Abstract
Communication and nutrition are major drivers of fitness in ants. While communication is paramount to colony cohesion, nutrition is decisive in regulating reproductive division of labor among colony members. However, neither of these has been studied from a molecular perspective in developing individuals. Here, we report the availability of the first transcriptome resources for larvae of the ant Formica fusca, a species with excellent discrimination abilities and thus the potential to become a model system for studying molecular mechanisms of communication. We generated a comprehensive, high-coverage RNA-seq data set using Illumina RNA-seq technology by sequencing 24 individual 1st - 2nd instar larvae collected from four experimental groups (6 samples per treatment, 49 million mean reads per sample, coverage between 194–253×). A total of 24,765 unigenes were generated using a combination of genome-guided and de novo transcriptome assembly. A comprehensive assembly pipeline and annotation lists are provided. This dataset adds valuable transcriptomic resources for further study of developmental gene expression, transcriptional regulation and functional gene activity in ant larvae.
Design Type(s) | stimulus or stress design • organism development design • transcription profiling by high throughput sequencing design • factorial design • sequence assembly objective |
Measurement Type(s) | transcription profiling assay |
Technology Type(s) | RNA sequencing |
Factor Type(s) | experimental condition |
Sample Characteristic(s) | Formica fusca |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Similar content being viewed by others
Background & Summary
The evolution of insect societies represents a major evolutionary transition comparable to the evolution of multicellular organisms from free-living cells1. Just like multicellular organisms, insect societies consist of individual somatic units (sterile workers) and individual germ-line units (reproductive queens and males) that together form a highly integrated system. In contrast to multicellular organisms, insect societies can easily be broken down into their separate components (i.e. individuals), which can then be studied and manipulated in isolation. This makes insect societies excellent models for studying biological processes across all levels of biological complexity, from genomes to holobiomes and social groups2.
Among social insects, ants are especially numerous and diverse, with over 12,000 described species worldwide3. Studies of individual ants have typically focused on traits of adults, and in particular on the genetics, morphology, physiology and behaviour of reproductive queens and sterile workers4. Conceptual advances in biology, sparked by seminal work on the role of development in evolution5, are causing this focus to shift toward developing individuals. Ants are holometabolous insects and their development goes through four distinct stages: egg, larva, pupa and adult. While eggs and pupae do not participate directly in colony life, larvae are actively engaged in crucial colony-level processes (e.g. food processing) and represent the developmental stage during which growth and determination of reproductive caste, i.e. queen or worker caste, occurs. As a result, what happens during larval development invariably affects both individual traits such as caste, body size and fecundity, as well as key colony-level traits such as overall productivity and caste ratios6. To better understand the regulation of ant development as well as the complex behavioural and physiological interactions between larvae and adult colony members, high-quality genomic resources are necessary.
The focus of this study was on two features known to play a crucial role in insect sociality – communication and nutrition – both of which act as major forces driving fitness in ants. First, we were interested in how social interaction affects larval gene expression, in particular in the context of chemical communication. Communication between individuals is involved in many aspects of social life, including reproductive division of labor, brood care, foraging and colony defence, and is paramount to colony organization and cohesion7,8. Not surprisingly, chemical communication among adult ants has been extensively studied8–11. However, although larvae represents the life stage in which individuals are first exposed to complex social interactions6, and during which their discrimination abilities are most likely primed for adult life12, very little is known about how larvae participate in colony communication. Second, we were interested in the effect of nutrition on larval gene expression, as nutrition has been identified as one of the key factors involved in reproductive caste determination in wasps and bees13–16. While it seems clear that nutrition is also the most important factor regulating reproductive division of labor via its effects on queen-worker caste determination in ants4,17, the molecular basis of nutritional signalling has not been studied in individual larvae.
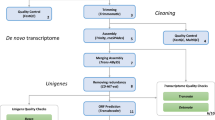
This study reports the first transcriptomic sequences from whole larvae of the black ant Formica fusca, a common pioneer species in temperate climates18. As illustrated in Fig. 1, sequenced samples were subjected to four treatments: I) social isolation, II) social stimulation, IIIa) nutritional stimulation – “fed”, and IIIb) nutritional stimulation – “starved”. We provide a high-quality transcriptome assembly and annotated results, enabling comparisons with previously generated social insect larvae transcriptomes (e.g.19). The bioinformatics pipeline steps used in this study are shown in Fig. 2. We obtained a total of 24,765 assembled unigenes ranging in size from 19,880 bp to 297 bp, with a mean length of 1723,45 bp (Table 1, Fig. 3). Overall, this dataset adds valuable transcriptomic resources for further study of molecular correlates of development, transcriptional regulation and functional gene activity in ant larvae.
Methods
Experimental design
Formica fusca is a common pioneer species, which acts as a host to several temporary social parasite ant species18. Perhaps due to high parasite pressure, F. fusca workers and larvae exhibit precise discrimination abilities against con- and heterospecific individuals20–25, making it an ideal species to study the molecular correlates of communication. In addition, F. fusca larvae have been shown to consume both con- and heterospecific eggs25,26, showing that even larvae are able to discriminate friend from foe. This provides the unique opportunity to study how nutritional stimulation in the form of egg consumption affects developmental gene expression.
To address these questions, we collected whole colonies of F. fusca ants (n = 8) containing queens, workers and brood in the spring of 2016 from a known population on the Hanko peninsula in southwestern Finland in the vicinity of Tvärminne Zoological Station (59°54′46.3′N 23°15′55.9′E). After collection, colonies were transferred to FluonTM coated laboratory nest boxes, which were kept at room temperature. Colonies were provided with Bhatkar--Whitcomb diet27 and water daily. Shortly after collection, queens started laying eggs. Young larvae (1-3 days post hatching) were removed from colony fragments and visually size-matched according to head capsule width. Although the number of larval development stages in F. fusca is not known, related species exhibit three (F. japonica) or four (F. polyctena) larval instars28. Based on our visual inspection of larvae (Supplementary Figures S1, S2), only young, i.e. first or second instar larvae, were included in the experiments.
Each larva was then placed on a petri dish lined with humid sponge cloth, where it was subjected to one of three treatments for 24 h. In the first treatment, individual larvae were isolated without contact to other brood or adults (“social isolation). In the second treatment, larvae were kept with four other nestmate larvae and five nestmate eggs (“social stimulation”). By comparing gene expression of larvae from treatments one and two, we aim to elucidate how expression of candidate communication-related genes, in particular chemosensory and odorant-binding proteins29,30, is affected by the social environment larvae encounter. In the third treatment, individual larvae were kept with two Formica pressilabris eggs (“nutritional stimulation”). For this treatment, heterospecific eggs were chosen as a food source since larval egg consumption has been demonstrated previously in Formica ants26. Larvae that had consumed an egg after 24 h were designated as “fed larvae” while those that had refrained from egg consumption were designated as “hungry larvae”. By comparing gene expression of fed and hungry larvae, we aim to investigate how nutrition links to the expression of genes known to be implicated in nutrient-signalling (e.g. insulin signalling genes, TOR pathway genes) and identify genes potentially involved in nutrition-mediated caste determination processes31.
After 24 h, six larvae each from treatments one and two, and 12 larvae from treatment three (6 fed larvae and 6 hungry larvae) were collected in individual Eppendorf tubes containing 200 μL Trisure (Bioline) and stored at −80 °C until RNA extraction. For treatment two, only larvae that had not consumed any eggs or larvae were chosen for sequencing. For each treatment, larvae from four to five different colonies (out of 8) were chosen for sequencing to minimize effects caused by inter-colony variation. In addition, all treatments were set up in parallel over the course of 3 days to avoid any sampling effects.
RNA extractions and library construction
Total RNA was extracted from the whole body of each individual larva using a standard Trizol protocol (TRIsure, Bioline). Subsequently, contaminating genomic DNA was removed by DNAse I digestion (Fermentas), and the RNA was purified using RNeasy MinElute kit (Qiagen), both following the protocol of the manufacturer. RNA was dissolved in 10 μL of milliQ water. The yield and quality of the RNA was verified by assessing the A280/A260 ratio (NanoDrop, Thermo Scientific) and inspected in a BioAnalyzer 2100 using RNA 6000 Nano kit (Agilent). Poly(A) RNA was isolated using the NEBNext Poly(A) mRNA Magnetic Isolation Module and libraries were prepared using the NEBNext Ultra Directional RNA Library Prep Kit for Illumina, both following the protocol of the manufacturer. The multiplexed samples were sequenced paired-end on five lanes of an Illumina HiSeqTM 2500 2 × 100 bp (~400 M PE reads for each lane) at the FuGU lab in Helsinki, Finland.
Transcriptome assembly
Approximately 2,640 million pairs of reads were generated using the Ilumina HiSeqTM 2500 platform. The quality of raw reads was assessed with FastQC tools (http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc) and MultiQC32. Raw reads were parsed through quality filtration (Trimmomatic33, options: LEADING:20 TRAILING:20 SLIDINGWINDOW:5:20 MINLEN:50). This program also searches for and removes any remaining TrueSeq Illumina adaptors in the reads. Unpaired reads were also discarded for the remainder of the assembly pipeline. After removal of low-quality reads, 2,383,894,158 clean reads (i.e. 90.3% of raw reads, Table 2) were retained and used in the transcriptome assembly pipeline described below. Genome-guided assembly usually provides high quality assembly of a reference transcriptome using the genome of a closely related species34. In order to recover a comprehensive set of transcripts, we used a combination of de novo assembly and genome-guided assembly known to give the best quality assembly35,36. We first performed a genome-guided assembly using Trinity software37 and the genome of the ant Formica exsecta, a closely related ant species (NCBI BioProject ID PRJNA393850 and BioSample: SAMN07344805). For this purpose, the trimmed reads were mapped to the F. exsecta genome using tophat software38. In parallel, we used the high-quality trimmed reads from all samples to perform a de novo assembly using Trinity software (trinityrnaseq-Trinity-v2.4.0) with 6 CPUS for the Inchworm and Butterfly steps, a maximum memory of 250 GB, a minimum t length of 200 bp, and the default K-mer of 25. The merged-assembly was built by combining the genome-guided and the de novo assembly. At this stage, a total of 412,776 transcripts were generated with a mean length of 1144.95 bp and an N50 of 2824 bp.
Transcript reconstruction
We used TransDecoder v3.0.1 (TransDecoder. https://transdecoder.github.io/) to identify potential coding regions within the final assembly set of transcripts, following three steps. First, TransDecoder.LongOrfs was used to select the single best open reading frame (ORF) per transcript longer than 100 amino acids. In the second step, we identified ORFs with homology to known proteins using BLAST search (Uniprot database, BLAST version 2.2.26+, BLASTp, with an e-value cut-off ≤ 10-5) and searched for protein signature in the Pfam-A database. In the last step, the program TransDecoder. Predict uses this information to predict the coding sequences. A total of 189,123 coding sequences were kept for further analysis. To obtain a set of non-redundant transcripts, we then clustered highly similar coding sequences using cd-hit v4.6.539 with an amino-acid sequence identity threshold of 0.99. A total of 61,036 coding sequences, belonging to 56,205 transcripts, were retained at this stage. Next, in order to obtain a set of unique, putative unigenes, transcripts containing these coding sequences were filtered to only retain the isoform with the highest expression, using the Trinity assembly information34. To do so, the cleaned reads were mapped to the cd-hit-filtered assembly using Bowtie240 and the abundance of each transcript was estimated using RSEM41. A total of 28,808 unigenes were found. Thereafter, we used RSEM_EVAL package distributed with DENOTATE42 to assess the quality of the assembly. We filtered the assembly by applying RSEM-EVAL’s contig impact score, and 231 unigenes with impact scores less than or equal to zero were removed from the assembly, as recommended by Li et al.42. Finally, the unigene list was cleaned from probable exogenous RNAs known to be abundant in social insect de novo transcriptomes43. To this end, we used BLAST to compare the above list of unigenes against virus, fungal, protozoan and bacterial genome databases downloaded from NCBI (BLAST version 2.2.26+, BLASTn and BLASTp, with an e-value cut-off ≤ 10−3). A total of 3,812 unigenes that showed a minimum of 70% amino acid identity with at least one of the databases were removed from the assembly. The final assembly includes 24,765 unigenes.
Transcriptome annotation and gene ontology
To provide comprehensive annotation of the final unigene set, we compared our final assembly to the NCBI non-redundant database (BLASTx with an e-value cut-off ≤ 103). Unigenes were also searched against the Swissprot database (e-value cut-off ≤ 103). A total of 19,688 unigenes returned a BLAST hit with the NR database (79.5%) and 13,129 (53%) with the Swissprot database. The annotations were submitted to the software Blast2GO (www.blast2go.com) to infer functional annotation and to obtain a list of gene ontology (GO) terms associated with the annotated genes. Of these unigenes, 11,898 contigs presented gene ontology (GO) annotation, with a mean GO level of 6.36 across biological process (BP), molecular function (MF) and cellular components (CC) categories (Supplementary Figures S3, S4). For biological process, 702 (5.9% of the number of sequences with GOterms) were in the oxidation-reduction process, 466 (3.7%) were in the proteolysis and 429 (3.6%) were in the regulation of transcription category. For cellular components, integral component of membrane represented the majority (3431, 28.6%), together with nucleus (755, 6.3%). For molecular function, 1104 (9.2%) ATP binding and 940 (7.84%) nucleic acid were highly represented (Supplementary Figures S5 (BP), S6 (MF), S7 (CC)). The number of unigenes annotated is shown in Table 1 and a complete list of BLAST and GOterm annotations are provided in Figshare (Annotation dataset, Data Citation 2).
Data Records
The raw read files from this study were deposited to the DDBJ Sequence Read Archive (Data Citation 1). The database contains 24 records. For each treatment, six replicates were sequenced, using a single larva. Furthermore, the final assembly has been uploaded to figshare (Transcriptome assembly, Data Citation 2), and the final annotation dataset was also uploaded to figshare (Annotation dataset, Data Citation 2).
Technical Validation
Quality of the reads validation
To assess total data quality, we performed quality check using FastQC and MultiQC for all samples before and after adaptor/sequences trimming. The mean read counts per quality scores were higher than 35 (Fig. 4a). The mean quality scores in each base position were higher than 35 (Fig. 4b). The mean sequence lengths were 95-100 bp (Fig. 4c). The mean per sequence GC content was 40% (Fig. 4d).
Transcriptome assembly validation
We ran the Trinity package utility script TrinityStats.pl, to obtain basic statistical information about the final assembly. The results of this evaluation are summarized in Table 1. Then, we quantified completeness of our final assembly by comparing the coding sequences of our final set of unigenes against a set of highly conserved hymenopteran single-copy orthologs using the BUSCO (Benchmarking Universal Single-Copy Orthologs) v2 pipeline44. The set of hymenopteran single-copy orthologs was downloaded from OrthoDB v9.1 database45. Following BUSCO recommendations, we calculated the number of complete/single-copy transcripts (2760, 62.5%), duplicated transcripts (1010, 22.9%), and fragmented transcripts (379, 8.6%). Only 266 (6%) single-copy orthologs were classified as missing from our final assembly. As one of the final steps, we ran RSEM Eval42 to evaluate our assembly and access how well the unigenes are supported by our RNA-Seq data. This step allowed us to filter unnecessary unigenes from our final dataset.
Additional information
How to cite this article: Morandin, C. et al. De novo transcriptome assembly and its annotation for the black ant Formica fusca at the larval stage. Sci. Data. 5:180282 doi: 10.1038/sdata.2018.282 (2018).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
References
Bourke, A. F. G. Principles of social evolution. (Oxford University Press, 2011).
Kennedy, P. et al. Deconstructing superorganisms and societies to address big questions in biology. Trends Ecol. Evol. 32, 861–872 (2017).
Moreau, C. S., Bell, C. D., Vila, R., Archibald, S. B. & Pierce, N. E. Phylogeny of the ants: Diversification in the Age of Angiosperms. Science. 312, 101–104 (2006).
Hölldobler, B. & Wilson, E. O. The Ants. (Belknap-Harvard University Press, 1990).
West-Eberhard, M. J. Developmental plasticity and evolution. (Oxford University Press, 2003).
Schultner, E., Oettler, J. & Helanterä, H. The role of brood in eusocial Hymenoptera. Q. Rev. Biol. 92, 39–78 (2017).
Le Conte, Y. & Hefetz, A. Primer pheromones in social hymenoptera. Annu. Rev. Entomol. 53, 523–542 (2008).
van Zweden, J. S., d’Ettorre, P. In Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology Blomquist G. C. & Bagnères A.-G. eds. 222–243 (Cambridge University Press, 2010).
Vargo, E. L. In Pheromone communication in social insects. Vander Meer R., Breed M., Espelie K. & Winston M. eds. 293–313 (Westview Press, 1998).
van Wilgenburg, E., Symonds, M. R. E. & Elgar, M. A. Evolution of cuticular hydrocarbon diversity in ants. J. Evol. Biol 24, 1188–1198 (2011).
Pask, G. M. et al. Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones. Nat. Commun. 8, 297 (2017).
Signorotti, L., Jaisson, P. & D’Ettorre, P. Larval memory affects adult nest-mate recognition in the ant Aphaenogaster senilis. Proc. R. Soc. B Biol. Sci 281, 20132579 (2014).
Haydak, M. H. Honey Bee Nutrition. Annu. Rev. Entomol. 15, 143–156 (1970).
O’Donnell, S. Reproductive caste determination in eusocial wasps (Hymenoptera: Vespidae). Annu. Rev. Entomol. 43, 323–346 (1998).
Schmidt, K. C., Hunt, B. G. & Smith, C. R. Queen, worker, and male yellowjacket wasps receive different nutrition during development. Insectes Soc. 59, 289–295 (2012).
Buttstedt, A., Ihling, C. H., Pietzsch, M. & Moritz, R. F. A. Royalactin is not a royal making of a queen. Nature 537, E10–E12 (2016).
Trible, W. & Kronauer, D. J. C. Caste development and evolution in ants: it’s all about size. J. Exp. Biol. 220, 53–62 (2017).
Punttila, P., Haila, Y., Pajunen, T. & Tukia, H. Colonisation of Clearcut Forests by Ants in the Southern Finnish Taiga: A Quantitative Survey. Oikos 61, 250–262 (1991).
Schrader, L., Simola, D., Heinze, J. & Oettler, J. Sphingolipids, transcription factors and conserved tool kit genes: developmental plasticity in the ant Cardiocondyla obscurior. Mol. Biol. Evol 32, 1474–1486 (2015).
Chernenko, A., Helanterä, H. & Sundström, L. Egg Recognition and Social Parasitism in Formica Ants. Ethology 117, 1081–1092 (2011).
Martin, S. J., Helantera, H. & Drijfhout, F. P. Is parasite pressure a driver of chemical cue diversity in ants? Proc. R. Soc. B Biol. Sci 278, 496–503 (2011).
Helanterä, H. & Sundström, L. Worker policing and nest mate recognition in the ant Formica fusca. Behav. Ecol. Sociobiol. 61, 1143–1149 (2007).
Helanterä, H., Martin, S. J. & Ratnieks, F. L. W. Prior experience with eggs laid by non-nestmate queens induces egg acceptance errors in ant workers. Behav. Ecol. Sociobiol. 62, 223–228 (2007).
Helanterä, H. & Ratnieks, F. L. W. Two independent mechanisms of egg recognition in worker Formica fusca ants. Behav. Ecol. Sociobiol. 63, 573–580 (2009).
Pullianien, U., Helanterä, H., Sundström, L. & Schultner, E. The possible role of ant larvae in the defense against social parasites. Submitted.
Schultner, E., Gardner, A., Karhunen, M. & Helanterä, H. Ant Larvae as Players in Social Conflict: Relatedness and Individual Identity Mediate Cannibalism Intensity. Am. Nat. 184, E161–E174 (2014).
Bhatkar, A. & Whitcomb, W. Artificial diet for rearing various species of ants. Florida Entomol 53, 229–232 (1970).
Solis, D. et al. Morphological description of the immatures of the ant, Monomorium floricola. J. Insect Sci 10, 15 (2010).
Kulmuni, J., Wurm, Y. & Pamilo, P. Comparative genomics of chemosensory protein genes reveals rapid evolution and positive selection in ant-specific duplicates. Heredity. 110 538–547 (2013).
McKenzie, S. K., Oxley, P. R. & Kronauer, D. J. C. Comparative genomics and transcriptomics in ants provide new insights into the evolution and function of odorant binding and chemosensory proteins. BMC Genomics 15, 718 (2014).
Corona, M., Libbrecht, R. & Wheeler, D. E. Molecular Mechanisms of Phenotypic Plasticity in Social Insects. Curr. Opin. Insect Sci. 13, 55–60 (2016).
Ewels, P., Magnusson, M., Lundin, S. & Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016).
Bolger, A. M., Lohse, M. & Usadel, B. Genome analysis Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Haas, B. J. et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 8, 1494–1512 (2013).
Bingxin, L. U., Zhenbing, Z. & Tieliu, S. H. I. Comparative study of de novo assembly and genome-guided assembly strategies for transcriptome reconstruction based on RNA-Seq. Life Sci. 56, 143–155 (2013).
Jain, P., Krishnan, N. M. & Panda, B. Augmenting transcriptome assembly by combining de novo and genome-guided tools. PeerJ 1, e133 (2013).
Grabherr, M. et al. Trinity: reconstructing a full-length transcriptome without a genome from RNA-Seq data Nat. Biotechnol. 29, 644–652 (2013).
Trapnell, C., Pachter, L. & Salzberg, S. L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Ã, W., L. & Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 (2018).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, B. & Dewey, C. N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323 (2011).
Li, B. et al. Evaluation of de novo transcriptome assemblies from RNA-Seq data. Genome Biol. 15, 1–21 (2014).
Johansson, H. et al. A metatranscriptomic approach to the identification of microbiota associated with the ant Formica exsecta. PLoS One 8, e79777 (2013).
Simão, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V. & Zdobnov, E. M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015).
Kriventseva, E. V. et al. OrthoDB v8: Update of the hierarchical catalog of orthologs and the underlying free software. Nucleic Acids Res. 43, D250–D256 (2015).
Data Citations
DNA DataBank of Japan DRP004341 (2018)
Morandin, C. et al. figshare https://doi.org/10.6084/m9.figshare.c.4172408 (2018)
Acknowledgements
We thank the Academy of Finland (grants, #252411, #284666 to the Centre of Excellence in Biological Interactions, and grant #289731 to NB), the University of Helsinki, and the Bayrisches Staatsministerium für Bildung und Kultus, Wissenschaft und Kunst (ES) for funding. We also thank the FuGU lab in Helsinki, Finland who performed sequencing.
Author information
Authors and Affiliations
Contributions
U.P., E.S.: conceived and conducted the experiments; C.M.: conducted the bioinformatics analyses, C.M., E.S.: wrote the manuscript; N.B., E.S.: coordinated and secured funding for the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Supplementary information accompanies this paper at
ISA-Tab metadata
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata files made available in this article.
About this article
Cite this article
Morandin, C., Pulliainen, U., Bos, N. et al. De novo transcriptome assembly and its annotation for the black ant Formica fusca at the larval stage. Sci Data 5, 180282 (2018). https://doi.org/10.1038/sdata.2018.282
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/sdata.2018.282
This article is cited by
-
Transcriptome profiling of differentially expressed genes in cytoplasmic male-sterile line and its fertility restorer line in pigeon pea (Cajanus cajan L.)
BMC Plant Biology (2020)
-
Genome-wide gene expression profiling of the melon fly, Zeugodacus cucurbitae, during thirteen life stages
Scientific Data (2020)
-
Liver transcriptome resources of four commercially exploited teleost species
Scientific Data (2020)