properties of tooth-coloured dental restorative materials
Scientific Reports (2022)
-
An Overview of Endocrine Disrupting Chemical Paraben and Search for An Alternative – A Review
Proceedings of the Zoological Society (2021)
-
Anti-biofilm efficacy of a medieval treatment for bacterial infection requires the combination of multiple ingredients
Scientific Reports (2020)