Abstract
Introduction:
Surfer’s myelopathy (SM) is a rare disorder described in subjects presenting with acute paraparesis while learning how to surf. It is thought to be secondary to spinal ischemia triggered by hyperextension. Spinal magnetic resonance imaging (MRI) shows changes consistent with spinal cord ischemia on T2-weighted and diffusion-weighted imaging (DWI).
Case Presentation:
We report two patients who presented with acute onset paraplegia shortly after spinal hyperextension. They had no physical or radiological evidence of soft tissue injury. Their clinical and imaging findings closely resemble those described in SM.
Discussion:
We propose the use of the term ‘acute hyperextension myelopathy’ to categorize patients with spinal cord infarction secondary to hyperextension. DWI sequencing on MRI should be considered to evaluate for early signs of spinal cord ischemia in these patients. Use of a broader term for diagnostic classification can help include patients with spinal cord infarction due to a common mechanism.
Similar content being viewed by others
Introduction
Surfer’s myelopathy (SM) is a rare disorder consistent of acute onset paraparesis in novice surfers. It was first described by Thompson et al.1 It is thought to be secondary to spinal ischemia triggered by hyperextension, but its exact pathophysiology remains unclear.1–3 The initial symptoms are typically back pain and lower limb weakness when the patient tries to get off the surfing board. Symptoms usually worsen over the next few hours. During that period, patients develop hyperalgesia, hyperesthesia with a spinal level, urinary retention and increasing weakness, sometimes to the point of paraplegia. Magnetic resonance imaging (MRI) of the spine typically shows increased signal on T2-weighted imaging and increased signal on diffusion-weighted imaging (DWI) with apparent diffusion coefficient mapping correlate. These findings are most likely secondary to cytotoxic edema due to spinal cord infarction. The usual cerebrospinal fluid findings are elevated protein, with a mild increase in both red and white blood cell counts.2
We report two patients who presented with the clinical picture and imaging findings described above but who did not have history of surfing. Both had a history of spinal hyperextension.
Case 1
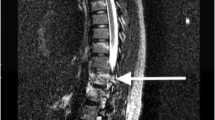
A 16-year-old girl with type 1 diabetes mellitus presented with back pain, urinary retention and progressive weakness and numbness distal to the umbilicus 3 h after performing a series of back handsprings during gymnastics practice. Physical examination on admission showed complete lower extremity paraplegia with a T5 sensory level. Rectal tone was decreased, and lower extremity reflexes were absent. Initial MRI of the thoracic spine revealed only subtle T2 hyperintensity but robust focal-restricted diffusion on DWI, consistent with cord infarction. Follow-up MRI 4 days later demonstrated progression of cord edema and swelling (Figure 1). Cerebrospinal fluid analysis showed normal cell count and protein. After 3 months of follow-up, she continued to be paraplegic, was now hyperreflexic in the lower extremities and had preserved temperature sensation below the level of T5.
Case 1. Cord Infarction. Initial MRI (bottom row) and 4-day follow-up (top row) demonstrating cord edema and swelling in the ventral cord (arrows) and corresponding restricted diffusion (asterisks). MR images include sagittal short inversion time inversion recovery (STIR), sagittal B-800 DWI with apparent diffusion coefficient (ADC) and axial T2W turbo spin echo (TSE) sequences.
Case 2
A healthy 13-year-old boy presented with acute onset of paraplegia. While playing on the day before admission, a younger boy jumped on his back while our patient was on his knees. As a result of the force, the spine was acutely hyperextended. He did not experience immediate pain, weakness or numbness, but developed pain later that evening, which interfered with sleep. The next morning, he was unable to walk and had urinary retention. At the local emergency department, thoracic and lumbar X-rays did not show any bony lesions. On arrival at our hospital, his examination demonstrated paraplegia with a sensory level around T5. MRI of thoracic and lumbar spine showed subtle changes in T2-weighted imaging, and DWI sequences showed scattered, non-confluent foci of restricted diffusion at the level of T5–6 and T7. MRI of the head and cervical spine were normal. Cerebrospinal fluid studies were unremarkable. Repeat MRI 72 h after the injury showed re-demonstration of the scattered foci of restricted diffusion more prominently in T5, T6 and T7, consistent with cord infarction (Figure 2). He had limited improvement in motor function after 2 weeks and was transferred to a spinal rehabilitation facility.
Case 2. Cord Infarction. Images demonstrate multifocal T2 hyperintense lesions, most conspicuously in the left lateral cord at T5-6 and right lateral cord at T7 (arrows) with corresponding restricted diffusion (asterisks). These slice positions highlight T5–6 level (top row) and T7 level (bottom row). MR images include sagittal short inversion time inversion recovery (STIR), sagittal B-1000 DWI and apparent diffusion coefficient (ADC), and axial T2W turbo spin echo (TSE) sequences.
Discussion
Our patients developed sudden onset of paraplegia in the absence of major trauma and with no radiographic evidence of osseous or soft tissue injury. Although our patients have no history of prior surfing or related injury, their symptoms and MRI findings closely resemble those described in patients with SM. Both patients had a history of cord hyperextension shortly before developing paraparesis, presuming a similar pathophysiology.
We are only aware of two other reports of SM without surfing. Wadia et al.4 presented a case similar to case 1. Their 7-year-old patient suffered paraparesis and urinary retention <24 h after performing backbends while cheerleading. As a result of her imaging findings and history, she was diagnosed with SM. Maharaj et al.5 reported a 51-year-old with the same clinical and MRI findings but without a history of spinal hyperextension.
Our patients and the one reported by Wadia et al.4 demonstrate that spinal cord ischemia can occur not only in surfers but also in other activities that involve spinal hyperextension. Given similarities in their clinical presentation and imaging findings, we believe that our patients and those diagnosed with SM share the same sequence of events and pathology for their spinal cord infarction, spinal hyperextension. Multiple possible pathophysiological mechanisms have been proposed. The first one is ischemia secondary to vasospasm of the artery of Adamkiewicz, affecting the watershed zone between the anterior and posterior spinal circulation. Other theories include ischemia resulting from avulsion of the perforating arteries, and tension of the spinal cord causing borderline hypoperfusion.2
Another potential etiology for SM is fibrocartilaginous embolism (FCE). Classically, FCE has not been deemed to be a likely etiology of SM because SM patients typically do not show any evidence of disc abnormalities. Also, their infarcts usually follow an anterior spinal artery distribution.6 However, to our knowledge, there are no reported cases of SM patients who have later undergone autopsy to evaluate for FCE.
Evidence obtained from 41 histopathologically confirmed reports of FCE to the spinal cord in humans demonstrates that the arguments used to exclude FCE as a potential etiology for SM are not robust. The majority of these cases (61%) were preceded by a minor incident such as carrying heavy objects, neck extension or falls. Forty percent of the patients had radiographic evidence of degenerative disc disease and/or Schmorl’s nodules. More than 75% of the cases had infarcts following an anterior spinal artery distribution.7 It appears that spinal hyperextension preceding SM may be enough to cause FCE, and for this reason this etiology cannot be excluded. If feasible, performance of autopsy studies in SM patients would provide further evidence of the presence or absence of FCE.
We suggest replacing the term SM with the broader term ‘acute hyperextension myelopathy’. This allows inclusion of all patients with spinal cord infarction and paraparesis due to acute spinal hyperextension.
We propose the following diagnostic criteria for the diagnosis of acute hyperextension myelopathy:
-
1
Young patients (age younger than 40 years).
-
2
Acute spinal hyperextension.
-
3
Paraparesis developing within 24 h of hyperextension.
-
4
Spinal MRI acutely demonstrating increased signal on DWI, with correlating decreased signal on apparent diffusion coefficient.
-
5
Subsequent MRI demonstrating increased signal on T2-weighted imaging.
We suggest excluding cases with known vascular risk factors or other identifiable causes for a spinal cord infarction. We proposed an upper age limit, hypothesizing that the requisite spinal flexibility for this injury disappears with age.
We recommend obtaining spinal cord DWI sequences in all suspected cases of acute hyperextension myelopathy as they may provide early evidence of spinal cord infarction and confirm the diagnosis. Although not performed for our patients, it is possible that spinal angiography may potentially demonstrate the underlying etiology of the spinal infarction such as vasospasm or vessel avulsion.
References
Thompson TP, Pearce J, Chang G, Madamba J . Surfer's myelopathy. Spine (Phila PA 1976) 2004; 29: E353–E356.
Conidi F . Some unusual sports-related neurologic conditions. Continuum (Minneap Minn) 2014; 20 (6 Sports Neurology): 1645–1656.
Freedman BA, Malone DG, Rasmussen PA, Cage JM, Benzel EC . Surfer's myelopathy: a rare form of spinal cord infarction in novice surfers: a systematic review. Neurosurgery 2016; 78: 602–611.
Wadia S, Padmanabhan P, Moeller K, Rominger A . Pediatric surfer's myelopathy. J Emerg Med 2015; 49: e143–e145.
Maharaj MM, Phan K, Hariswamy S, Rao PJ . Surfer's myelopathy: a rare presentation in a non-surfing setting and review of the literature. J Spine Surg 2016; 2: 222–226.
Chang CW, Donovan DJ, Liem LK, O'Phelan KH, Green DM, Bassin S et al. Surfers' myelopathy: a case series of 19 novice surfers with nontraumatic myelopathy. Neurology 2012; 79: 2171–2176.
AbdelRazek MA, Mowla A, Farooq S, Silvestri N, Sawyer R, Wolfe G . Fibrocartilaginous embolism: a comprehensive review of an under-studied cause of spinal cord infarction and proposed diagnostic criteria. J Spinal Cord Med 2016; 39: 146–154.
Acknowledgements
We thank the Departments of Neuroradiology and Pediatric Critical Care at the University of Kentucky for their ongoing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Albuja, A., Qaiser, S., Lightner, D. et al. Surfer’s myelopathy without surfing: a report of two pediatric patients. Spinal Cord Ser Cases 3, 17008 (2017). https://doi.org/10.1038/scsandc.2017.8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.8
This article is cited by
-
Acute hyperextension myelopathy in children: Radiographic predictors of clinical improvement
Spinal Cord (2022)
-
Is Surfer’s myelopathy an acute hyperextension-induced myelopathy? A systematic synthesis of case studies and proposed diagnostic criteria
Journal of Neurology (2022)
-
Surfer’s myelopathy: an atypical case presentation
Spinal Cord Series and Cases (2020)
-
Spinal cord injury with central cord syndrome from surfing
Spinal Cord Series and Cases (2018)
-
Traumatic spinal cord injury due to human tower accident in Catalonia
Spinal Cord Series and Cases (2018)