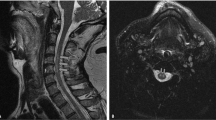
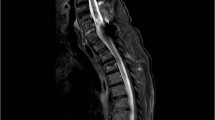
Abstract
Introduction:
This single-subject case report aims to describe and discuss a case of a patient with established C5 tetraplegia with acute coronary syndrome presenting with left upper quadrant pain and tenderness.
Case presentation:
A 65-year-old male with chronic C5 American Spinal Injury Association Impairment Scale (AIS) A tetraplegia presented to the emergency department with severe left upper quadrant pain radiating across the chest to the right upper limb with associated dyspnoea and diaphoresis. Prior to his emergency department admission, he had experienced progressive worsening of left upper quadrant pain and tenderness over several months. He was a non-smoker and swam regularly. He underwent coronary angiography and was found to have significant coronary artery disease. Drug-eluting stents were placed to critical coronary artery lesions followed by an uneventful hospital course with complete symptom resolution and discharge home.
Discussion:
Patients with tetraplegia are known to have higher rates of cardiovascular disease compared to ambulatory patients. Their cardiovascular risk profile may include atypical risk factors, for example, sleep disordered breathing, relative immobility and autonomic dysfunction. A high index of suspicion for cardiac pathology is warranted in those with cervical tetraplegia with persistent ‘atypical’ symptoms, including within the abdomen (especially when no specific abdominal organ dysfunction is evident). Sleep apnoea and significantly impaired mobility are potential cardiac risk factors in this patient group and should alert the emergency physician to cardiac disease (as in this case) irrespective of their complex past medical history and symptomatology.
Similar content being viewed by others
Introduction
Patients with tetraplegia present diagnostic challenges to the treating medical practitioner with impaired ability to localise and sense pain (including cardiac)1 compared to the non-spinal cord injured.
Abdominal pain as a presenting symptom of acute coronary syndrome is a further diagnostic challenge for the treating clinician with delay in diagnosis and specific management due to exploration of abdominal aetiology as in the case described. Atypical or asymptomatic cardiac ischaemia (including abdominal presentation)2,3 is associated with higher rates of morbidity and mortality even when occurring in ambulatory patients.
Case presentation
We present the case of a 65-year-old Caucasian male with C5 American Spinal Injury Association Impairment Scale (AIS) A tetraplegia, 31 years post traumatic spinal cord injury (SCI), who presented to the emergency department with 30 min of severe left upper quadrant pain radiating across his stomach around the chest and into the right shoulder associated with dyspnoea and diaphoresis. Pain was intermittent, dull and sharp, and some relief was obtained with intranasal Fentanyl 360 mcg administered during ambulance transfer. Pain was reportedly worse after meals, however also occurred outside of mealtimes. During 8 weeks prior to his emergency presentation, he had experienced intermittent left upper quadrant pain increasing in severity and becoming constant. It worsened particularly after meals with sharp pain on the background of constant discomfort. His sleep was disturbed due to the pain. He also reported generalised increase in fatigue. He had been seen twice during this period by his treating spinal cord injury rehabilitation physician and undergone colonoscopy and abdomino-pelvic scanning, both unremarkable and non-contributory to his diagnosis.
He denied weight loss, nausea or vomiting. Abdominal computed tomography scanning was unremarkable. Electrocardiogram (ECG) performed as an outpatient revealed first degree heart block (no change from ECG previously performed when well). Fasting glucose, cholesterol and triglycerides reported at symptom onset were 5.5 mmol l−1 (3.0–5.4), 4.5 mmol l−1 (<5.5) and 1.9 mmol l−1 (<2.0), respectively. High-density lipoprotein was 1.0 mmol l−1 (0.9) and low-density lipoprotein was 2.6 mmol l−1 (<3.5).
Comorbidities experienced by the patient included: severe right upper limb burning neuropathic pain present since the time of initial injury requiring pharmacological therapy and intermittent review by the multidisciplinary pain management team; moderate sleep apnoea managed with non-invasive nocturnal ventilation autoset continuous positive airway pressure, diagnosed in 2007 and previous left upper quadrant pain in 2011 with normal colonoscopy (internal haemorrhoid banding was performed in 2005).
He was a non-smoker and drank one glass of wine per night. He was actively employed as a solicitor and swam 1 km twice per week (modified backstroke). He maintained a healthy diet and weighed 80 kg with a calculated body mass index of 24.2 kg m−2.
In the emergency department, he was reported to be ‘looking well’ with the positive finding of left upper quadrant tenderness on deep palpation only. He exhibited dual heart sounds with no murmurs and clear auscultation of chest.
Initial investigations revealed ECG changes with T wave inversion in lead I, aVL and V2 with no ST changes. Troponin level was 79 ng l−1 (normal high <26 ng l−1) and C-reactive protein 4.5 mg l−1 (normal high<5).
The initial impression was of dynamic non-specific ECG changes with troponin leak managed with paracetamol 1 g per oral, aspirin 300 mg and clopidogrel. The initial recommendation was for discharge and follow-up in the clinic setting in light of chronicity of symptoms. The treating spinal cord injury rehabilitation physician was contacted, however, and advised further investigation of pain and symptomatology as an inpatient. Referral for cardiology consultation ensued and they found the patient to be haemodynamically stable with no prior cardiac history. The presumption was that the presenting pain was unlikely to be cardiac; however, based on ECG changes and troponin rise, a coronary angiogram was recommended. Persistent left upper quadrant pain was present while the patient was awaiting angiography with elevation in his systolic blood pressure to 156/70 from his usual 90 systolic. No other system dysfunction explained blood pressure rise. Bowels were moving, the suprapubic catheter was draining and no other pressure injuries or sources of dyreflexia were found. Heart rate was 80 beats per minute (b.p.m.).
On day 2 post admission, the patient experienced 30 s syncope while being showered on his commode chair. Sinus bradycardia with a rate of 57 b.p.m., troponin elevated to 231 ng l−1 and oxygen saturation 95% was noted. No pain was reported at the time of event. The patient felt ‘back to normal’ when he returned to bed
On day 3 post admission coronary angiography was performed, with ECG showing sinus bradycardia at 48 b.p.m. and first-degree atrioventricular block. Left ventricular hypertrophy and incomplete right bundle branch block (12 September 2016) were noted and the patient was diagnosed with a critical proximal left anterior descending coronary artery lesion with heavy thrombus burden and a subtotally occluded first diagonal artery (D1). The proximal left anterior descending artery was predilated and stented with a 3.5×18 mm drug-eluting stent. It was post-dilated with a 4.0 non-compliant balloon with good final result.
The left circumflex artery showed a tight ostial lesion with diffuse moderate, mid and distal disease. In the proximal circumflex artery, an ostial lesion was predilated and stented with a 2.75 mm drug-eluting stent with good final result.
The right coronary artery was dominant and showed only minor atheroma.
The conclusion of the coronary angiogram was critical proximal left anterior descending artery and tight ostial left circumflex lesion, which were both treated with drug-eluting stents (Figures 1, 2, 3, 4).
On day 4, the patient’s course was remarkable for asymptomatic intermittent bradycardia.
The patient was discharged on day 10 with complete resolution of all symptoms of his left upper quadrant pain and a reported sensation of renewed energy and less fatigue. The patient continues to remain asymptomatic 4 months post intervention experiencing no abdominal pain and minimal fatigue.
Discussion
Cardiac disease presenting with abdominal pain has been reported to be associated with increased morbidity and mortality.2,3,4 SC persons with tetraplegia and impaired cardiac sensory supply present a dual challenge for diagnosing acute coronary disease. Moreover, routine screening does not form part of standard surveillance for persons with SCIs.5
Acute coronary syndrome with atypical/silent presentations is not well described.6Abdominal pain as a presenting symptom is not always gastrointestinal in origin.2,7 Reassessing the patient and prioritising life-threatening time critical conditions is crucial.3 Challenges are faced by physical medicine and general physicians, as well as patients who may have no symptoms or vague symptoms at best.1,8,9,10 Access to SCI clinicians who are focused on cardiac and comorbid health condition presentations played a role in this patient, and emphasises the valuable role of tertiary spinal care centres with colocation of emergency and specialised cardiac services.5 In this case, there was a delay in cardiac diagnosis, despite a compliant patient. The patient’s symptomatology and history of previous colonoscopies for similar abdominal pain prioritised gastrointestinal over cardiac evaluation to all but the SCI clinician.7
This patient’s cardiac risk factors were: age, time post injury, moderate sleep apnoea, blood pressure variability and complete neurological cervical SC limiting exercise and heart rate variability.1,11 Previous studies highlighted that positive cardiac disease testing was more common in persons with SCIs with rostral, complete, cervical and increased age and time post injury.1
Our patient’s ability to further modify his lifestyle risk factors appeared limited: he was working full time, swimming twice per week, health focused (proactive) with optimal self-care with respect to infection prevention, compliant with sleep disordered breathing management and living local to his tertiary spinal service. He was a non-smoker and not diabetic.
The usual aetiology of abdominal pain in the person with SCI is bladder or bowel pathology, and in this case the previous history of abdominal pain delayed cardiac investigations. The patient was provided with information and direction to present to the emergency department if his symptoms did not abate and this was followed.
As recommended in previous studies optimising modifiable risk factors is worthwhile4,8,12,13,14, 15 and this should include (as in this patient) sleep apnoea, weight and so on. Sleep apnoea and SCI has been increasingly researched with Sankari et al.16 suggesting that sleep disordered breathing may contribute to cardiovascular mortality. In non-spinal cord injured patients17,18,19 sleep disordered breathing has been linked to cardiovascular disease, hypertension and elevated blood sugar levels. In persons with SCI, there may be more than physical immobility to explain the long-term increased risk of cardiovascular disease.16 The unmodifiable risk factors remained age and SCI level, and completeness.
An atypical risk profile in conjunction with abdominal pain as a presenting cardiac symptom complicated this case, however, the literature remains limited with respect to guidelines and large-scale screening of SCI patients who are asymptomatic6 despite reviews highlighting increased rates of cardiovascular disease in individuals with SCI.12,20 Acute coronary syndrome guidelines fail to include SCI as a high-risk category (the elderly, females and diabetics are well studied, and due emphasis is placed on this group when presenting to emergency departments).21,22
This case is an example of a patient with chronic cervical SCI with multiple comorbidities who presented to the emergency department with a history of left upper quadrant pain and tenderness (not typically classical central chest pain with radiation to left arm and jaw2,21). His presentation to the emergency department with relatively few ‘hard’ signs almost led to his being discharged. Further assessment revealed cardiac pathology requiring specific intervention and treatment with complete symptom resolution and minimal morbidity. The main lesson learned from this case was to consider all serious time critical pathology, for example, cardiac prior to dismissing complex persons with SCI who are unable to accurately localise their pain due to neurological impairment. Persons with SCIs should be considered high risk for cardiac disease despite the absence of recognised modifiable risk factors. Prior knowledge of the patient was the key to advocating on their behalf as in this case where the patient had new and progressively more severe pain, which was not consistent with his previous pain history and character. There should be active collaboration with cardiology and vascular physicians when assessing patients with chronic cervical SCI to identify potentially modifiable vascular disease either by increased surveillance screening or angiographic intervention. Spinal rehabilitation physicians should aim to emphasise both patient and all clinicians that early onset vascular pathology may be asymptomatic or atypical in nature and requires closer surveillance especially with ageing.4,12,13,14,15 Larger studies looking at potential contributions to cardiovascular pathology as a result of SCI level and completeness in addition to sleep disordered breathing would be of great interest and will stimulate discussion (especially in ‘asymptomatic’ patients to determine who, how and when to screen or investigate based on what objective criteria).
Conclusion
In the patient with tetraplegia, we should assume high risk for cardiac/coronary artery disease independent of their symptom constellation even if the standard risk factors, for example, diabetes, smoking and positive family history are absent. We should consider increased surveillance in patients with chronic complete tetraplegia as they age and be alert to new symptomatology. Close collaboration between SCI services and the emergency and cardiology departments should occur with access to relevant SCI patient medical records and knowledge of the patient when well. Empowering patients to seek further medical review if persistently unwell and to think of cardiac disease is vital if we are to prevent significant morbidity and mortality in the SCI population from missed cardiovascular pathology.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G . The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 2001; 39: 310–317.
Tsipouras S . Nonabdominal causes of abdominal pain--finding your heart in your stomach!. Aust Fam Physician 2008; 37: 620–623.
Goodman DA, Kavsak PA, Hill SA, Worster A . Presenting characteristics of patients undergoing cardiac troponin measurements in the emergency department. CJEM 2015; 17: 62–66.
Bauman WA, Spungen AM . Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord 2008; 46: 466–476.
Finnie AK, Buchholz AC, Martin Ginis KA, Group SSR. Current coronary heart disease risk assessment tools may underestimate risk in community-dwelling persons with chronic spinal cord injury. Spinal Cord 2008; 46: 608–615.
Lee CS, Lu YH, Lee ST, Lin CC, Ding HJ . Evaluating the prevalence of silent coronary artery disease in asymptomatic patients with spinal cord injury. Int Heart J 2006; 47: 325–330.
Bhuiya FA, Pitts SR, McCaig LF . Emergency department visits for chest pain and abdominal pain: United States, 1999-2008. NCHS Data Brief 2010; 43: 1–8.
Akbal A, Kurtaran A, Selcuk B, Akyuz M . H-FABP, cardiovascular risk factors, and functional status in asymptomatic spinal cord injury patients. Herz 2013; 38: 629–635.
Groah SL, Menter RR . Long-term cardiac ischemia leading to coronary artery bypass grafting in a tetraplegic patient. Arch Phys Med Rehabil 1998; 79: 1129–1132.
Walker WC, Khokhar MS . Silent cardiac ischemia in cervical spinal cord injury: case study. Arch Phys Med Rehabil 1992; 73: 91–94.
Weaver LC, Fleming JC, Mathias CJ, Krassioukov AV . Disordered cardiovascular control after spinal cord injury. Handb Clin Neurol 2012; 109: 213–233.
Chen YK, Hung TJ, Lin CC, Yen RF, Sung FC, Lee WY et al. Increased risk of acute coronary syndrome after spinal cord injury: a nationwide 10-year follow-up cohort study. Int J Cardiol 2013; 168: 1681–1682.
Cragg JJ, Noonan VK, Krassioukov A, Borisoff J . Cardiovascular disease and spinal cord injury: results from a National Population Health Survey. Neurology 2013; 81: 723–728.
Cragg JJ, Stone JA, Krassioukov AV . Management of cardiovascular disease risk factors in individuals with chronic spinal cord injury: an evidence-based review. J Neurotrauma 2012; 29: 1999–2012.
de Groot S, Post MW, Snoek GJ, Schuitemaker M, van der Woude LH . Longitudinal association between lifestyle and coronary heart disease risk factors among individuals with spinal cord injury. Spinal Cord 2013; 51: 314–318.
Sankari A, Martin JL, Badr M . A retrospective review of sleep-disordered breathing, hypertenstion and cardiovascular diseases in spinal cord injury patients. Spinal Cord 2015; 53: 496–497.
Back M, Stanke-Labesque F . Obstructive sleep apnoea and cardiovascular calcification. Thorax 2015; 70: 815–816.
Pafili K, Steiropoulos P, Papanas N . The relationship between obstructive sleep apnoea and coronary heart disease. Curr Opin Cardiol 2015; 30: 439–446.
Utriainen KT, Airaksinen JK, Polo O, Laitio R, Pietila MJ, Scheinin H et al. Sleep apnoea is associated with major cardiac events in peripheral arterial disease. Eur Respir J 2014; 43: 1652–1660.
Miyatani M, Szeto M, Moore C, Oh PI, McGillivray CF . Catharine Craven B exploring the associations between arterial stiffness and spinal cord impairment: a cross-sectional study. J Spinal Cord Med 2014; 37: 556–564.
El-Menyar A, Zubaid M, Sulaiman K, AlMahmeed W, Singh R, Alsheikh-Ali AA et al. A typical presentation of acute coronary syndrome: a significant independent predictor of in-hospital mortality. J Cardiol 2011; 57: 165–171.
Hwang SY, Park EH, Shin ES, Jeong MH . Comparison of factors associated with atypical symptoms in younger and older patients with acute coronary syndromes. J Korean Med Sci 2009; 24: 789–794.
Acknowledgements
We acknowledge all of the clinicians involved in the admission, discussion, investigation and treatment of our patient. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nier, L., Hansen, P. Coronary artery disease presenting with left upper quadrant pain in a patient with chronic cervical tetraplegia. Spinal Cord Ser Cases 3, 17048 (2017). https://doi.org/10.1038/scsandc.2017.48
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.48