Abstract
The availability of data on the feeding habits of species of conservation value may be of great importance to develop analyses for both scientific and management purposes. Stomach flushing is a harmless technique that allowed us to collect extensive data on the feeding habits of six Hydromantes species. Here, we present two datasets originating from a three-year study performed in multiple seasons (spring and autumn) on 19 different populations of cave salamanders. The first dataset contains data of the stomach content of 1,250 salamanders, where 6,010 items were recognized; the second one reports the size of the intact prey items found in the stomachs. These datasets integrate considerably data already available on the diet of the European plethodontid salamanders, being also of potential use for large scale meta-analyses on amphibian diet.
Design Type(s) | observation design • time series design • species comparison design |
Measurement Type(s) | Gastric Content |
Technology Type(s) | light microscopy |
Factor Type(s) | temporal_interval • Season • organism • geographic location |
Sample Characteristic(s) | Hydromantes ambrosii • Hydromantes flavus • Hydromantes genei • Hydromantes imperialis • Hydromantes sarrabusensis • Hydromantes supramontis • cave |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Similar content being viewed by others
Background & Summary
The European cave salamanders, (Hydromantes, for further taxonomy information see, (ref. 1)) are the only plethodontid salamanders occurring in Europe2. The genus Hydromantes includes three species endemic to California and eight species endemic (or sub-endemic) to Italy: five (H. flavus, H. supramontis, H. imperialis, H. sarrabusensis, H. genei) are endemic to Sardinia, the remaining three (H. italicus, H. ambrosii, H. strinatii) are distributed along Apennine one of the mainland species (H. strinatii) is also present in a small part of SW France2–4. Moreover, some individuals have been introduced in some European countries5–7. Several European Hydromantes are all listed as vulnerable or endangered species (I.U.C.N. Red List) and therefore strictly protected by the European laws8.
Hydromantes species are fully terrestrial salamanders able to exploit several environments, from forest floors to cracks and bare rocks2,9,10. However, when local climate becomes unsuitable (too hot and/or harsh), Hydromantes salamanders seek refuge underground, where microclimatic conditions are generally suitable all year round11–14. In underground environments Hydromantes species show stable populations, reaching high densities and being able to carry out most of their biological functions15–17. The elusive behavior of these salamanders, combined with the intrinsic complexity of underground environments, often strongly reduces feasibility of data collection; in fact, there is still a paucity of information about biology, ecology and behavior of most of the species of the genus2. For example, until recently, the reproduction of Hydromantes salamanders was only observed in controlled conditions2,18,19; just in the last few years the first observations and researches on Hydromantes nesting ecology in natural environments have been performed16,17.
One of the most important aspects that need to be studied concerns feeding ecology and diet composition. Until today, studies on the diet of Hydromantes were performed only on three European species20–22, while for others there is no information2. Diet is a dynamic feature characterizing individuals throughout their life23,24. Resource requirements depend on life stage and therefore individuals focus their feeding activity on specific food resources25–27. Within species range, populations likely occupy areas characterized by different resource assemblages, which in turn can shape species diet at local level28. Moreover, seasonality may produce food resource fluctuations, forcing species to adapt their feeding habits to the available ones29,30. Finally, individual diet can be affected by the presence of competitors31–33. The availability of exhaustive data on the different species diet may be of fundamental importance for ecological and zoological researches in order to define species patterns and strategies.
In the present work, we report a large dataset on the diet of six European Hydromantes species (H. flavus, H. supramontis, H. imperialis, H. sarrabusensis, H. genei and H. ambrosii), considering different seasons and numerous populations. For most of these species, as mentioned above, no data on the diet and feeding behavior are available. During a three-year survey, we analysed the stomach contents of different salamanders populations, sampling individuals from underground environments. We produced two different datasets: one contains data on the salamanders’ stomach contents, while the second the maximum length of the intact prey items. Future studies are planned to include data on H. strinatii and H. italicus in the dataset, and increase the number of sampled populations. The datasets can also be combined with those of other amphibian species, in order to compare local and macro-scale information.
Methods
Experimental design
We adopted the following methodology to collect the data of the two datasets (Data Citation 1):
-
1
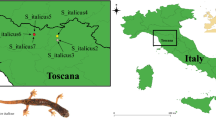
We sampled at least 3 populations per species, for a total of 19 different underground sites (i.e., caves) (Fig. 1).
Figure 1: Map of the study area. The distribution of each studied species (polygons obtained combining published and unpublished data2,40) and the studied populations (stars). Maps were created with the program QGIS using data from http://www.diva-gis.org/gdata.
-
2
Multiple sampling was repeated from 2015 to 2017.
-
3
Sampling was performed in different seasons: May/June 2016 and 2017 (beginning of the hot season; hereafter spring) and early September 2015 and 2016 (end of the hot season; hereafter autumn). In these periods, salamanders intensify their foraging activity in- and outside the caves10,11 for both, the upcoming aestivation and to recover after the summer inactivity period2.
-
4
For each population, we performed a minimum of 3 samplings, in different years and seasons.
-
5
We sampled at least 170 individuals per species.
-
6
In each site, we sampled males, females and juveniles (see Salamanders sampling).
-
7
Stomach contents were obtained by stomach flushing and contents preserved in ethanol 75% (see Stomach flushing).
-
8
Stomach contents were examined at an optic microscope and, when possible, prey items were counted and ascribed to the lower possible taxonomic level (see Stomach contents analysis).
-
9
Intact prey items, were measured at the maximum length (see Stomach contents analysis).
Salamander sampling
All surveys were performed during day time (9 a.m. – 6 p.m.). Caves where explored entirely or up to the point reachable without speleological equipment. We divided the cave environments into parts of 3 linear meters each (hereafter, sector) using a laser meter (Anself RZE-70, accuracy 2 mm), starting from the cave entrance to the maximum reachable point (for further explanation see (ref. 13)). We actively searched and captured salamanders using sterile disposable gloves. Each survey ended when salamanders were no longer observed for 15 min. After capture, salamanders were temporarily placed in sterile fauna boxes until stomach flushing was performed (maximum 2 h); when possible, we registered the position (the cave sector) of the captured salamanders. Salamanders’ snout-vent length (SVL) was measured using a transparent plastic ruler where salamanders. Salamanders were sexed by checking the presence/absence of males’ secondary sexual characters (mental gland and pre-maxillary teeth)2. Individuals below the size of the smallest male were considered juveniles, as females differ from juveniles only by body-size, without providing any other recognisable feature2. The smallest recorded male’ SVL was 40 mm for H. ambrosii and H. genei, while 45 mm for all other species.
Stomach flushing
We used stomach flushing to obtain salamander stomach contents; a harmless technique already tested on Hydromantes salamanders34. We used a 5 ml syringe filled with tap water; where the needle was replaced by a plastic pipe of 1 mm diameter. Salamander was first positioned upside down (Fig. 2a) and the free extremity of the pipe was carefully inserted into its mouth, reaching the stomach. Once the pipe was in position, water was gently injected while salamander’s belly was massaged; the reflux was collected in a collection tube using a small funnel. Stomach flushing was performed only on salamanders with SVL≥30 mm, as this method could be too invasive for small individuals. In larger salamanders (SVL≥70 mm) when the first reflux was without content, the flushing was repeated one more time in order to confirm the stomach emptiness. The stomach content was fixed in 75% ethanol. After flushing, salamanders were released at the point of captured.
Stomach contents analysis
Stomach contents were examined in the lab using an optical microscope. Prey items were recognised (when possible) at the order level, with the exception of the Staphylinidae (among Coleoptera) and Formicidae (among Hymenoptera) which were considered separately because of their peculiar ecology along with easy morphological identification; the general works of Sabelli and Chinery35,36 were taken as guiding references for the identification of the different consumed prey items. In some cases, it was also possible to distinguish arthropods' at their different stages of development (for more information see Data Records). We defined three different categories of "stomach content": "empty", without prey; "not-identifiable", when the advanced stage of digestion prevented any identification to at least the order level; "full", when at least one prey item was recognizable. For each full stomach content the minimum number of recognizable items was counted according to (ref. 5). For each typology of prey item, we counted the prey items as the sum of a) whole prey, b) heads, c) residual of single abdomens and single heads (only when abdomens > heads). Considering that stomach contents often contained numerous prey segments, when single appendices were recognisable with confidence (e.g., pincers, elytra), and no head/abdomen were matching with them, we added to the previous count the half (rounded up) of the appendices sum. Using a digital microscope (MAOZUA 5MP 20×–300×) we took pictures of the intact prey items and measured the maximum length (mm) using a built-in software (Fig. 3).
Code availability
No code was used in this study.
Data Records
The first dataset (Data on the diet of Hydromantes salamanders, Data Citation 1) consists of:
-
1
1,250 salamander samples from 6 different Hydromantes species (average 208.33±10.35 individuals per species), divided in a) 319 individuals with empty stomach (average±SD per species; 53.17±38.93), b) 370 with non-recognizable contents (61.67±39.93) and c) 561 individuals with full stomach (93.5±48.74). Given that each population was sampled up to 4 times, and individuals were not marked, each individual could be present more than ones in the dataset.
-
2
5,996 recognized invertebrate prey items (average±ES per individual, 10.69±0.70) belonging to 40 different taxa (Pulmonata, Sarcoptiformes, Mesostigmata, Trombidiformes, Araneae, Pseudoscorpiones, Opiliones, Lithobiomorpha, Geophilomorpha, Scolopendromorpha, Julida, Polydesmida, Isopoda, Symphypleona, Poduromorpha, Entomobryomorpha, Zygentoma, Ephemeroptera, Odonata_ninfa, Orthoptera, Blattodea, Psocoptera, Hemiptera, Endopterygota_larva, Hymenoptera, Hymenoptera_Formicidae, Coleoptera, Coleoptera_Staphylinidae, Coleoptera_larva, Neuroptera, Trichoptera_larva, Lepidoptera, Lepidoptera_larva, Diptera, Diptera_larva, Archaeognatha, Tricladida, Gordea, Nematoda, Haplotaxida).
-
3
10 recognized Vertebrate's prey items: 6 skin residuals, 3 eggs and 1 Hydromantes juvenile (Fig. 2b–d).
-
4
NA means no specific data existing. SVL and position were not always recorded; in case of empty stomachs, NA was added to all other columns; if contents were not identifiable, NA was added to all prey typologies.
Detailed explanation of dataset "Data on the diet of Hydromantes salamanders" (Data Citation 1) is given in Table 1.
The second dataset ("Measures of intact prey items", Data Citation 1) consists of:
-
1
352 intact invertebrate prey items measured.
Detailed explanation of dataset "Measures of intact prey items" (Data Citation 1) is given in Table 2.
Technical Validation
Sites were surveyed once per season to avoid individual resampling. During each season, all surveys were performed within 30 days to limit variation of climate conditions, which can in turn affect prey composition. Considering differences in environmental conditions characterizing the studied sites, populations could show different phenology; indeed, during our surveys, within the same period in some cases we observed high population densities while in others no individuals were observed. Surveys on multiple years and season were performed to avoid biased data collection37. Blinded stomach contents analyses were performed to further reduce any possible bias38.
Usage Notes
Dataset is provided in CSV format, which can be used with the free statistic program R39. Before starting the analyses, the linear distance of individuals from the cave entrance should be square-root transformed (hereafter, depth), while the number of prey items should be log transformed to improve normality and reduce skewness. Precise coordinates of the studied caves are not shown as species are strictly protected.
Additional information
How to cite this article: Lunghi E. et al. Field-recorded data on the diet of six species of European Hydromantes cave salamanders. Sci. Data 5:180082 doi: 10.1038/sdata.2018.83 (2018).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
References
Wake, D. B. The enigmatic history of the European, Asian and American plethodontid salamanders. Amphibia-Reptilia 34, 323–336 (2013).
Lanza, B., Pastorelli, C., Laghi, P. & Cimmaruta, R. A review of systematics, taxonomy, genetics, biogeography and natural history of the genus Speleomantes Dubois, 1984 (Amphibia Caudata Plethodontidae). Atti Mus. Civ. Stor. Nat. Trieste 52, 5–135 (2006).
Sillero, N. et al. Updated distribution and biogeography of amphibians and reptiles of Europe. Amphibia-Reptilia 35, 1–31 (2014).
Chiari, Y. et al. Phylogeography of Sardinian cave salamanders (genus Hydromantes) is mainly determined by geomorphology. PLoS ONE 7, e32332 (2012).
Lunghi, E., Guillaume, O., Blaimont, P. & Manenti, R. The first ecological study on the oldest allochthonous population of European cave salamanders (Hydromantes sp.). Amphibia-Reptilia 39, 113–119 (2018).
Lucente, D. et al. A new population of European cave salamanders (genus Hydromantes) from west-central France: relict or introduction? Herpetol. Bull 138, 21–23 (2016).
Cimmaruta, R., Forti, G., Lucente, D. & Nascetti, G. Thirty years of artificial syntopy between Hydromantes italicus and H. ambrosii ambrosii (Amphibia, Plethodontidae). Amphibia-Reptilia 34, 413–420 (2013).
Stoch, F. & Genovesi, P. Manuali per il monitoraggio di specie e habitat di interesse comunitario (Direttiva 92/43/CEE) in Italia: specie animali (ISPRA, 2016).
Manenti, R. Dry stone walls favour biodiversity: a case-study from the Appennines. Biodivers. Conserv. 23, 1879–1893 (2014).
Costa, A., Crovetto, F. & Salvidio, S. European plethodontid salamanders on the forest floor: local abundance is related to fine-scale environmental factors. Herpetol. Conserv. Biol. 11, 344–349 (2016).
Lunghi, E., Manenti, R. & Ficetola, G. F. Seasonal variation in microhabitat of salamanders: environmental variation or shift of habitat selection? PeerJ 3, e1122 (2015).
Ficetola, G. F., Pennati, R. & Manenti, R. Do cave salamanders occur randomly in cavities? An analysis with Hydromantes strinatii. Amphibia-Reptilia 33, 251–259 (2012).
Lunghi, E., Manenti, R. & Ficetola, G. F. Do cave features affect underground habitat exploitation by non-troglobite species? Acta Oecol. 55, 29–35 (2014).
Culver, D. C. & Pipan, T. The biology of caves and other subterranean habitats (Oxford Unuiversity Press, 2009).
Roth, G. Experimental analysis of the prey catching behavior of Hydromantes italicus Dunn (Amphibia, Plethodontidae). J. Comp. Physiol. A Sens. Neural. Behav. Physiol 109, 47–58 (1976).
Lunghi, E. et al. Nesting of cave salamanders (Hydromantes flavus and H. italicus) in natural environments. Salamandra 50, 105–109 (2014).
Lunghi, E. et al. First data on nesting ecology and behaviour in the Imperial cave salamander Hydromantes imperialis. North-West. J. Zool. 11, 324–330 (2015).
Oneto, F., Ottonello, D., Pastorino, M. V. & Salvidio, S. Posthatching parental care in salamanders revealed by infrared video surveillance. J. Herpetol. 44, 649–653 (2010).
Oneto, F., Ottonello, D., Pastorino, M. V. & Salvidio, S. in Scripta Herpetologica. Studies on Amphibians and Reptiles in honour of Benedetto Lanza (eds Capula M. & Corti C. ) 129–138 (Edizioni Belvedere, 2014).
Vignoli, L., Caldera, F. & Bologna, M. A. Trophic niche of cave populations of Speleomantes italicus. J. Nat. Hist 40, 1841–1850 (2006).
Salvidio, S. Diet and food utilization in the European plethodontid Speleomantes ambrosii. Vie Milieu 42, 35–39 (1992).
Salvidio, S. et al. Consistency in trophic strategies between populations of the Sardinian endemic salamander Speleomantes imperialis. Animal. Biol 67, 1–16 (2017).
Chase, J. M. & Leibold, M. A. Ecological Niches. Linking classical and contemporary approaches (The University of Chicago Press, 2003).
Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115–1123 (2007).
Juáres, M. A., Santos, M., Mennucci, J. A., Coria, N. R. & Mariano-Jelicich, R. Diet composition and foraging habitats of Adélie and gentoo penguins in three different stages of their annual cycle. Mar. Biol. 163, 105 (2016).
Showalter, A. M., Vanni, M. J. & González, M. J. Ontogenetic diet shifts produce trade-offs in elemental imbalance in bluegill sunfish. Freshwater Biol. 61, 800–813 (2016).
Brunkow, P. E. & Collins, J. P. Effects of individual variation in size on growth and development of larval salamanders. Ecology 77, 1483–1492 (1996).
Bašić, T. & Britton, J. R. Characterizing the trophic niches of stocked and resident cyprinid fishes: consistency in partitioning over time, space and body sizes. Ecol. Evol 6, 5093–5104 (2016).
de la Vega, C. et al. Seasonal variation of Harbor Seal's diet from the Wadden Sea in relation to prey availability. PLoS ONE 11, e0155727 (2016).
Romero, S. A. & Harwood, J. D. Diel and seasonal patterns of prey available to epigeal predators: evidence for food limitation in a linyphiid spider community. Biol. Control. 52, 84–90 (2010).
Arif, S., Adams, D. C. & Wicknick, J. A. Bioclimatic modelling, morphology, and behaviour reveal alternative mechanisms regulating the distributions of two parapatric salamander species. Evol. Ecol. Res 9, 834–854 (2007).
Cloyed, C. S. & Eason, P. K. Niche partitioning and the role of intraspecific niche variation in structuring a guild of generalist anurans. Royal Soc. Open Sci 4, 170060 (2017).
Araújo, M. S. et al. Network analysis reveals contrasting effects of intraspecific competition on individual vs. population diets. Ecology 89, 1981–1993 (2008).
Crovetto, F., Romano, A. & Salvidio, S. Comparison of two non-lethal methods for dietary studies in terrestrial salamanders. Wildlife Res. 39, 266–270 (2012).
Sabelli, B. Atlante di diversità e morfologia degli invertebrati (Piccin-Nuova Libraria, 2009).
Chinery, M. Insects of Britain and Western Europe. 3rd Edition (Field Guide) (A&C Black, 2012).
Novak, M. & Tinker, M. T. Timescales alter the inferred strength and temporal consistency of intraspecific diet specialization. Oecol 178, 61–74 (2015).
MacCoun, R. & Perlmutter, S. Hide results to seek the truth. Nature 526, 187–189 (2015).
Core Team., R R: A language and environment for statistical computing. (2016).
de Pous, P., Speybroeck, J., Bogaerts, S., Pasmans, F. & Beukema, W. A contribution to the atlas of the terrestrial herpetofauna of Sardinia. Herpetol. Notes 5, 391–405 (2012).
Data Citations
Lunghi, E. figshare https://doi.org/10.6084/m9.figshare.c.3968970 (2018)
Acknowledgements
Data collection was authorized by Italian Ministry of Environment (9384/PNM of 12/05/2015) and by Regione Autonoma della Sardegna (n° 6312 of 27/03/2017). A special thank goes to S. Salvidio for guidance on how to perform the stomach flushing.
Author information
Authors and Affiliations
Contributions
E.L., M.M., R.C., B.B., L.C., D.A., R.M. and G.F.F. participated in data collection; E.L., F.Cia. and F.Cec. determined stomach contents; E.L. prepared tables, figures and first draft of the manuscript; F.Cia, F.Cec, M.V., R.M., G.F.F. and C.C contributed in manuscript writing; L.C. took photo 2a; E.L. took photos 2b–c; F.Cia took photo 2d.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
ISA-Tab metadata
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata files made available in this article.
About this article
Cite this article
Lunghi, E., Cianferoni, F., Ceccolini, F. et al. Field-recorded data on the diet of six species of European Hydromantes cave salamanders. Sci Data 5, 180083 (2018). https://doi.org/10.1038/sdata.2018.83
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/sdata.2018.83
This article is cited by
-
The trophic niche of subterranean populations of Speleomantes italicus
Scientific Reports (2022)
-
Updating salamander datasets with phenotypic and stomach content information for two mainland Speleomantes
Scientific Data (2021)
-
Diet diversity and environment determine the intestinal microbiome and bacterial pathogen load of fire salamanders
Scientific Reports (2021)
-
Photographic database of the European cave salamanders, genus Hydromantes
Scientific Data (2020)