Key Points
-
The practice of BTA is effective in the field of facial aesthetics over placebo, and lasts between 4-6 months.
-
There are few adverse effects of BTA if injected using careful technique.
-
The incidence of blepharoptosis, associated with injecting BTA in glabellar lines, ranges between 0-5.4%.
-
Further randomised controlled studies are necessary to establish long term effects of BTA and the effects of repeated injections.
Abstract
Background The use of botulinum toxin type A (BTA) in facial aesthetics for the treatment of wrinkles has recently become more popular as an alternative to surgical techniques. However, its true efficacy and potential adverse effects are still unclear.
Objectives The primary objective of this study was to review the efficacy of BTA in facial aesthetics. A secondary objective was to determine whether there are any adverse effects associated with the procedure of using BTA in facial aesthetics.
Search strategy We conducted literature searches on Medline (1977 to January 2009), Cochrane Controlled Trials Register (CENTRAL), EMBASE (1977 to January 2009) and CINAHL (1977 to January 2009). The search strategy also included reference lists of located articles and hand searching for randomised controlled trials (RCTs). We contacted authors of studies for further information where required.
Selection criteria Randomised studies comparing BTA with placebo in facial aesthetics in a double-blind and crossover or parallel group design.
Data collection and analysis Two reviewers independently assessed trial quality and extracted data. The area of face injected, assessment methods, outcome measures, duration of action of BTA and associated adverse effects were reviewed.
Results and discussion A total of eleven RCTs involving 1,603 subjects were found, of which 1,203 were enrolled for treatment with BTA. The 11 trials were not directly comparable to each other due to differences in the areas of the face injected with BTA, length of study period, concentration of BTA used and outcome measures. The studies showed similar trends. The use of BTA showed improvements in facial wrinkles over placebo, with a peak effect reported at around one month and the effects lasting between 4-6 months. No studies reported any severe adverse effects. The incidence of blepharoptosis in glabellar lines treated with BTA was reported to be between 0-5.4%, and may be related to the technique of injection into the muscles. The incidence of other side-effects such as headache, pain at injection site and mild bruising was similar in both the BTA and placebo groups.
Authors' conclusions The use of BTA in facial aesthetics is more effective than placebo. The incidence of adverse effects associated with BTA is similar to placebo, with the exception of blepharoptosis which is reported to be 0-5.4% after treatment of glabellar lines with BTA.
Similar content being viewed by others
Background
Botulinum neurotoxin is produced by the gram-negative anaerobic bacterium Clostridium botulinum. There are eight serologically distinct botulinum neurotoxins; types A (BTA) and B (BTB) are most commonly used in human medicine for the treatment of conditions such as dystonia, hyperhidrosis, strabismus, gustatory sweating syndrome, alleviation of pain, overactive bladder, achalasia and anal fissure.1,2 The use of BTA in the orofacial region, such as masseteric hypertrophy, Frey's syndrome, sialorrhoea, chronic facial pain and hemifacial spasm has also been reported in literature.3 Other uses of botulinum toxin are being explored including its use in cancer therapy.4
A wide range of non-surgical techniques, including BTA, are becoming increasingly popular for the treatment of facial lines (wrinkles).5 The wrinkles are a consequence of ageing of the facial tissues, which involves combined changes in skin quality, volume depletion, decreased tissue elasticity, redistribution of subcutaneous fullness, progressive bone resorption and gravitational descent.6 The use of BTA allows a low cost and accessible alternative to surgery. In turn, many health professionals including plastic surgeons, GPs, dentists and beauticians practice facial aesthetics within their profession. The most common applications for BTA in the head and neck area are in the horizontal forehead lines, glabellar (frown) lines, lateral canthal lines (crow's feet), lips and platysma muscle in the neck.7
The Independent Healthcare Advisory Service (IHAS) defines cosmetic treatments as non-surgical, non-incisional (although in some cases piercing of the skin may be involved) procedures that revise or change the appearance, colour, texture or structure of bodily features to achieve what patients perceive to be more desirable.8 The use of BTA for facial aesthetics is increasing in popularity and this has opened the debate on whether this can be considered as part of the practice of dentistry. This article will determine the effectiveness and adverse effects associated with the practice of BTA in facial aesthetics.
Objectives
The primary objective of this study was to review the efficacy of botulinum toxin A (BTA) in facial aesthetics. A secondary objective was to determine whether there are any adverse effects associated with the procedure of using BTA in facial aesthetics.
Criteria for considering studies for this review
Inclusion
The studies included in our review were randomised controlled trials that compare BTA to placebo in facial aesthetics in a double-blind and crossover or parallel group design.
Exclusion
-
1
Randomised controlled trials using BTA as a therapeutic agent in correcting medical conditions
-
2
Randomised controlled trials comparing two BTA manufacturers
-
3
All other studies not classified as RCT, ie case reports, opinions etc.
Types of participants
We included trials recruiting patients of either sex (male or female), and we did not apply any age restrictions. A baseline record of the severity of the wrinkles at the start of trials must be specified in the papers for inclusion.
Types of intervention
Intramuscular injection of BTA or placebo. We allowed all administration schedules, manufacturers and injection techniques, with or without electromyography (EMG) guidance.
Assessment and outcome measures
For each trial, we identified the number of patients originally allocated to each treatment group. For each outcome measure, we tried to determine the numbers improving in the placebo and active BTA treatment groups. A brief description of the assessment of the facial wrinkles and outcome measures that were used in the RCTs is shown in Tables 1 and 2 respectively.
Search methods for identification of studies
We conducted searches from 1977, which was the first year when botulinum toxin was used therapeutically. The search was carried out using the keywords botox and dentistry; botulinum toxin and dentistry; botulinum toxin type A and dentistry; botox and facial aesthetics; botulinum toxin and facial aesthetics; botulinum toxin type A and facial aesthetics; facial aesthetics and dentistry; adverse effects of facial aesthetics; adverse effects of botox; adverse effects of botulinum toxin; and adverse effects of botulinum toxin type A.
A literature search for the relevant trials was carried out from the following sources:
-
1
MEDLINE (1977 to January 2009)
-
2
Cochrane Controlled Trials Register (CENTRAL) (1977 to January 2009)
-
3
EMBASE (1977 to January 2009)
-
4
CINAHL (1977 to January 2009).
We screened titles, keywords and abstracts of the citations downloaded from the electronic searches and obtained full copies of reports of potentially relevant trials for further assessment.
The search strategy also included:
-
1
Reference lists of located trials and botulinum toxin review articles
-
2
Hand-search for randomised controlled trials comparing botulinum toxin A and placebo
-
3
Where necessary, we contacted authors of published trials for further information via email.
Method of review
The studies were appraised by two independent assessors (KG and ADW) using a simple checklist adapted and modified from the Critical Appraisal Skills Program (CASP) available on the NHS Public Health Resource Unit Appraisal Tool website.9 We resolved disagreements about inclusion and exclusion by discussion.
The two authors independently assessed full papers of all included RCTs for any risk of bias using the Cochrane collaboration tool.10 The RCTs were assessed for methodological quality by extracting details of randomisation methods, blinding of treatments and assessments, whether or not intention-to-treat analysis was possible from the published data, definition of outcome, and entry and exclusion criteria. We also looked for any causes of bias in these papers. Both authors discussed the quality of papers and resolved any disagreements by discussion. Where disagreements occurred due to differences in interpretation, further information was requested from the authors of the trials.
The methodology, assessment tools, outcome measures, and adverse effects of the RCTs were analysed and discussed to allow comparison of the RCTs. Any data collected in an open-phase period was not included in this review.
Meta-analysis was not carried out to compare the RCTs due to the differences in the manufacturers and concentration of BTA used, the area of face treated and differences in assessment methods and outcome measures.
Results
Results of the search
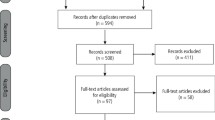
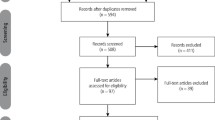
Using the search criteria for the keywords described above, all studies using BTA in non-facial areas and in the treatment of medical conditions were excluded. After duplicate studies were removed, the above search resulted in a collection of 64 papers reflecting the use of BTA in the facial area; only seven of these were randomised controlled trials that compared the use of BTA over placebo in facial aesthetics.11,12,13,14,15,16,17 Full-length articles of these 64 papers were obtained and assisted in a hand search to locate any other randomised controlled trials comparing BTA to a placebo in facial aesthetics. The hand search resulted in a further four double-blind RCTs to be included in our study.18,19,20,21
Description of studies
The 11 RCTs included in our review paper and their backgrounds are summarised in Table 3.
These studies used either Botox (Allergan, Inc, Irvine, CA, USA) or Dysport (Ipsen Ltd, Slough, UK) in the following facial areas:
-
Glabellar lines (n = 6)
-
Crow's feet (n = 2)
-
Chin (n = 1)
-
Glabella and central forehead region (n = 1)
-
Crow's feet and forehead (n = 1).
The concentration of the BTA in Allergan cannot be compared to or converted into units of BTA in Dysport due to differences in the assays used by the manufacturers. The placebo commonly used was either sterile preservative-free normal saline (n = 7), or all constituents present in the active group without the active ingredient BTA (n = 4). In two papers, the BTA manufacturer used was not specified.20,21 The studies were conducted in countries including the USA, Canada, France, UK, Germany and Belgium.
The mean age and standard deviation (sd) of patients in each of the studies included in this review is summarised in Table 4. The mean ages of patients included in this review ranged between 31 and 50.9 years. The sex and race distribution of the participants in the studies is shown in Table 3. Most patients included in this study were females (mean percentage range: 66.67%-100%) and Caucasian (mean percentage range 61.67%-100%). The term 'unknown' is recorded where the mean age, sd or sex is not specified in the papers.
The RCTs included were all short term (4 to 24 weeks). In two studies, patients receiving placebo treatment crossed over to receive the BTA treatment. The results beyond this point were recorded in an open phase period of the trial, and therefore not included in our review. In none of the studies did patients cross over from the active BTA treatment group to receive placebo. The remaining nine studies were of the parallel-group design. In one study, the injection was EMG guided and in the others they were freehand. The total dose used per participant varied between trials.
Risk of bias in included studies
The risk of bias assessment, as agreed by both authors, is illustrated in Figures 1 and 2. These were generated by using Review Manager, [(RevMan) Version 5.0, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008].
A green circle marked with '+' sign shows the RCT has mentioned clearly the randomisation process, indicating low risk of bias. A red circle marked with '−' shows the RCT has not mentioned the randomisation protocol, indicating high risk of bias. Where the information is unclear, the box is not marked. Generated by RevMan Version 5.0, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2008
Further information on the quality of the studies was requested from four studies; response to our email requests was obtained from authors of only one of these.20 Two studies had only enrolled female patients in their study.18,21 In one study on forehead and crow's feet, the authors revealed that the sides of the forehead to be injected with the BTA or placebo were randomised; however at one week there was very little blinding since the results of treatment became obvious, with one side of the forehead having little motion while the other had normal motion.21
Effects of interventions
a) Glabellar lines
Two formulations of BTA were commonly used in the glabellar area, as seen in Table 3: Botox and Dysport. Four out of the seven studies compared Botox formulations to placebo (Table 5) while three out of the seven compared Dysport formulation to placebo (Table 6).
Reduction in glabellar lines from BTA use occurred within one week post-injection, both at maximum frown and at rest. The magnitude of effect at week one was marginally smaller than the peak effect, which was at around four weeks post-injection. The studies report that the effect of BTA appeared to gradually reduce over 3-6 months. The reduction in glabellar line severity was more pronounced at maximum contraction than the reduction in severity at rest. However, the reduction in glabellar line severity at rest was sustained longer than the reduction in severity at maximum frown. The studies also show a good agreement between investigators' ratings and those of the participants, where both outcome measures were used.
The results from one study on glabellar lines show a mean severity score at baseline as 4.0 and this changes to 1.8 at week six following BTA administration.19 Baseline score for the placebo group was 3.86 and at week six was 3.78. The RCT uses a scale of 0 to 3 for evaluation of wrinkle severity, therefore it was not clear how a baseline score exceeded the maximum value on the assessment scale. Further correspondence with the author clarified that the scores were a combined severity score at rest and frown, ie, a possible maximum of 6.
b) Lateral canthal lines (crow's feet), forehead and chin
The peak benefit of BTA in crow's feet was seen between weeks four to eight post-injection and the effect lasted up to 4 months. Patients noted an improvement and satisfaction with treatment. The studies noted little difference between 12U and 18U BTA used in crow's feet and recommend 12U application. The 18U BTA can be used in patients with very severe crow's feet for optimum results. However, no additional safety concerns were raised at the higher dose group of 18U. The effect of BTA injection showed greater improvement at maximum contraction; however, the effect of BTA is sustained longer at rest compared to maximum contraction.
When BTA was used in the forehead the mean reduction of wrinkles in the forehead at rest and maximum contraction was higher than placebo. The data on the scores as recorded by the blinded assessors was requested from the authors; however they did not have access to the data as the study was over 15 years old.20
The maximum improvement in chin wrinkles also occurred four weeks post-injection of BTA. The effect of BTA over placebo was prominent when assessment was carried out at maximum contraction, but this effect diminished slowly over the 12-week recall period.
c) Adverse effects
The incidence of any adverse event was monitored and recorded in both the active and placebo groups. An adverse effect was classified as 'serious' if it was life threatening or resulted in death, hospitalisation, or a persistent or significant disability or incapacity considered to be related to BTA treatment. The patients were asked about any symptoms or unexpected occurrences. Of the 11 studies included in this review, one paper had not recorded or reported any adverse effects.21 In some studies, the relationship of the adverse effect to the study was assessed as probable, possible, not related or not assessable. The intensity was assessed as mild, moderate, severe or not assessable. Some of the vital signs, such as blood pressure, heart rate, haematology and blood biochemistry were also recorded.
The incidence of the adverse effects reported is shown in Table 7. The results of any statistical analysis to compare the incidence of adverse effects in active vs placebo groups were noted. The reported adverse effects in all the included studies appeared to occur without any statistically significant difference between the active (BTA) group and placebo, with the exception of blepharoptosis in glabellar lines as discussed below.
Four out of the seven papers studying the effect of BTA in glabellar lines reported incidences of blepharoptosis (drooping of the upper eyelid) of between 0% and 5.4%.12,13,14,17 In the first paper, blepharoptosis was resolved with an average duration of 20-40 days.12 In the second paper, the incidence of blepharoptosis was 1% and classified as mild, but there was no mention of how soon it was resolved.13 The third paper had a very low incidence of blepharoptosis affecting 1 in 73 patients (0.01%) that started 13 days after injections, was considered mild, had improved by week four and was not visible by week 12.14 The fourth paper reported an incidence of 0.8% and classified it as mild. There was no indication of the length of resolution period.17 A fifth paper also reported blepharoptosis, however this occurred in an open-phase study following the period of the double-blind randomised study and hence was not reported here.14
Discussion
Quality of evidence
This review found that there is a low number of randomised controlled trials of the use of BTA in facial aesthetics, which is the gold-standard evaluation method for evidence-based medicine and dentistry. We found a total of 11 RCTs that we considered suitable for this review. In general, the quality of included trial was good as assessed by the Jadad Score.22 Two of the studies may be criticised for enrolling only female subjects.18,21 Three studies may also be criticised for using the same patients to receive active BTA on one side of the midline and the placebo on the other side; this reduces blinding and increases the bias during assessment of facial wrinkles when one side responds more than the other.16,20,21 Four of the included studies can be criticised for providing inadequate data on sequence generation, allocation concealment, blinding process or variable reporting of data on a per-protocol or intention-to-treat basis.14,17,20,21
The mean age of patients included in this review ranged between 31 and 50.9 years. Most patients included in this study were females (mean percentage range: 66.67%-100%) and Caucasian (mean percentage range 61.67%-100%). This reflects the aesthetic nature of the procedure and may bias results, as it is a self-selecting group and not a reflection of the population at large.
Methodology
The literature search was carried out using four different English-speaking databases. Studies relevant to the keywords were also hand-searched to improve chances of finding more randomised controlled trials. The included studies were all short term (4-24 weeks), double-blind, parallel studies performed in a randomised control fashion. Any results from a cross-over design or second injection of BTA were from an open phase study, and therefore excluded from our review. The RCTs were not directly comparable to each other due to different areas of the face being injected with BTA, different lengths of study period, different manufacturers and concentrations of BTA, and different outcome measures. Therefore no meta-analysis was performed. In clinical practice, the concentration of Dysport (Ipsen) cannot be compared to Botox (Allergan) due to differences in the assays used by the manufacturers.23
Findings
The use of BTA in glabellar lines, crow's feet, forehead and chin showed similar and consistent results. The effect of BTA was seen immediately within one or two weeks of injection, with a peak effect at an average four weeks. The effect of BTA was sustained for between 4-6 months; this effect was higher when the facial wrinkles were assessed at rest compared to at maximum contraction. We did not find any studies that looked at the effects of repeated injection with BTA in a double-blind randomised fashion.
Adverse events associated with the use of BTA were low in the studies included in our review when compared with placebo. Blepharoptosis, which was often associated with use of BTA in glabellar lines, was reported in four out of the seven papers, ranging between 0% and 5.4% of patients. There was no report of permanent ptosis; one paper showed resolution after between 20-40 days. The dose levels used, the low injection volume and the anatomic location of the injections may have minimised the effects of diffusion to the nearby levator muscles of the upper eyelids, thus avoiding ptosis. This, however, remains a risk factor if the BTA is not administered appropriately. Following all the other adverse effects reported in the studies, the patients recovered without sequelae. The use of BTA treatment had few or no side-effects in the immediate vicinity of the injections and no systemic effects. One of the papers suggested injecting into the corrugator muscle 1 cm above the supraorbital ridge and avoiding injecting near the levator palpebrae superioris as precautions to reduce the incidence of blepharoptosis.12 Headaches and other adverse effects seemed to occur in both the treatment groups and are therefore more likely to be related to the overall effect of the injection procedure than to BTA treatment or other factors.
Authors' conclusion
The use of BTA in non-surgical facial cosmetic procedures produces high rates of improvement with rapid onset and long duration of action (longer than four months for some patients) when compared to placebo. The effects of BTA were sustained for longer at rest than at maximum muscle contraction. The incidence of adverse effects associated with BTA is similar to placebo, with the exception of blepharoptosis which is reported to be 0-5.4% after treatment of glabellar lines with BTA. Further studies are necessary to fully understand the long-term duration of effect of botulinum toxin and any effects associated with repeated injections. This will also help clinicians determine a suitable inter-injection interval.
References
Truong D D, Jost W H . Botulinum toxin: clinical use. Parkinsonism Relat Disord 2006; 12: 331–355.
Kostrzewa R M, Segura-Aguilar J. Botulinum neurotoxin: evolution from poison, to research tool – onto medicinal therapeutic and future pharmaceutical panacea. Neurotox Res 2007; 12: 275–290.
Bhogal P S, Hutton A, Monaghan A . A review of the current uses of botox for dentally-related procedures. Dent Update 2006; 33: 165–168.
Ansiaux R, Gallez B . Use of botulinum toxins in cancer therapy. Expert Opin Investig Drugs 2007; 16: 209–218.
Wise J B, Greco T . Injectable treatments for the aging face. Facial Plast Surg 2006; 22: 140–146.
Coleman S R, Grover R . The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthet Surg J 2006; 26(1S): S4–S9.
Fedok F G. Advances in minimally invasive facial rejuvenation. Curr Opin Otolaryngol Head Neck Surg 2008; 16: 359–368.
Independent Healthcare Advisory Services. Standards for injectable cosmetic treatments. London: Independent Healthcare Advisory Services, 2008. Available online at http://www.independenthealthcare.org.uk (accessed 1 January 2009).
NHS Public Health Resource Unit. Appraisal tools webpage. http://www.phru.nhs.uk/Pages/PHD/resources.htm (accessed 10 January 2009).
Higgins J P T, Green S (eds). Cochrane handbook for systematic reviews of interventions. http://www.cochrane-handbook.org/ (accessed 10 January 2009).
Lowe N J, Ascher B, Heckmann M, Kumar C, Fraczek S, Eadie N ; Botox Facial Aesthetics Study Team. Double-blind, randomized, placebo-controlled, dose-response study of the safety and efficacy of botulinum toxin type A in subjects with crow's feet. Dermatol Surg 2005; 31: 257–262.
Carruthers J A, Lowe N J, Menter M A et al.; BOTOX Glabellar Lines I Study Group. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol 2002; 46: 840–849.
Carruthers J D, Lowe N J, Menter M A et al. Double-blind, placebo controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg 2003; 112: 1089–1098.
Rzany B, Ascher B, Fratila A, Monheit G, Talarico S, Sterry W . Efficacy and safety of 3- and 5-injection patterns (30 and 50 U) of botulinum toxin A (Dysport) for the treatment of wrinkles in the glabella and the central forehead region. Arch Dermatol 2006; 142: 320–326.
Ascher B, Zakine B, Kestemont P et al. A multicenter, randomized, double-blind, placebo controlled study of efficacy and safety of 3 doses of botulinum toxin A in the trearment of glabellar lines. J Am Acad Dermatol 2004; 51: 223–233.
Lowe N J, Lask G, Yamauchi P, Moore D . Bilateral, double-blind, randomized, comparison of 3 doses of botulinum toxin type A and placebo in patients with crow's feet. J Am Acad Dermatol 2002; 47: 834–840.
Monheit G, Carruthers A, Brandt F, Rand R . A randomized, double-blind, placebo-controlled study of botulinum toxin type A for the treatment of glabellar lines: determination of optimal dose. Dermatol Surg 2007; 33(1 Spec No.): S51–S59.
Fagien S, Cox S E, Finn J C, Werschler P, Kowalski J W . Patient reported outcomes with botulinum toxin type A treatment of glabellar rhytids. A double blind, randomised, placebo-controlled study. Dermatol Surg 2007; 33(1 Spec No.): S2–S9.
Lowe N J, Maxwell A, Harper H . Botulinum A exotoxin for glabellar folds: a double-blind, placebo-controlled study with an electromyographic injection technique. J Am Acad Dermatol 1996; 35: 569–572.
Keen M, Blitzer A, Aviv J et al. Botulinum toxin A for hyperkinetic facial lines: results of a double blind, placebo controlled study. Plast Reconstr Surg 1994; 94: 94–99.
Beer K, Yohn M, Closter J . A double-blinded, placebo-controlled study of Botox for the treatment of subjects with chin rhytids. J Drugs Dermatol 2005; 4: 417–422.
Jadad A R, Moore R A, Carroll D et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12.
Sampaio C, Costa J, Ferreira J J . Clinical comparability of marketed formulations of botulinum toxin. Mov Disord 2004; 19(Suppl 8): S129–S136.
Acknowledgements
The authors wish to acknowledge the help and assistance of the British Dental Association Library, in particular Helen Nield and Damyanti Raghvani, and the editors and reviewers of the British Dental Journal for their encouragement and review of the work that was undertaken.
Both authors had full access to all the data in the study and the final responsibility for the decision to submit for publication rested with the corresponding author. Mr Gadhia is employed as a Senior House Officer at Birmingham Dental Hospital and Professor Walmsley is Professor of Restorative Dentistry and is employed by the University of Birmingham. He is also Scientific Advisor to the British Dental Journal and British Dental Association. Neither author has any financial or personal relationships with the products mentioned in this article and has no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed paper
Rights and permissions
About this article
Cite this article
Gadhia, K., Walmsley, A. Facial aesthetics: is botulinum toxin treatment effective and safe? A systematic review of randomised controlled trials. Br Dent J 207, E9 (2009). https://doi.org/10.1038/sj.bdj.2009.813
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2009.813
This article is cited by
-
A systematic review of botulinum toxin in the management of patients with temporomandibular disorders and bruxism
British Dental Journal (2019)
-
Clinical uses of botulinum toxin A in smile aesthetic modification
British Dental Journal (2018)
-
Are Nurse Injectors the New Norm?
Aesthetic Plastic Surgery (2014)
-
A comparative assessment of three formulations of botulinum toxin A for facial rhytides: a systematic review and meta-analyses
Systematic Reviews (2013)
-
Summary of: 'Facial aesthetics: is botulinum toxin treatment effective and safe? A systematic review of randomised controlled trials'
British Dental Journal (2009)