Abstract
The U.S. Geological Survey (USGS) maintains a place-based research program in San Francisco Bay (USA) that began in 1969 and continues, providing one of the longest records of water-quality measurements in a North American estuary. Constituents include salinity, temperature, light extinction coefficient, and concentrations of chlorophyll-a, dissolved oxygen, suspended particulate matter, nitrate, nitrite, ammonium, silicate, and phosphate. We describe the sampling program, analytical methods, structure of the data record, and how to access all measurements made from 1969 through 2015. We provide a summary of how these data have been used by USGS and other researchers to deepen understanding of how estuaries are structured and function differently from the river and ocean ecosystems they bridge.
Design Type(s) | time series design • observation design |
Measurement Type(s) | chlorophyll a • dissolved oxygen concentration • waterborne particulate matter • photoabsorption • water salinity • temperature of water • nutrient level |
Technology Type(s) | data acquisition system |
Factor Type(s) | spatiotemporal_interval |
Sample Characteristic(s) | San Francisco Bay • estuarine water |
Machine-accessible metadata file describing the reported data (ISA-Tab format)
Similar content being viewed by others
Background & Summary
On April 10 and 11, 1969 oceanographers from the U.S. Geological Survey (USGS) conducted the first hydrographic research cruise along the salinity gradient of San Francisco Bay (SFB)—one of the largest estuaries on the west coast of the Americas. Although it was not the researchers’ original intention, that survey launched an observational program that continues and expanded into a program of long-term ecosystem research that has contributed to the development of estuarine oceanography as a scientific discipline. In that era little was known about how estuaries function as transitional ecosystems between land and sea, where seawater and fresh water meet. Early USGS studies focused on: estuarine circulation where surface waters flow seaward over a landward-flowing bottom layer1; sediment accumulation in an estuarine turbidity maximum2; geomorphology3; marsh vegetation and land forms4; biogeochemistry of nutrients, oxygen and carbon5; benthic invertebrate communities6; urban pollution7, and its flushing by river inflows8.
Over time the research expanded into new domains to measure, model and understand: tidal circulation and transport processes9–11; human modifications of sediment supply12 and geomorphology13; sediment-water nutrient exchanges14,15; microbial biogeochemistry16,17; bioaccumulation and cycling of contaminants including petroleum hydrocarbons18, metals19, mercury20, PCBs21, and selenium22–24; disturbance by introduced species25,26; ecosystem metabolism27; phytoplankton communities28, productivity29, and regulating processes30,31; zooplankton ecology32; responses to climate variability33,34 and climate change35.
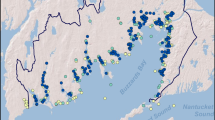
Central to this research is a core set of measurements repeated over time at a network of sampling sites (Fig. 1, Table 1) spaced along the estuarine salinity gradient. The San Francisco Bay system has been a useful place for studying estuarine dynamics because it includes two different estuary types. South Bay (stations 21–36) is an urbanized marine lagoon, and North Bay (stations 15–657) is the estuary of California’s two largest rivers, the Sacramento and San Joaquin. Central Bay connects South and North Bays to each other and to the coastal Pacific Ocean (Fig. 1). Thus, one goal of USGS research has been to compare two different estuary types36. The data set includes measurements of salinity, temperature, suspended particulate matter, light penetration, dissolved oxygen, chlorophyll-a as an indicator of phytoplankton biomass, and concentrations of dissolved inorganic N, P and Si. The sampling program maps longitudinal and vertical distributions of these estuarine properties and captures their variability at seasonal, annual and decadal time scales.
Station coordinates are given in Table 1.
The data described here were collected for one research purpose—to measure and understand how an estuarine ecosystem changes in response to human activities and the climate system. However, we recognized from the beginning of this effort that the data have value beyond this one purpose. We have encouraged and supported use of these data by others, and the diversity of applications of this data set has been both surprising and gratifying. We illustrate this diversity with examples of scientific articles (Table 2 (available online only)) that used the data for purposes we could not have imagined, ranging across disciplines of archaeology, geochemistry, hydrodynamics, ecotoxicology, conservation biology, sediment dynamics, and biology of organisms from microbes to seabirds. Some of these publications were collaborations with visiting scientists, postdocs and graduate students. Others were done independently of USGS research. The collective knowledge accumulated from this research over decades has contributed to the global understanding of estuaries as ecosystems situated where land, ocean, atmosphere and people converge. Our purpose here is to widen accessibility of these data so their value continues to grow.
Methods
USGS water-quality studies in San Francisco Bay include two types of measurements: (1) laboratory analyses of discrete water samples collected aboard ship (chlorophyll-a, dissolved oxygen, suspended particulate matter, dissolved inorganic nutrients), and (2) shipboard or submersible sensors to measure salinity, temperature, chlorophyll fluorescence, dissolved oxygen, turbidity, and light attenuation. The analyses of discrete water samples were used to calibrate the chlorophyll fluorescence, dissolved oxygen, and turbidity sensors, with individual calibrations for each sampling cruise, and often separate calibrations for each bay region. Therefore, the data record includes both discrete measurements (e.g., Discrete_Chlorophyll-a) and sensor-based in-situ measurements (e.g., Calculated_Chlorophyll-a).
From 1969 through March 1987 the discrete water samples were collected by submersible pump that delivered bay water to a shipboard fluorometer, nephelometer, thermistor, and conductivity sensor37. Vertical profiles were obtained by lowering the pump to prescribed depths, typically 0, 2, 5, 10, 20 m. Since April 1987 the discrete water samples have been collected near surface (~1.5 m) by pump and ~1 m above bottom with a Niskin bottle, and vertical profiles of salinity and temperature have been obtained with a Sea-Bird Electronics SBE-9 CTD. In 1988 turbidity, chlorophyll-a fluorescence, and photosynthetically active radiation (PAR) sensors were added to the CTD package, and in 1993 a dissolved oxygen sensor was added. In 2002 we started using a Sea-Bird Electronics SBE-9plus CTD. The individual sensors on the CTDs changed over time as new technologies emerged (see below). The CTD system is lowered through the water column at a rate<1 m s−1, collecting >24 samples/meter for Seabird sensors and >5 samples/meter for third party sensors. The values we report are averages over 1-m depth bins centered at the depth reported (i.e., CTD values are means of all measurements made 0.5 m above and 0.5 m below the reported depth).
Sampling design
This data set was acquired as a component of a research program whose goals evolved over time, so the frequency, spatial coverage, and makeup of water-quality measurements varied from year to year. We characterize sampling effort for five constituents, measured as the number of samples binned by station, month, and year (Fig. 2). Sampling effort was greatest in South Bay and during March and April, reflecting a key research objective to follow dynamics and ecological and biogeochemical consequences of the spring phytoplankton bloom30. The record reveals multi-year gaps in measurements of SPM and dissolved oxygen; that chlorophyll-a measurements first began in 1977; and that nutrient (e.g., phosphate) concentrations were measured less frequently than other constituents (Fig. 2). Sampling became more regular, and all constituents were measured each cruise starting in 1993 when this program became incorporated into the Regional Monitoring Program for Water Quality in San Francisco Bay (http://www.sfei.org/rmp).
Depth
Pump depth was determined using a pressure transducer with an accuracy of +/− 1 m. Readings at zero meters are representative of a 0.4 m intake depth. Beginning March 1987, depth was determined with a Paroscientific Digiquartz (http://www.paroscientific.com/) pressure transducer as part of a Sea-Bird Electronics CTD package.
Discrete_Chlorophyll-a
Chlorophyll-a measurements began in 1977, and methods changed as new instrumentation and widely-accepted standard methods emerged. Samples were collected onto Gelman GFF (glass fiber) filters and pigments were extracted with 90% acetone. The absorbance of the extracts was measured with a Varian 635D spectrophotometer following Strickland and Parsons38. Chlorophyll-a concentrations were calculated using the SCOR-UNESCO trichromatic equations39. Beginning in 1983, we used Lorenzen’s40 spectrophotometric equations. In 1992 we began using a Hewlett Packard 8452A diode array spectrophotometer. In 1999 we began measuring chlorophyll-a concentrations fluorometrically using the acidification method on a Turner Designs TD-700 fluorometer calibrated with chlorophyll-a standard41,42. Since 2011 we have used a Turner Designs Trilogy fluorometer. After each method change we compared results of the older and newer approach on replicate samples across a range of chlorophyll-a concentrations to verify that bias was not introduced as new instruments and methods were used.
Calculated_Chlorophyll-a
Vertical profiles of chlorophyll-a were derived from calculated concentrations based on calibrations of an in-vivo fluorometer done each cruise to account for variability of phytoplankton species assemblages and the relationship between chlorophyll-a and fluorescence43. Calibrations were linear regressions of Discrete_Chlorophyll-a (above) and in-vivo fluorescence measured initially with a Turner Designs Model 10 fluorometer connected to a pumped stream of bay water. Beginning in 1988, profiles were obtained with a SeaTech fluorometer connected to a Sea-Bird Electronics CTD. This was replaced with a Turner Designs SCUFA fluorometer in 2002, and with a Turner Designs C7 fluorometer in May 2004.
Discrete_DO
Water samples for dissolved oxygen measurement were collected into 300-ml BOD bottles that were filled from the bottom and allowed to overflow at least 3 times their volume. Winkler reagents38 were added immediately and bottles were stored capped with water in their cap-wells. In the laboratory, 100.2 ml of acidified sample was titrated manually following Carpenter44. Beginning in 1993, the samples were analyzed with a Metrohm 686 titroprocessor autotitrator38 using the potentiometric titration method of Granéli and Granéli45. Potassium iodate standardization of the sodium thiosulfate was conducted (Knapp et al., 1991). In 2007 the autotitrator was replaced with a Metrohm Titrino 798.
Calculated_DO
In 1993 we added a Sea-Bird Electronics SBE-13 sensor to the CTD package to obtain vertical profiles of dissolved oxygen. The sensor was calibrated prior to each cruise with 100% and zero saturation endpoints, and additionally with Discrete_DO measurements (above) each cruise. In 2002 we began using a Sea-Bird Electronics SBE-43 oxygen sensor calibrated each cruise with Discrete_DO measurements.
Discrete_SPM
Suspended particulate matter was measured gravimetrically as mass collected onto pre-weighed 0.45 μm pore-size silver filters (1969–1984) or polycarbonate 0.4 μm pore-size membrane filters (1993–2015). A correction was made for mass of salt retained on the filter46.
Calculated_SPM
Vertical profiles of suspended particulate matter were calculated concentrations derived from individual calibrations of a nephelometer or optical backscatter sensor each cruise. Calibrations were linear regressions of Discrete_SPM (above) and voltage output from a Turner Designs Model 10 fluorometer configured as a nephelometer connected to the pumped stream of bay water37. Beginning 1993, SPM profiles were obtained with a D&A Instrument Company (now Campbell Scientific) OBS-3 optical backscatter sensor as part of a Sea-Bird Electronics CTD package.
Extinction_coefficient
PAR (mol quanta m−2 s−1) was measured using a Li-Cor Biosciences LI-192 underwater quantum sensor (1977–1982, 1988–2015). The light extinction coefficient (k) was computed from the slope of the regression of ln(PAR) against water depth. Measurements were initially made at 6–7 depths per station. From 1983 through 1987 the light extinction coefficient was computed from Secchi depth SD (m) using an empirical relationship derived for San Francisco Bay: k=0.4+109/SD47. Beginning in 1988, the LI-192 sensor was deployed as part of a Sea-Bird Electronics CTD package collecting at least 28 measurements per meter, generating high-resolution vertical profiles of PAR.
Salinity
Salinity was initially measured with an Industrial Instruments RS5-3 induction salinometer (accuracy of 0.3 PSU), and beginning December 1969 with a CM2 model 516 CTD probe48. Output from that probe was verified each cruise with 6–12 duplicate water samples analyzed in the laboratory with a Beckman RS7-B salinometer calibrated with Copenhagen water (agreement of 0.2 PSU)48. Beginning July 1974, salinity was measured with an electrodeless induction salinometer, with outputs validated each cruise with duplicate samples run on the Beckman salinometer (agreement of 0.05 PSU)48. In March 1987 we began measuring salinity with a Sea-Bird Electronics SBE-4 conductivity sensor as part of the CTD package, and since 2002 with a SBE-4C conductivity sensor. Salinity was computed from conductivity, temperature, and pressure48.
Temperature
Temperature was initially measured with linear thermistors calibrated at ice point and near 20 °C each cruise49. Beginning March 1987, temperature was measured with a Sea-Bird Electronics SBE-3 temperature sensor as part of the CTD package, and since 2002 with a SBE-3plus temperature sensor.
Nutrients
Samples for dissolved inorganic nutrient analyses were filtered through polycarbonate 0.4-μm pore size membrane filters into bottles previously acid washed with 10% HCL. From 1971–2003, most sample bottles were pre-washed with acetone and 2.5 meq l−1 sodium bicarbonate instead of 10% HCL, with blank analyses confirming undetectable nutrient concentration. Sample filtrates were either analyzed immediately, refrigerated and analyzed within 48 h, or frozen until analysis. Frozen samples were allowed to thaw at room temperature for at least 14 hours before analysis. Samples were analyzed with a Technicon II AutoAnalyzer for: dissolved silica using Technicon Industrial Method 105-71WB50, dissolved reactive phosphate using the method of Atlas et al.51 with ascorbic acid as a reductant, nitrate+nitrite using Technicon Industrial Method AII 100-70 W52, and ammonium using the method of Solorzano53 and starting in 1980 with color development at 37 °C following Berg and Abdullah54. Standards for each analyte were prepared in artificial river water and artificial seawater38. Beginning in April 1991, the ammonium method was modified to improve precision as detailed in Hager46. Beginning in 2006, nutrients were stored frozen until analysis by the Richard Dugdale laboratory at San Francisco State University using a Bran and Luebbe AutoAnalyzer II for all nutrients except ammonium, which was analyzed by spectrophotometer. Nitrate, nitrite, and phosphate were determined according to Whitledge et al.55. Silicate was determined with Bran Luebbe AutoAnalyzer Method No. G-177-96 (ref. 56). Ammonium was determined with the method of Solorzano53. Beginning in March 2014, nutrients were analyzed by the USGS National Water Quality Laboratory with a Thermo Scientific Aquakem 600 automated discrete analyzer using methods of Fishman and Friedman57 for nitrite, phosphate, and silicate, the method of Patton and Kryskalla58 for nitrate, and the Solorzano method53 for ammonium with a salt correction factor applied59.
Data Records
The dataset includes the following fields for each record:
Date: format MM/DD/YY
Station_Number: locations shown in Fig. 1 and provided in Table 1
Depth: sampled depth below the surface (m)
Discrete_Chlorophyll-a: chlorophyll-a measured in a water sample (μg l−1)
Calculated_Chlorophyll-a: chlorophyll-a calculated from in-vivo fluorescence (μg l−1)
Discrete_Oxygen: dissolved oxygen measured in a water sample (mg l−1)
Calculated_Oxygen: dissolved oxygen calculated from an oxygen sensor (mg l−1)
Discrete_SPM: suspended particulate matter measured in a water sample (mg l−1)
Calculated_SPM: suspended particulate matter calculated from a turbidity sensor (mg l−1)
Extinction_Coefficient: light extinction coefficient (m−1)
Salinity: Practical Salinity Units (PSU)
Temperature: water temperature (°C)
Nitrite: nitrite concentration (μM)
Nitrate+Nitrite: sum of nitrate and nitrite concentration (μM)
Ammonium: ammonium concentration (μM)
Phosphate: phosphate concentration (μM)
Silicate: silicate concentration (μM)
Data record 1
The dataset includes 210,826 records, each representing a water sample from a unique date, station, and depth. All measurements made between 4/10/69 and 12/16/15 are available in one csv file (SanFranciscoBayWaterQualityData1969-2015v3.csv) uploaded to the USGS ScienceBase repository (Data Citation 1). An xml-formatted metadata file is also available at that repository.
Technical Validation
Results from each sampling cruise were examined carefully by at least two members of the research team to ensure that all values fell within expected ranges, to verify that calibration regressions were an acceptable basis for computing quantities from shipboard sensor measurements, to ensure completeness of each cruise data report, and to verify that values transcribed from field notes were accurate. The complete 1969–2015 data set was validated with three steps: (1) range tests to ensure that the measured values fell within ranges that are plausible and consistent with knowledge of San Francisco Bay and other estuaries; (2) pattern tests of time series of all measurements to ensure they followed plausible and understandable patterns of variability over time; (3) pattern tests of all measurements by sampling station to ensure they followed plausible and understandable spatial patterns.
Sea-Bird Electronics sensors were calibrated annually by the manufacturer and have initial accuracies of: temperature=±0.001 °C, conductivity=±0.0003 mS m−1, pressure=±0.015% of full range, dissolved oxygen=±2% of saturation (http://www.seabird.com). Li-Cor LI192 sensors were calibrated by the manufacturer and sensitivity is typically 4 μA per 1,000 μmol m−2 s−1 (https://www.licor.com). Cruise-specific calibrations of shipboard fluorometers, nephelometer/optical backscatter, and oxygen sensors yielded highly significant (P<10−16) linear relationships between all discrete and calculated concentrations of chlorophyll-a, SPM and DO (Fig. 3). Median absolute deviations between discrete and calculated concentrations were: 0.40 μg l−1 for chlorophyll-a; 2.10 mg l−1 for SPM; 0.10 mg l−1 for DO. Linear regressions yielded residual standard errors between discrete and calculated concentrations of: 1.36 μg l−1 for chlorophyll-a; 8.2 mg l−1 for SPM; 0.16 mg l−1 for DO (Fig. 3).
Discrete measurements are laboratory analyses of water samples; calculated values are derived from ship-based sensors calibrated each sampling cruise with discrete measurements. Red lines are linear regressions; n, number of paired samples; R2, adjusted correlation coefficient from linear regression; RSE is the residual standard error of the regression, an estimator of error in calculated concentrations from sensors.
Discrete chlorophyll-a values are mean concentrations in replicate (2, 3, or 4) aliquots from each sample. If the replicate results differed by more than 10% of their mean the results were not included in the data set. The mean coefficient of variation between replicate aliquots from 3,564 chlorophyll-a samples collected between 2005 and 2013 was 2.4%. Agreement between all replicates was within the recommended guideline for the method: >90% of the coefficients of variation (CV) between samples are <5% (ref. 42). Discrete suspended particulate matter precision was 1%-10%. Analytical precision of the potentiometric DO method is <0.3% (ref. 45).
Nutrients analysed at the USGS Menlo Park (USGS-MP) laboratory from 1969–2005 had a typical precision of 0.02–0.2 μM for ammonium, 0.04–0.17 μM for nitrate+nitrite, 0.01–0.05 μM for nitrite, 0.01–0.05 μM for phosphate, and 0.06–1.0 μM for silicate46,60–63. Beginning in 2006, nutrients were analyzed by the Richard Dugdale laboratory at San Francisco State University (SFSU). That transition began after verification of acceptable agreement in analyses of nutrient standards or San Francisco Bay samples by the USGS-MP and SFSU laboratories (Fig. 4). Ammonium was analysed using different methodologies, but replicate samples analysed in each laboratory confirmed consistency between them (Fig. 4). The SFSU laboratory reported detection limits as 0.05 μM for ammonium, nitrite, nitrate+nitrite and phosphate, and 0.1 μM for silicate.
As a preliminary step in the 2014 transition from SFSU to the USGS National Water Quality Laboratory (USGS-NWQL), we collected triplicate water samples along the salinity gradient of San Francisco Bay to compare analyses by SFSU, USGS-NWQL, and the Chesapeake Biological Laboratory (CBL) as an independent laboratory. We continued analysis of duplicate samples by USGS-NWQL and CBL through 2015. We compare results of the three laboratories in Fig. 5. The USGS-NWQL has the following minimum reporting levels: 0.7 μM ammonium, 0.1 μM nitrite and phosphate, 0.7 μM nitrate+nitrite when total<10 μM, 2.9 μM when total >10 μM, and 1.0 μM silicate. Replicate samples are intermittently analysed by USGS-NWQL to measure precision. Replicates have mean coefficients of variation<5% for all nutrients: nitrite=3.1%, nitrate+nitrite=2.3%, ammonium=4.6%, phosphate=1.6%, and silicate=0.01%.
USGS National Water Quality Laboratory (USGS NWQL), San Francisco State University (SFSU), and Chesapeake Biological Laboratory (CBL). Each comparison includes the linear regression equation, adjusted R2, and Residual Standard Error (RSE) as an estimator of the mean difference between laboratories. Comparisons of silicate analyses were only done for USGS NWQL and CBL.
Although nutrient methods changed over time, routine analyses of blanks and standards confirmed that methods changes did not reduce analytical precision or accuracy.
Usage Notes
This Data Descriptor identifies a csv file that contains the complete record of USGS water-quality measurements made in San Francisco Bay from 1969–2015. Users may prefer to access the data from our project web page that includes a database from which queries can be made to select and download subsets of the full data record (https://sfbay.wr.usgs.gov/access/wqdata/index.html ). This web page also provides visual displays of water-quality spatial variability for each sampling cruise, and more detail about the research project and team members.
Additional Information
How to cite this article: Schraga, T. S. & Cloern, J. E. Water quality measurements in San Francisco Bay by the U.S. Geological Survey, 1969–2015. Sci. Data 4:170098 doi: 10.1038/sdata.2017.98 (2017).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
References
Conomos, T. J., Peterson, D. H., Carlson, P. R. & McCulloch, D. S. A preliminary study of the effects of water circulation in the San Francisco Bay estuary - Movement of sea bed drifters in the San Francisco Bay estuary and the adjacent Pacific Ocean. USGS Publications Warehouse https://pubs.er.usgs.gov/publication/cir637AB U.S. Geological Survey Circular (1970).
Peterson, D. H., Conomos, T. J., Broenkow, W. W. & Doherty, P. C. Location of the non-tidal current null zone in northern San Francisco Bay. Estuarine and Coastal Marine Science 3, 1–11 (1975).
Rubin, D. M. & McCulloch, D. S. in San Francisco Bay—The Urbanized Estuary (ed. Conomos, T. J.) 97–114 (American Association for the Advancement of Science, Pacific Division, 1979).
Atwater, B. F. et al. in San Francisco Bay, the Urbanized Estuary (ed. Conomos, T. J. ) 347–386 (American Association for the Advancement of Science, Pacific Division, 1979).
Peterson, D. H. in San Francisco Bay—The Urbanized Estuary (ed. Conomos, T. J. 175–194 (American Association for the Advancement of Science, Pacific Division, 1979).
Nichols, F. H. in San Francisco Bay—The Urbanized Estuary (ed. Conomos, T. J. ) 409–426 (American Association for the Advancement of Science, Pacific Division, 1979).
Luoma, S. N. & Cain, D. J. in San Francisco Bay—The Urbanized Estuary (ed. Conomos, T. J. 231–246 (American Association for the Advancement of Science, Pacific Division, 1979).
McCulloch, D. S., Peterson, D. H., Carlson, P. R. & Conomos, T. J. A preliminary study of the effects of water circulation in the San Francisco Bay estuary - Some effects of fresh-water inflow on the flushing of south San Francisco Bay. USGS Publications Warehouse https://pubs.er.usgs.gov/publication/cir637AB (1970).
Cheng, R. T., Casulli, V. & Gartner, J. W. Tidal, residual, intertidal mudflat (TRIM) model and its applications to San Francisco Bay, California. Estuarine, Coastal and Shelf Science 36, 235–280 (1993).
Cheng, R. T. & Casulli, V. On Lagrangian residual currents with applications in south San Francisco Bay, California. Water Resources Research 18, 1652–1662 (1982).
Walters, R. A., Cheng, R. T. & Conomos, T. J. Time scales of circulation and mixing processes of San Francisco Bay waters. Hydrobiologia 129, 13–36 (1985).
Wright, S. A. & Schoellhamer, D. H. Trends in the sediment yield of the Sacramento River, California, 1957-2001. San Francisco Estuary and Watershed Science 2, 1–14 (2004).
Jaffe, B. E., Smith, R. E. & Foxgrover, A. C. Anthropogenic influence on sedimentation and intertidal mudflat change in San Pablo Bay, California: 1856–1983. Estuarine Coastal and Shelf Science 73, 175–187 (2007).
Grenz, C., Cloern, J., Hager, S. & Cole, B. Dynamics of nutrient cycling and related benthic nutrient and oxygen fluxes during a spring phytoplankton bloom in South San Francisco Bay (USA). Marine Ecology-Progress Series 197, 67–80 (2000).
Caffrey, J. M. Spatial and seasonal patterns in sediment nitrogen remineralization and ammonium concentrations in San Francisco Bay, California. Estuaries 18, 219–233 (1995).
Oremland, R. S., Marsh, L. & Polcin, S. Methane production and simultaneous sulfate reduction in anoxic, salt marsh sediments. Nature 296, 143–145 (1982).
Miller, L. G., Oremland, R. S. & Paulsen, S. Measurement of N20 reductase activity in aquatic sediments. Applied and Environmental Microbiology 51, 18–24 (1986).
Pereira, W. E., Hostettler, F. D. & Rapp, J. B. Bioaccumulation of hydrocarbons derived from terrestrial and anthropogenic sources in the Asian clam, Potamocorbula amurensis, in San Francisco Bay estuary. Marine Pollution Bulletin 24, 103–109 (1992).
Luoma, S., van Geen, A., Lee, B. & Cloern, J. Metal uptake by phytoplankton during a bloom in South San Francisco Bay: Implications for metal cycling in estuaries. Limnology and Oceanography 43, 1007–1016 (1998).
Marvin-DiPasquale, M. C., Agee, J. L., Bouse, R. M. & Jaffe, B. E. Microbial cycling of mercury in contaminated pelagic and wetland sediments of San Pablo Bay, California. Environmental Geology 43, 260–267 (2003).
McLeod, P. B., Van Den Heuvel-Greve, M. J., Luoma, S. N. & Luthy, R. G. Biological uptake of polychlorinated biphenyls by Macoma balthica from sediment amended with activated carbon. Environ. Toxicol. Chem. 26, 980–987 (2007).
Johns, C., Luoma, S. N. & Elrod, V. Selenium accumulation in benthic bivalves and fine sediments of San Francisco Bay, the Sacramento-San Joaquin Delta, and selected tributaries. Estuarine, Coastal and Shelf Science 27, 381–396 (1988).
Luoma, S. N. et al. Determination of selenium bioavailability to a benthic bivalve from particulate and solute pathways. Environmental Science and Technology 26, 485–491 (1992).
Stewart, A. R., Luoma, S. N., Schlekat, C. E., Doblin, M. A. & Hieb, K. A. Food web pathway determines how selenium affects aquatic ecosystems: A San Francisco Bay case study. Environmental Science and Technology 38, 4519–4526 (2004).
Alpine, A. E. & Cloern, J. E. Trophic interactions and direct physical effects control phytoplankton biomass and production in an estuary. Limnology and Oceanography 37, 946–955 (1992).
Thompson, J. K. in The Comparative Roles of Suspension Feeders in Ecosystems (eds Olenin, S. & Dame, R. ) 291–316 (Springer, 2005).
Caffrey, J., Cloern, J. & Grenz, C. Changes in production and respiration during a spring phytoplankton bloom in San Francisco Bay, California, USA: implications for net ecosystem metabolism. Marine Ecology-Progress Series 172, 1–12 (1998).
Cloern, J. & Dufford, R. Phytoplankton community ecology: principles applied in San Francisco Bay. Marine Ecology-Progress Series 285, 11–28 (2005).
Cole, B. E. & Cloern, J. E. Significance of biomass and light availability to phytoplankton productivity in San Francisco Bay. Marine Ecology Progress Series 17, 15–24 (1984).
Cloern, J. Phytoplankton bloom dynamics in coastal ecosystems: A review with some general lessons from sustained investigation of San Francisco Bay, California. Rev. Geophys. 34, 127–168 (1996).
Lucas, L., Koseff, J., Monismith, S., Cloern, J. & Thompson, J. Processes governing phytoplankton blooms in estuaries. II: The role of horizontal transport. Marine Ecology-Progress Series 187, 17–30 (1999).
Ambler, J. W., Cloern, J. E. & Hutchinson, A. in Temporal Dynamics of an Estuary: San Francisco Bay Developments in Hydrobiology (eds Cloern, J. E. & Nichols, F. H. ) 177–197 (Springer Netherlands, 1985).
Peterson, D., Cayan, D., Dileo, J., Noble, M. & Dettinger, M. The role of climate in estuarine variability. American Scientist 83, 58–67 (1995).
Cloern, J. et al. Biological communities in San Francisco Bay track large-scale climate forcing over the North Pacific. Geophysical Research Letters 37, L21602 (2010).
Cloern, J. E. et al. Projected evolution of California’s San Francisco Bay-Delta-River System in a century of climate change. PLoS ONE 6, e24465 (2011).
Cloern, J. E. & Nichols, F. H. Temporal Dynamics of an Estuary: San Francisco Bay Developments in Hydrobiology Volume 30 (Springer, 1985).
Schemel, L. E. & Dedini, L. A. A Continuous Water-sampling and Multi-parameter Measurement System for Estuaries. USGS Publications Warehouse https://pubs.er.usgs.gov/publication/ofr79273 (1979).
Strickland, J. D. H. & Parsons, T. R. A Practical Handbook of Seawater Analysis, Bulletin 167 2nd edn (Fisheries Research Board of Canada Bulletin, 1972).
SCOR-UNESCO. Determination of Photosynthetic Pigments in Seawater, Monographs on Oceanographic Methodology 1 (UNESCO 1966).
Lorenzen, C. J. Determination of chlorophyll and phaeopigments: spectrophotometric equations. Limnology And Oceanography 12, 343–346 (1967).
Jeffrey, S. W., Mantoura, R. F. C. & Wright, S. W . Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods Vol. 10 (UNESCO, 1997).
Arar, E. & Collins, G. Method 445 0: In-Vitro Determination of Chlorophyll a and Pheophytin a in Marine and Freshwater Algae by Fluorescence. US Environmental Protection Agency https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=309417 (1997).
Alpine, A. E. & Cloern, J. E. Differences in In-vivo fluorescence yield between 3 phytoplankton size classes. Journal of Plankton Research 7, 381–390 (1985).
Carpenter, J. H. The Chesapeake Bay Institute Technique for the Winkler oxygen method. Limnology and Oceanography 10, 141–143 (1965).
Granéli, W. & Granéli, E. Automatic potentiometric determination of dissolved oxygen. Mar. Biol. 108, 341–348 (1991).
Hager, S. W. Dissolved nutrient and suspended particulate matter data for the San Francisco Bay Estuary, California, October1988 through September 1991. USGS Publications Warehouse https://pubs.er.usgs.gov/publication/ofr9357 (1993).
Alpine, A. A., Wienke, S. M., Cloern, J. E. & Cole, B. E. Plankton studies in San Francisco Bay IX, chlorophyll distributions and hydrographic properties of South San Francisco Bay, 1984-86. USGS Publications Warehouse https://pubs.er.usgs.gov/publication/ofr88319 (1988).
Fofonoff, N. P. & Millard, R. C. Jr. Algorithms for Computation of Fundamental Properties of Seawater. (UNESCO, 1983).
Smith, R. E., Herndon, R. E. & Harmon, D. D. Physical and chemical properties of San Francisco Bay waters, 1969–1976. USGS Publications Warehouse https://pubs.er.usgs.gov/publication/ofr79511 (1979).
Technicon Industrial Systems. Silicates in water and wastewater. Industrial Method Number, 105-71WB (Technicon Industrial Systems, Tarrytown, NY., 1976).
Atlas, E. L., Hager, S. W., Gordon, L. I. Park, P. K. and Oregon State University of Corvallis Dept. of Oceanography. A Practical Manual For Use Of The Technicon Autoanalyzer in Seawater Nutrient Analyses: Revised (Defence Technical Information Center, (1971).
Technicon Industrial Systems. Nitrate and nitrite in water and wastewater. Industrial Method Number, 100-70W (Technicon Industrial Systems, Tarrytown, NY., 1973).
Solorzano, L. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnology and Oceanography 14, 799–801 (1969).
Berg, B. R. & Abdullah, M. I. An automated method on the determination of ammonia in seawater. Water Research 2, 637–638 (1977).
Whitledge, T. E., Malloy, S. C., Patton, C. J. & Wirick, C. D . Automated nutrient analysis in seawater, Report BNL 51398 216 (Brookhaven National Laboratory, 1981).
Bran Luebbe Inc. Bran Luebbe Autoanalyzer Applications: AutoAnalyzer Method No. G-177-96 Silicate in Water and Seawater. (Bran Luebbe, Inc, 1999).
Fishman, M. J. & Friedman, L. C. Methods for determination of inorganic substances in water and fluvial sediment. USGS Publications Warehouse. https://pubs.er.usgs.gov/publication/twri05A1_1979 (1989).
Patton, C. J. & Kryskalla, J. R. Methods of analysis by the U.S. Geological Survey National Water Quality Laboratory: evaluation of alkaline persulfate digestion as an alternative to Kjeldahl digestion for determination of total and dissolved nitrogen and phosphorus in water. USGS Publications Warehouse. https://pubs.er.usgs.gov/publication/wri034174. (2003).
Stewart, B. M. & Elliott, P. A. W. Systematic salt effects in the automated determination of nutrients in seawater. Water Research 30, 869–874 (1996).
Ota, A. Y., Schemel, L. E. & Hager, S. W. Physical and chemical data for northern San Francisco Bay, California, September through November, 1984. USGS Publications Warehouse.s https://pubs.er.usgs.gov/publication/ofr86228 (1986).
Ota, A. Y., Schemel, L. E. & Hager, S. W. Dissolved nutrient and suspended particulate matter data for the San Francisco Bay estuary, California, October 1988 through September 1991. USGS Publications Warehouse https://pubs.er.usgs.gov/publication/ofr9357 (1993).
Hager, S. W. Dissolved nutrient data for the San Francisco Bay estuary, California, February through November 1994. USGS Publications Warehouse https://pubs.er.usgs.gov/publication/ofr9717 (1997).
Hager, S. W. & Schemel, L. E. Dissolved nutrient data for the San Francisco Bay estuary, California, January through November 1995. USGS Publications Warehouse. https://pubs.er.usgs.gov/publication/ofr97359 (1997).
Eerkens, J. W., Byrd, B. F., Spero, H. J. & Fritschi, A. K. Stable isotope reconstructions of shellfish harvesting seasonality in an estuarine environment: implications for Late Holocene San Francisco Bay settlement patterns. Journal of Archaeological Science 40, 2014–2024 (2013).
Luengen, A. C., Raimondi, P. T. & Flegal, A. R. Contrasting biogeochemistry of six trace metals during the rise and decay of a spring phytoplankton bloom in San Francisco Bay. Limnology and Oceanography 52, 1112–1130 (2007).
Smith, S. V. & Hollibaugh, J. T. Water, salt, and nutrient exchanges in San Francisco Bay. Limnology and Oceanography 51, 504–517 (2006).
Luengen, A. C. & Flegal, A. R. Role of phytoplankton in mercury cycling in the San Francisco Bay estuary. Limnology and Oceanography 54, 23–40 (2009).
Schaefer, S. C. & Hollibaugh, J. T. Temperature decouples ammonium and nitrite oxidation in coastal waters. Environmental Science & Technology 51, 3157–3164 (2017).
Nicolini, M. H. & Penry, D. L. Spawning, fertilization, and larval development of Potamocorbula amurensis (Mollusca:Bivalvia) from San Francisco Bay, California. Pac. Sci. 54, 377–388 (2000).
Parchaso, F. & Thompson, J. K. Influence of hydrologic processes on reproduction of the introduced bivalve Potamocorbula amurensis in northern San Francisco Bay, California. Pacific Science 56, 329–345 (2002).
De la Cruz, S. E. W. et al. Resource selection and space use by sea ducks during the non-breeding season: Implications for habitat conservation planning in urbanized estuaries. Biological Conservation 169, 68–78 (2014).
Kimmerer, W. Open water processes of the San Francisco Estuary: From physical forcing to biological responses. San Francisco Estuary and Watershed Science 2 (2004).
Spencer, M., Fisher, N. S., Wang, W. X. & Ferson, S. Temporal variability and ignorance in Monte Carlo contaminant bioaccumulation models: A case study with selenium in Mytilus edulis. Risk Analysis 21, 383–394 (2001).
Lee, B. G. & Luoma, S. N. Influence of microalgal biomass on absorption efficiency of Cd, Cr, and Zn by two bivalves from San Francisco Bay. Limnology and Oceanography 43, 1455–1466 (1998).
Chen, L., Meseck, S. L., Roy, S. B., Grieb, T. M. & Baginska, B. Modeling fate, transport, and biological uptake of Selenium in North San Francisco Bay. Estuaries and Coasts 35, 1551–1570 (2012).
Kimmerer, W. J. Response of anchovies dampens effects of the invasive bivalve Corbula amurens is on the San Francisco Estuary foodweb. Marine Ecology Progress Series 324, 207–218 (2006).
Loboschefsky, E. et al. Individual-level and population-level historical prey demand of San Francisco Estuary striped bass using a bioenergetics model. San Francisco Estuary and Watershed Science 10, 1–23 (2012).
Lesen, A. E. Sediment organic matter composition and dynamics in San Francisco Bay, California, USA: Seasonal variation and interactions between water column chlorophyll and the benthos. Estuarine, Coastal and Shelf Science 66, 501–512 (2006).
Bessinger, B. et al. A kinetic model of copper cycling in San Francisco Bay. San Francisco Estuary and Watershed Science 4, 1–27 (2006).
Bundy, R. M. et al. Iron-binding ligands and humic substances in the San Francisco Bay estuary and estuarine-influenced shelf regions of coastal California. Marine Chemistry 173, 183–194 (2015).
McLaughlin, K., Kendall, C., Silva, S. R., Young, M. & Paytan, A. Phosphate oxygen isotope ratios as a tracer for sources and cycling of phosphate in North San Francisco Bay, California. Journal of Geophysical Research 111, 1–12 (2006).
Davis, J. A. The long-term fate of polychlorinated biphenyls in San Francisco Bay (USA). Environ. Toxicol. Chem. 23, 2396–2409 (2004).
Hatje, V., Bruland, K. W. & Flegal, A. R. Increases in anthropogenic gadolinium anomalies and rare Earth element concentrations in San Francisco Bay over a 20 year record. Environ Sci Technol 50, 4159–4168 (2016).
Chua, V. P. & Fringer, O. B. Sensitivity analysis of three-dimensional salinity simulations in North San Francisco Bay using the unstructured-grid SUNTANS model. Ocean Modelling 39, 332–350 (2011).
Chua, V. P. & Xu, M. Impacts of sea-level rise on estuarine circulation: An idealized estuary and San Francisco Bay. Journal of Marine Systems 139, 58–67 (2014).
Fram, J. P., Martin, M. A. & Stacey, M. T. Dispersive fluxes between the coastal ocean and a semienclosed estuarine basin. Journal of Physical Oceanography 37, 1645–1660 (2007).
Ganju, N. K. & Schoellhamer, D. H. Calibration of an estuarine sediment transport model to sediment fluxes as an intermediate step for simulation of geomorphic evolution. Continental Shelf Research 29, 148–158 (2009).
Gross, E. S., MacWilliams, M. L. & Kimmerer, W. J. Three-dimensional modeling of tidal hydrodynamics in the San Francisco Estuary. San Francisco Estuary and Watershed Science 7, 1–37 (2009).
MacWilliams, M. L. et al. Three-dimensional modeling of hydrodynamics and salinity in the san francisco estuary: An evaluation of model accuracy, X2, and the low salinity zone. San Francisco Estuary and Watershed Science 13, 1–37 (2015).
Monismith, S. G., Kimmerer, W., Burau, J. R. & Stacey, M. T. Structure and flow-induced variability of the subtidal salinity field in northern San Francisco Bay. Journal of Physical Oceanography 32, 3003–3019 (2002).
Lesen, A. E. Relationship between benthic foraminifera and food resources in South San Francisco Bay, California, USA. Marine Ecology Progress Series 297, 131–145 (2005).
Mosier, A. C. & Francis, C. A. Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environmental Microbiology 10, 3002–3016 (2008).
Murrell, M. C., Hollibaugh, J. T., Silver, M. W. & Wong, P. S. Bacterioplankton dynamics in northern San Francisco Bay: Role of particle association and seasonal freshwater flow. Limnology and Oceanography 44, 295–308 (1999).
Damashek, J., Casciotti, K. L. & Francis, C. A. Variable nitrification rates across environmental gradients in turbid, nutrient-rich estuary waters of San Francisco Bay. Estuaries and Coasts 39, 1050–1071 (2016).
Murrell, M. C. & Hollibaugh, J. T. Microzooplankton grazing in northern San Francisco Bay measured by the dilution method. Aquatic Microbial Ecology 15, 53–63 (1998).
Lesen, A. E. & Lipps, J. H. What have natural and human changes wrought on the foraminifera of San Francisco Bay late Quaternary estuaries? Quaternary Research 76, 211–219 (2011).
Esparza, M. L. et al. Impact of atypical ammonium concentrations on phytoplankton abundance and composition in fresh versus estuarine waters. Aquatic Biology 21, 191–204 (2014).
Kimmerer, W. Long-term changes in apparent uptake of silica in the San Francisco estuary. Limnology and Oceanography 50, 793–798 (2005).
Newcomer, M. E. et al. Estuarine sediment deposition during wetland restoration: a GIS and remote sensing modelling approach. Geocarto International 29, 451–467 (2014).
Boroumand, A. & Rajaee, T. Discrete entropy theory for optimal redesigning of salinity monitoring network in San Francisco bay. Water Science and Technology: Water Supply 17, 606–612 (2017).
Goodwin, D. H., Cohen, A. N. & Roopnarine, P. D. Forensics on the half shell: A sclerochronological investigation of a modern biological invasion in San Francisco Bay, United States. Palaios 25, 742–753 (2010).
Goodwin, D. H., Gillikin, D. P. & Roopnarine, P. D. Preliminary evaluation of potential stable isotope and trace element productivity proxies in the oyster Crassostrea gigas. Palaeogeography Palaeoclimatology Palaeoecology 373, 88–97 (2013).
Bever, A. J. & MacWilliams, M. L. Simulating sediment transport processes in San Pablo Bay using coupled hydrodynamic, wave, and sediment transport models. Marine Geology 345, 235–253 (2013).
Manning, A. J. & Schoellhamer, D. H. Factors controlling floc settling velocity along a longitudinal estuarine transect. Marine Geology 345, 266–280 (2013).
McGann, M., Erikson, L., Wan, E., Powell, C. & Maddocks, R. F. Distribution of biologic, anthropogenic, and volcanic constituents as a proxy for sediment transport in the San Francisco Bay Coastal System. Marine Geology 345, 113–142 (2013).
Shellenbarger, G. G., Wright, S. A. & Schoellhamer, D. H. A sediment budget for the southern reach in San Francisco Bay, CA: Implications for habitat restoration. Marine Geology 345, 281–293 (2013).
Schoellhamer, D. H. Sudden clearing of estuarine waters upon crossing the threshold from transport to supply regulation of sediment transport as an erodible sediment pool is depleted: San Francisco Bay, 1999. Estuaries and Coasts 34, 885–899 (2011).
Chang, A. L. et al. Tackling aquatic invasions: risks and opportunities for the aquarium fish industry. Biological Invasions 11, 773–785 (2009).
Schoellhamer, D. H. Teaching estuarine hydrology with online data. Estuaries and Coasts 32, 1069–1078 (2009).
Gifford, S. M., Rollwagen-Bollens, G. & Bollens, S. M. Mesozooplankton omnivory in the upper San Francisco Estuary. Marine Ecology Progress Series 348, 33–46 (2007).
Rollwagen Bollens, G. C. & Penry, D. L. Feeding dynamics of Acartia spp. copepods in a large, temperate estuary (San Francisco Bay, CA). Marine Ecology Progress Series 257, 139–158 (2003).
Rollwagen-Bollens, G. C., Bollens, S. M. & Penry, D. L. Vertical distribution of micro- and nanoplankton in the San Francisco Estuary in relation to hydrography and predators. Aquatic Microbial Ecology 44, 143–163 (2006).
Rollwagen-Bollens, G., Gifford, S. & Bollens, S. M. The role of protistan microzooplankton in the upper San Francisco Estuary planktonic food web: source or sink? Estuaries and Coasts 34, 1026–1038 (2011).
Hooff, R. C. & Bollens, S. M. Functional response and potential predatory impact of Tortanus dextrilobatus, a carnivorous copepod recently introduced to the San Francisco Estuary. Marine Ecology Progress Series 277, 167–179 (2004).
Bollens, S. M., Breckenridge, J. K., Vanden Hooff, R. C. & Cordell, J. R. Mesozooplankton of the lower San Francisco Estuary: spatio-temporal patterns, ENSO effects and the prevalence of non-indigenous species. Journal of Plankton Research 33, 1358–1377 (2011).
Data Citations
Cloern, J. E., & Schraga, T. S. U.S. Geological Survey https://doi.org/10.5066/F7TQ5ZPR787700 (2016)
Acknowledgements
Long-term USGS research in San Francisco Bay has been supported by: USGS National Research Program; USGS Priority Ecosystem Science; Regional Monitoring Program for Water Quality in San Francisco Bay; and the San Francisco Bay Nutrient Management Strategy.
Author information
Authors and Affiliations
Contributions
T.S.S and J.E.C. wrote the manuscript. Both authors directed the research program that acquired and organized the data record and made it available at the USGS ScienceBase repository.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
ISA-Tab metadata
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ The Creative Commons Public Domain Dedication waiver http://creativecommons.org/publicdomain/zero/1.0/ applies to the metadata files made available in this article.
About this article
Cite this article
Schraga, T., Cloern, J. Water quality measurements in San Francisco Bay by the U.S. Geological Survey, 1969–2015. Sci Data 4, 170098 (2017). https://doi.org/10.1038/sdata.2017.98
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/sdata.2017.98
This article is cited by
-
Evolutionary divergence and adaptive capacity in morphologically distinct song sparrow subspecies
Conservation Genetics (2023)
-
Paired Synoptic and Long-Term Monitoring Datasets Reveal Decadal Shifts in Suspended Sediment Supply and Particulate Organic Matter Sources in a River-Estuarine System
Estuaries and Coasts (2023)
-
Escape from the heat: thermal stratification in a well-mixed estuary and implications for fish species facing a changing climate
Hydrobiologia (2022)
-
In-depth Spatiotemporal Characterization of Planktonic Archaeal and Bacterial Communities in North and South San Francisco Bay
Microbial Ecology (2021)
-
Increasing threat of coastal groundwater hazards from sea-level rise in California
Nature Climate Change (2020)