Abstract
Larval fishes are a useful metric of marine ecosystem state and change, as well as species-specific patterns in phenology. The high level of taxonomic expertise required to identify larval fishes to species level, and the considerable effort required to collect samples, make these data very valuable. Here we collate 3178 samples of larval fish assemblages, from 12 research projects from 1983-present, from temperate and subtropical Australian pelagic waters. This forms a benchmark for the larval fish assemblage for the region, and includes recent monitoring of larval fishes at coastal oceanographic reference stations. Comparing larval fishes among projects can be problematic due to differences in taxonomic resolution, and identifying all taxa to species is challenging, so this study reports a standard taxonomic resolution (of 218 taxa) for this region to help guide future research. This larval fish database serves as a data repository for surveys of larval fish assemblages in the region, and can contribute to analysis of climate-driven changes in the location and timing of the spawning of marine fishes.
Design Type(s) | database creation objective • data integration objective • biodiversity assessment objective |
Measurement Type(s) | Taxon |
Technology Type(s) | digital curation |
Factor Type(s) | specimen collection time measurement datum • temperature • depth • geographic location |
Sample Characteristic(s) | New South Wales • Tasmania • State of Victoria • Eastern Indian Ocean coastal waters of Western Australia • ocean biome |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Similar content being viewed by others
Background & Summary
The early life history of most marine fishes occurs in the upper water column, with eggs and larvae developing as part of the plankton, before leaving the plankton for settlement as post-larvae. Surveys of larval fishes (which, together with fish eggs, are termed ichthyoplankton) are one tool for monitoring marine ecosystems and fish communities1. Surveys of larval fishes are valuable for ecosystem monitoring because many oceanographic, biological, and anthropogenic processes influence their distribution, abundance, and survival2–6. Survey data has been used for monitoring spawning habitats7,8, and changes in phenology9 and the spawning biomass of adult populations10, and may be useful in this Australian region for helping interpret ecosystem changes in a climate change hotspot11,12 undergoing substantial biological changes13,14.
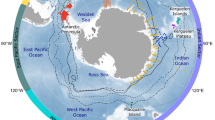
Ichthyoplankton have been surveyed in Australia since the early 20th century, with a 1910 survey of three species in Port Phillip Bay15, and surveys in the 1930–50 s of larvae and eggs of sardine (Sardinops sagax) and anchovy (Engraulis australis)16–18. Descriptions of larvae for various species occurred from the 1950s onwards, and in the 1980s surveys of larval fish assemblages began in earnest in Australian temperate marine waters (reviewed in19). Since 2014, larval fishes have been routinely collected at five reference stations (Fig. 1), with samples sorted and larval fishes identified at the three east coast stations. Abundances of larval fishes from numerous research voyages were collated in a 2002 report on ~45 commercially-important fish species20, with the data provided on a compact disc. There have otherwise been no attempts at collating and sharing larval fish assemblage data from temperate and subtropical Australia.
See Table 1 for project descriptions. The five National Reference Stations (NRS) at which ~monthly sampling of larval fishes is ongoing (the NIMO program) are indicated. Samples at the three NRS on the east coast are currently being sorted and identified, with the remainder stored for future sorting and identification.
One challenge with collating larval fish data is a difference in taxonomic resolution among studies, which limits the ability to make robust comparisons of larval fish assemblages. Few scientists have the ability to identify many taxa to species level, and given the progressive loss of taxonomic expertise (and that a large proportion of fish species are endemic to southern Australia)21, there is some uncertainty as to whether this expertise will extend to a new generation of marine scientists22. This suggests that the taxonomic resolution of future surveys of larval fishes could decline without some guidance. DNA barcoding methods can greatly enhance the identification of ichthyoplankton23,24, and potentially reduce the reliance on taxonomic experts; but for generating larval fish count data from large surveys, DNA methods are currently complimentary to morphological identification methods.
This study had two aims: 1) to collate marine larval fish assemblage data from the 1980s onwards for temperate and subtropical Australian waters; and 2) to create a standard taxonomic resolution for these data, which can act as a target resolution for future larval fish research in the region (when identifying all taxa to species is not feasible). The data collated here have come from research voyages (1983-2015), as well as current monitoring at three coastal reference stations (2014 onwards; Table 1, Fig. 1). It consists of 3178 samples and >490,000 identifications. The research voyages were done by a variety of universities and government agencies, and often with environment- or species-specific research objectives: e.g. monitoring the impact of sewage ocean outfalls on larval fishes25. These various objectives are reflected in the broad range of spatial and temporal scales of these surveys (Table 1). The recent monitoring data are an initiative under the auspices of the Australian Integrated Marine Observing System (IMOS), which monitors multiple physical and bio-chemical properties at seven National References Stations (NRS). This monitoring initiative has been called ‘NIMO’ (National Ichthyoplankton Monitoring and Observing) and began at some of these NRS in late 201426. Together, data from the collated voyages and recent monitoring create a broad understanding of the larval fish assemblages of temperate and subtropical Australia.
This paper begins a database for Australian larval fish assemblages, which acts as a repository for future larval fish surveys and monitoring in this region. The expert-derived taxonomic resolution used for these data can also act as a guide for a minimum resolution of future surveys, and is aimed at resolving all common, commercially-important, and readily identifiable marine fish taxa in this region, while remaining accessible to taxonomists and sorters beyond the few experts who created the species list used in this database. This database joins only a few data sets1,27 in providing regional ichthyoplankton survey data collected over a relatively long period.
This Australian larval fish database will be available through the Australian Ocean Data Network portal (AODN: https://portal.aodn.org.au/), the main repository for marine data in Australia. The Australian larval fish database will be maintained and updated through the Commonwealth Scientific and Industrial Research Organisation (CSIRO) data centre, with periodic updates sent to the AODN. A snapshot of the Australian larval fish database at the time of this publication has been assigned a DOI and will be maintained in perpetuity by the AODN (Data Citation 1).
Methods
Larval fish sampling
There are a variety of methods for surveying larval fish in the pelagic environment, including towing nets at specific depths, obliquely across a depth range, or at the surface28–30. In this database, all larval fishes were sampled by vessel-towed plankton nets towed obliquely or at constant near-surface depths. Upon net retrieval of a single ‘tow’, all plankton were fixed immediately in ~4% formalin in seawater (and often buffered with sodium borate or sodium carbonate to avoid sample degradation). The volume sampled by the net for each tow was determined, typically using a flowmeter attached to the mouth of the net, which was used to standardise larval fish counts to volume of water sampled. The types of nets used and the depths surveyed varied among studies, and are detailed in Table 1 (refer to key references for each project for more information).
Larval fish identification
In the laboratory, larval fishes in each sample were sorted, enumerated, and identified. Identification of species was done using key reference guides19,31–36 (and others22), and frequently through direct contact with experts. For example, AGM or FJN (who co-edited19) were involved in the identification of some species in nearly every project in this study. Larval fishes were then stored in ethanol for later reference, and a subset has been archived with the Australian Museum22. A list of best reference guides for the identification of each taxon in this study is provided alongside this database (see ‘Data Records’).
Taxonomic resolution
The taxonomic resolution often varies among projects, which can complicate comparison of larval fish assemblages. An aim of this study was to create a standard taxonomic resolution for surveys of larval fish assemblages in temperate and subtropical Australia. We created a database species list to act as a guide for an ideal minimum resolution for surveys of larval fishes in this Australian region. The goals of this species list were: 1) that it included all common and as many commercially important species as possible; 2) that this taxonomic level could be achieved with a reasonable level of training and reference to existing guides. A working group led by AGM, FJN, JML, and JK, met at the University of Tasmania on 7–9th December 2015, and the resulting species list (with some subsequent revision) is used in this data paper and stored online as associated metadata. The species list consists of a higher order division (usually family), a genus and species (when appropriate), and a common name. Each taxon is also identified with a unique CAAB number. CAAB (Codes for Australian Aquatic Biota) is an 8-digit coding system maintained by CSIRO (http://www.marine.csiro.au/caab/). There are an additional three groups in the species list identified with text: ‘Unknown’, ‘Damaged’, and ‘Other’. To create a single matrix file, each taxon is identified with a single header consisting of ‘Family_Species_CAAB’. If a taxon is only identified to Family, the header is ‘Family_CAAB’. ‘Other’ is used as a ‘Species’ term to indicate when a taxon contains all other species of that family. For example, the taxon ‘Acropomatidae_other_37311956’ includes all species in Acropomatidae except ‘Acropomatidae_Synagrops.spp_37311949’ and ‘Acropomatidae_Apogonops.anomalus_37311053’.
All projects in this study have been aligned to this database species list. Given that each of the projects included here had identification input from the same few experts (AGM, FJN), most taxa could be matched directly. Taxonomic resolutions were occasionally simplified (e.g. multiple species grouped in a single family), and in all cases AGM ensured the alignment was accurate. In rare cases, AGM examined stored samples from specific projects to ensure identifications were accurate.
Project selection
For this database, we selected a range of projects from temperate and subtropical Australia that surveyed marine pelagic larval fish assemblages and had high taxonomic resolution, and could be aligned to our common database species list with accuracy. This led to 11 projects suitable for inclusion, with the 12th project being the ongoing NIMO monitoring program (Fig. 1, Table 1).
The projects reported in this study are not an exhaustive list of larval fish surveys in Australia. There have been numerous surveys in more tropical areas (e.g.37–40); some with species-specific surveys (e.g. sardine, mackerel, blue grenadier41–44), and some surveys using vertical hauls (e.g.4). These (and others, see20) were not included either because they were outside the geographic area of interest, were not at the desired taxonomic resolution, or required investment (in species alignment and quality control) beyond what this study could achieve. However, it is likely that some existing data sets could be added to this database given investment by the data custodians.
Environmental data
Water temperature and salinity were often collected with each plankton tow, usually measured using a CTD. In some cases these were reported separately and needed to be aligned with the larval fish records. In all cases, except the NRS monitoring and project P3 (Table 1), water temperature of the surface (<10m) waters was available at each tow. Surface salinity for each record was also available for six of the projects.
Data Records
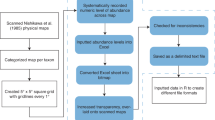
All data are combined into a single data set, with data distinguished by ‘project’ (Table 1). A project is defined as a set of data records that have been collected together, usually as a voyage or study, and have the same sampling and analysis methods and the same person(s) identifying the larval fishes.
Each record in a project represents a single plankton net tow, with larval fishes reported as counts per tow. Each record has a unique identifier called ‘Tow_ID’ (Table 2), consisting of the Project_ID (Table 1) followed by a consecutive record number within that project. Tow volumes (m3) are reported for each record, to allow standardisation to unit volume (per m3). Larval fish abundance is sometimes reported per unit area over the sampled depth range (per m2) by multiplying by the depth surveyed5, and this could be calculated for each record using the information on depths sampled.
Most metadata (e.g. tow depth, water temperature) are provided alongside each record; these record-specific metadata are defined in Table 2. Project-specific metadata (e.g. net type, mesh size) are provided in Table 1, and a non-exhaustive list of published studies are referenced for each project, and can be referred to for further project information (Table 1). Original sample ID codes and survey-specific metadata are used where appropriate to retain traceability with the original data. Missing or non-applicable data are left blank.
Key personnel are listed for each project (Table 1), and these people were involved in data collection and processing, and are usually the original custodians of the samples and data. The database species list to which all data were aligned is also provided as a stand-alone file (https://catalogue-imos.aodn.org.au/geonetwork/srv/en/metadata.show?uuid=2d2b2f92-12fa-4330-a480-94f0892c2b72). Within this file is also a list of best reference texts and guides for the identification of each species in this list, with priority given to references that identify the species, then to references that identify only the genera or family.
Technical Validation
The original identification of larval fishes in each survey was done with reference to key reference texts (see ‘Larval fish identification’ above). Every dataset presented here has been re-examined by AGM to ensure all identifications are within expected spatial and temporal domains. The alignment of taxa to the common species list was done by JAS in consultation with AGM to ensure the alignment was consistent (given revisions in taxonomy).
Usage Notes
This dataset snapshot is freely available from the following metadata record at (https://catalogue-imos.aodn.org.au/geonetwork/srv/en/metadata.show?uuid=2d2b2f92-12fa-4330-a480-94f0892c2b72). Larval fish monitoring at the IMOS National Reference Stations (NIMO) is ongoing (Fig. 1), and these data will continue to be updated for the duration of the NIMO program. Contact IMOS for status of these data.
Our goal is that the species list in this database remains fixed, but minor updates that do not alter the ability to compare the datasets presented here may occur. Any changes to the species list will be updated on the NRS data files and on the database version of the species list. When data are added to this database, the taxonomic resolution should ideally include all taxa within this species list (to maintain this minimum taxonomic resolution) to enable comparison of all datasets.
Additional information
How to cite this article: Smith, JA et al. A database of marine larval fish assemblages in Australian temperate and subtropical waters. Sci. Data. 5:180207 doi: 10.1038/sdata.2018.207 (2018).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
References
Koslow, J. A. & Wright, M. Ichthyoplankton sampling design to monitor marine fish populations and communities. Mar. Policy 68, 55–64 (2016).
Hsieh, C.-h. et al. Fishing elevates variability in the abundance of exploited species. Nature 443, 859–862 (2006).
Cowen, R. K., Gawarkiewicz, G. G., Pineda, J., Thorrold, S. R. & Werner, F. E. Population connectivity in marine systems: an overview. Oceanography 20, 14–21 (2007).
Keane, J. P. & Neira, F. J. Larval fish assemblages along the south-eastern Australian shelf: linking mesoscale non-depth-discriminate structure and water masses. Fish. Oceanogr 17, 263–280 (2008).
Koslow, J. A., Goericke, R. & Watson, W. Fish assemblages in the Southern California Current: relationships with climate, 1951–2008. Fish. Oceanogr 22, 207–219 (2013).
Holliday, D. et al. Larval fish assemblages and particle back-tracking define latitudinal and cross-shelf variability in an eastern Indian Ocean boundary current. Mar. Ecol. Prog. Ser. 460, 127–144 (2012).
Muhling, B. A. et al. Reproduction and larval biology in tunas, and the importance of restricted area spawning grounds. Rev. Fish Biol. Fish 27, 697–732 (2017).
Suca, J. J., Rasmuson, L. K., Malca, E., Gerard, T. & Lamkin, J. T. Characterizing larval swordfish habitat in the western tropical North Atlantic. Fish. Oceanogr 27, 246–258 (2018).
Asch, R. G. Climate change and decadal shifts in the phenology of larval fishes in the California Current ecosystem. Proc. Natl. Acad. Sci. USA 112, E4065–E4074 (2015).
Smith, P. E. & Moser, H. G. Long-term trends and variability in the larvae of Pacific sardine and associated fish species of the California Current region. Deep-Sea Res. Pt II 50, 2519–2536 (2003).
Hobday, A. J. & Pecl, G. T. Identification of global marine hotspots: sentinels for change and vanguards for adaptation action. Rev. Fish Biol. Fish 24, 415–425 (2014).
Wernberg, T. et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Change 3, 78–82 (2013).
Last, P. R. et al. Long-term shifts in abundance and distribution of a temperate fish fauna: a response to climate change and fishing practices. Global Ecol. Biogeogr 20, 58–72 (2011).
Vergés, A. et al. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. Lond., Ser. B: Biol. Sci 281, 20140846 (2014).
Regan, C. T. British Antarctic (“Terra Nova”) Expedition, 1910: Larval and post-larval fishes. Zoology 1, 125–156 (1916).
Dakin, W. J. & Colefax, A. N. The eggs and early larval stages of the Australian pilchard Sardinia neopilchardus (Steind.). Rec. Aust. Mus. 19, 136–140 (1934).
Blackburn, M. Age, rate of growth, and general life-history of the Australian pilchard (Sardinops neopilchardus) in New South Wales waters. Bulletin Council for Scientific and Industrial Research 242, 1–86 (1949).
Blackburn, M. A biological study of the anchovy, Engraulis australis in Australian waters. Mar. Freshw. Res 1, 3–84 (1950).
Neira, F. J., Miskiewicz, A. G. & Trnski, T. Larvae of Temperate Australian Fishes: Laboratory Guide for Larval Fish Identification. (University of Western Australia Press, 1998).
Bruce, B. D. & Bradford, R. W. A synthesis of existing data on the early life history of southern Australian finfish, Report No. FRDC 98/103 (CSIRO Marine Research, 2002).
Costello, M. J. et al. A census of marine biodiversity knowledge, resources, and future challenges. PLOS ONE 5, e12110 (2010).
Leis, J. M. Taxonomy and systematics of larval Indo-Pacific fishes: a review of progress since 1981. Ichthyol. Res. 62, 9–28 (2015).
Ahern, A. L. M et al. DNA sequencing of fish eggs and larvae reveals high species diversity and seasonal changes in spawning activity in the southeastern Gulf of California. Mar. Ecol. Prog. Ser. 592, 159–179 (2018).
Harada, A. E. et al. Monitoring spawning activity in a Southern California marine protected area using molecular identification of fish eggs. PLOS ONE 10, e0134647 (2015).
Gray, C. A., Otway, N. M., Laurenson, F. A., Miskiewicz, A. G. & Pethebridge, R. L. Distribution and abundance of marine fish larvae in relation to effluent plumes from sewage outfalls and depth of water. Mar. Biol. 113, 549–559 (1992).
Smith, J. A. et al. An Evaluation of Ichthyoplankton Monitoring at National Reference Stations. Final Report to the Australian Fisheries Management Authority, Report No. 2015/0819. (UNSW: Sydney, Australia, 2016).
Koslow, J. A. & Couture, J. Pacific Ocean observation programs: Gaps in ecological time series. Mar. Policy 51, 408–414 (2015).
Smith, P. E. & Richardson, L. E. Standard techniques for pelagic fish egg and larva surveys, FAO Fish. Tech. Pap., Report No. 175 (1977).
Habtes, S., Muller-Karger, F. E., Roffer, M. A., Lamkin, J. T. & Muhling, B. A. A comparison of sampling methods for larvae of medium and large epipelagic fish species during spring SEAMAP ichthyoplankton surveys in the Gulf of Mexico. Limnol. Oceanogr. Methods 12, 86–101 (2014).
Heath, M. R. In Field Investigations of the Early Life Stages of Marine Fish (eds J. H. S. Blaxter & A. J. Southward) 1–174 (Academic Press, 1992).
Moser, H. G. et al. Ontogeny and Systematics of Fishes. Report No. Species Publication No. 1. (American Society of Icthyologists and Herpotologists, 1984).
Moser, H. G. The Early Stages of Fishers in the California Current Region. Report No. CalCOFI Atlas No 33 (1996).
Moser, H. G. & Watson, W. Preliminary Guide to the Identification of the Early Life History Stages of Myctophiform Fishes of the Western Central Atlantic, NOAA Technical Memorandum, Report No. NMFS-SEFSC-453 (2001).
Leis, J. M. & Carson-Ewart, B. M. The Larvae of Indo-Pacific Coastal Fishes: an Identification Guide to Marine Fish Larvae. 2nd edn, (Brill, 2004).
Richards, W. J. Early Stages of Atlantic Fishes - An Identifciation Guide for the Western Central North Atlantic. (Taylor and Francis, 2006).
Okiyama, M. An Atlas of the Early Stages Fishes in Japan. 2nd edn, (Tokai University Press, 2014).
Leis, J. M. Vertical and horizontal distribution of fish larvae near coral reefs at Lizard Island, Great Barrier Reef. Mar. Biol. 90, 505–516 (1986).
Young, P. C., Leis, J. M. & Hausfeld, H. F. Seasonal and spatial distribution of fish larvae in waters over the north west continental shelf of Western Australia. Mar. Ecol. Prog. Ser. 31, 209–222 (1986).
Leis, J. M. Larval fish assemblages near Indo-Pacific coral reefs. Bull. Mar. Sci. 53, 362–392 (1993).
Holliday, D., Beckley, L. E., Weller, E. & Sutton, A. L. Natural variability of macro-zooplankton and larval fishes off the Kimberely, north-western Australia: preliminary findings. J. R. Soc. West. Aust 94, 181–195 (2011).
Thresher, R. E., Bruce, B. D., Furlani, D. M. & Gunn, J. S. Distribution, advection, an growth of larvae of the southern temperate gadoid, Macruronus novaezelandiae (Teleostei: Merlucciidae), in Australian coastal waters. Fish. Bull. 87, 29–48 (1988).
Young, J. W. & Davis, T. L. O. Feeding ecology and interannual variations in diet of larval jack mackerel, Trachurus declivis (Pisces: Carangidae), from coastal waters of eastern Tasmania. Mar. Biol. 113, 11–20 (1992).
Ward, T. & Staunton-Smith, J. Comparison of the spawning patterns and fisheries biology of the sardine, Sardinops sagax, in temperate South Australia and sub-tropical southern Queensland. Fish. Res 56, 37–49 (2002).
Ward, T. M. et al. Pelagic ecology of a northern boundary current system: effects of upwelling on the production and distribution of sardine (Sardinops sagax), anchovy (Engraulis australis) and southern bluefin tuna (Thunnus maccoyii) in the Great Australian Bight. Fish. Oceanogr 15, 191–207 (2006).
Miskiewicz, A., Bruce, B. & Dixon, P. Distribution of tailor Pomatomus saltatrix larvae along the coast of New South Wales, Australia. Mar. Freshw. Res 47, 331–336 (1996).
Jordan, A., Pullen, G., Marshall, J. & Williams, H. Temporal and spatial patterns of spawning in jack mackerel, Trachurus declivis (Pisces: Carangidae), during 1988-91 in eastern Tasmanian waters. Mar. Freshw. Res 46, 831–842 (1995).
Gray, C. A. & Miskiewicz, A. G. Larval fish assemblages in south-east Australian coastal waters: seasonal and spatial structure. Estuar. Coast. Shelf Sci. 50, 549–570 (2000).
Smith, K. A., Gibbs, M. T., Middleton, J. H. & Suthers, I. M. Short term variability in larval fish assemblages of the Sydney shelf: tracers of hydrographic variability. Mar. Ecol. Prog. Ser. 178, 1–15 (1999).
Smith, K. A. & Suthers, I. M. Displacement of diverse ichthyoplankton assemblages by a coastal upwelling event on the Sydney shelf. Mar. Ecol. Prog. Ser. 176, 49–62 (1999).
Neira, F. J., Jenkins, G. P., Longmore, A. & Black, K. P. Spawning and larval recruitment processes of commercially important species in coastal waters off Victoria., Report No. Final Report FRDC Project No. 96/116 (Marine and Freshwater Resources Institute, Queenscliff: Victoria, Australia, 1998).
Uehara, S., Syahailatua, A. & Suthers, I. M. Recent growth rate of larval pilchards Sardinops sagax in relation to their stable isotope composition, in an upwelling zone of the East Australian Current. Mar. Freshw. Res. 56, 549–560 (2005).
Syahailatua, A., Roughan, M. & Suthers, I. M. Characteristic ichthyoplankton taxa in the separation zone of the East Australian Current: Larval assemblages as tracers of coastal mixing. Deep-Sea Res. Pt II 58, 678–690 (2011).
Syahailatua, A., Taylor, M. D. & Suthers, I. M. Growth variability and stable isotope composition of two larval carangid fishes in the East Australian Current: The role of upwelling in the separation zone. Deep-Sea Res. Pt II 58, 691–698 (2011).
Muhling, B. A. et al. Spawning, larval abundance and growth rate of Sardinops sagax off southwestern Australia: influence of an anomalous eastern boundary current. Mar. Ecol. Prog. Ser. 364, 157–167 (2008).
Muhling, B. A. & Beckley, L. E. Seasonal variation in horizontal and vertical structure of larval fish assemblages off south-western Australia, with implications for larval transport. J. Plankton Res. 29, 967–983 (2007).
Koslow, J. A. et al. The effect of the Leeuwin Current on phytoplankton biomass and production off Southwestern Australia. J. Geophys. Res. (Oceans) 113 (2008).
Muhling, B. A., Beckley, L. E., Koslow, J. A. & Pearce, A. F. Larval fish assemblages and water mass structure off the oligotrophic south-western Australian coast. Fish. Oceanogr 17, 16–31 (2008).
Mullaney, T. J., Miskiewicz, A. G., Baird, M. E., Burns, P. T. P. & Suthers, I. M. Entrainment of larval fish assemblages from the inner shelf into the East Australian Current and into the western Tasman Front. Fish. Oceanogr 20, 434–447 (2011).
Weller, E., Holliday, D., Feng, M., Beckley, L. E. & Thompson, P. A. A continental shelf scale examination of the Leeuwin Current off Western Australia during the austral autumn–winter. Cont. Shelf Res. 31, 1858–1868 (2011).
Sutton, A. L. & Beckley, L. E. Influence of the Leeuwin Current on the epipelagic euphausiid assemblages of the south-east Indian Ocean. Hydrobiologia 779, 193–207 (2016).
Matis, P. A. et al. Cyclonic entrainment? The ichthyoplankton attributes of three major water mass types generated by the separation of the East Australian Current. ICES J. Mar. Sci 71, 1696–1705 (2014).
Data Citations
Smit, J. A. et al. Australian Ocean Data Network https://doi.org/10.4225/69/5ab33c62f9c52 (2018)
Acknowledgements
We acknowledge the contributions of all collaborators and their institutions. We acknowledge the Australian Fisheries Management Authority who funded part of this study (grant: 2015/0819), as well as the Marine National Facility and the Australian Research Council who provided funding in support of many of these projects. We acknowledge the support of the Integrated Marine Observing System (IMOS), which currently undertakes the larval fish monitoring at the National Reference Stations (NRS; project P12). We are grateful to the personnel who collect these NRS samples each month. If using data from project P12 please add the following acknowledgement: “Data were sourced from the Integrated Marine Observing System (IMOS) – IMOS is a national collaborative research infrastructure, supported by the Australian Government.”
Author information
Authors and Affiliations
Contributions
J.A.S. drafted the manuscript with input from all authors; J.A.S., A.G.M., I.M.S. collated the data; J.K., J.M.L., A.G.M., F.J.N., I.M.S. created the database species list; J.A.S., J.D.E., J.K., A.L-L., J.M.L., A.G.M., F.J.N., A.R., I.M.S., K.S., T.W., P.v.R. contributed to development of this study and the NIMO program; L.E.B., V.G., C.A.G., A.R.J., P.A.M., A.G.M., B.A.M., F.J.N., I.M.S., K.A.S., A.S., M.D.T. collected and provided data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
ISA-Tab metadata
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata files made available in this article.
About this article
Cite this article
Smith, J., Miskiewicz, A., Beckley, L. et al. A database of marine larval fish assemblages in Australian temperate and subtropical waters. Sci Data 5, 180207 (2018). https://doi.org/10.1038/sdata.2018.207
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/sdata.2018.207
This article is cited by
-
Modelling the distribution of larval fish in a western boundary current using a multi-voyage database
Reviews in Fish Biology and Fisheries (2021)
-
Spatial and interannual variability of presettlement tropical fish assemblages explained by remote sensing oceanic conditions
Marine Biodiversity (2020)
-
A database of zooplankton biomass in Australian marine waters
Scientific Data (2020)