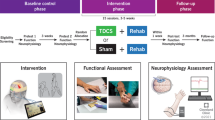
Abstract
Introduction:
This is a prospective clinical pilot case series. Improvement of arm and hand functions after spinal cord injury (SCI) is one of the major rehabilitation goals. Electrical stimulation of the primary motor cortex via transcranial direct current stimulation (tDCS) coupled with high-intensity repetitive motor training may have potential to facilitate improvement in motor function in chronic, incomplete cervical SCI. We investigated the relationship between motor recovery and changes in white matter integrity in response to treatment intervention. This study was conducted in The Institute for Rehabilitation and Research Memorial Hermann, Houston, USA.
Case Presentation:
Four right-handed adults with chronic, incomplete cervical SCI (age, 36–63 years, American Spinal Injury Association Impairment Scale grade C–D) were enrolled in 10 sessions of anodal tDCS at 2 mA versus sham tDCS followed by 1 h of robotic-assisted arm training. Changes in arm and hand function were measured with Jebsen–Taylor Hand Function Test and Motor Activity Log-Amount of Use. Diffusion tension imaging was used to measure changes in fractional anisotropy (FA) of corticospinal tracts (CSTs).
Discussion:
After 10 sessions of treatment, we found greater improvement in hand function and hand usage in patients who received active tDCS treatment versus sham treatment. There was an overall positive change in FA values across all patients. We show changes in arm and hand function associated with changes in CST tractographic mapping to quantify the motor system components in chronic incomplete cervical SCI.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) can cause substantial motor deficits in arm and hand functions that can result in significant loss in performance of daily activities for many years. The extent of neurological impairment is the major contributing factor to recovery from injury.1 Even small improvements in arm and hand function may lead to improvements in self-care, transfers and quality of life.2,3,4
There are a number of factors that explain recovery of sensorimotor functions after injury. For example, preservation of longitudinally oriented axonal tracts in white matter, as well as proper conductance through spared white matter axons, has been directly related to neurological recovery.5 In the chronic stage of injury, a number of pathologic changes such as spinal cord atrophy,6 reduced axonal integrity,7 demyelination8 and remyelination9 have been documented. Although most of these changes occur at the spinal cord level, both animal and human studies have also demonstrated structural and functional reorganization at remote cortical and subcortical structure.10,11,12,13 Some of the structural changes in the white matter can be detectable by diffusion tensor imaging (DTI). DTI is an advanced magnetic resonance imaging (MRI) technique used to quantify fiber orientation properties and integrity of white matter pathways within neural networks. Fractional anisotropy (FA), an index of the diffusion characteristics of water molecules directed along the axis of axonal pathways, can detect microstructural changes. In chronic SCI, FA values of the cervical spinal cord have been shown to be correlated with motor scores in patients with chronic cervical injury14,15,16 and suggested to be non-invasive imaging biomarkers for SCI.17
Rehabilitation protocols that have been in use to improve upper-limb sensorimotor functions are sparse and have provided modest functional benefits. Traditional muscle strengthening, range of motion, massed practice,18 electrical stimulation19,20 and more recently robotic-assisted exercises21,22,23 are used alone or in combination. Despite the fact that restoration of arm and hand function is an important part of rehabilitation, there is still no consensus in management of the tetraplegic upper limb. Most aforementioned approaches focus on the extremity muscles, and only a few studies have attempted to combine motor cortex stimulation with peripheral training.24,25,26 In a recent study, we demonstrated greater improvement in arm and hand functions in the active anodal stimulation group compared with the sham stimulation group in incomplete cervical SCI.27 Although combination therapies have emerged as an alternative approach to facilitate neurorecovery and improve motor skill performance, the accompanying neural changes for improved functions still remain unknown in SCI. Moreover, understanding the mechanism of recovery will force clinical researchers to develop rehabilitation protocols that aim to facilitate recovery from paralysis with less focus on compensatory techniques.
Therefore, in the current study, we report findings of a case–control study to demonstrate structural white matter changes in the corticospinal tract (CST), the primary structure for motor control, especially for fine motor control of the hand.
Methods
Participants
Four participants with chronic, incomplete cervical SCI were randomly assigned into active and control groups. Main enrollment criteria were as follows: (1) age between 18 and 65 years; (2) diagnosis of chronic, incomplete cervical SCI as defined by the American Spinal Injury Association Impairment scale classification at least 6 months before the study; (3) no neuropsychiatric comorbidities, including traumatic brain injury, evidenced by loss of consciousness because of brain trauma, post-traumatic amnesia or skull fracture, or objective neurological findings that can be reasonably attributed to traumatic brain injury; (4) no contraindications to transcranial direct current stimulation (tDCS) and MRI; (5) no prior history of seizure or family history of seizure disorder in a first degree relative; and (6) no use of medications containing a sodium-channel blocker such as carbamazepine.
This study was approved by the Committee for the Protection of Human Subjects of the University of Texas Health Science Center at Houston. All participants gave written consent before participation.
Intervention
All participants completed 10 sessions of treatment over a 2-week period. After randomization, they received either anodal tDCS or sham tDCS over M1 at 2 mA for 20 min, which was immediately followed by 1 h of robotic-assisted arm training (RAT). Participant #1 and Participant #2 received anodal tDCS+RAT. Participant #3 and Participant #4 received sham tDCS+RAT.
tDCS is a form of non-invasive cortical stimulation and has the potential to alter corticospinal excitability. Direct current is transferred by two saline-soaked surface sponge electrodes (7×5 cm=35 cm2 active areas) and delivered by a battery-driven stimulator device (medical tDCS for clinical trials device, Soterix Medical, New York, NY, USA). To stimulate the primary motor cortex (M1), the anode electrode (increasing cortical excitability) was placed over C3/C4 (according to the 10–20 international electroencephalogram electrode system) contralateral to the targeted arm. The cathode (reference) electrode was placed over the contralateral supraorbital area.
Immediately after cortical stimulation, repetitive movement training was provided using the MAHI Exo-II exoskeleton28 operated in active-constrained mode. In this mode, the robotic device is commanded to display a force field proportional to the movement velocity at the intended joint that serves to oppose the subject’s movement toward a fixed target displayed on the computer screen. Single degree of freedom movements for elbow, forearm and wrist were repeated at high intensity, with a visual display of the real-time cursor position and target position visible to the participant. Treatment was progressed gradually by increasing the number of repetitions and amount of resistance applied to each movement. On a computer screen, graphical feedback of the number of repetitions achieved was displayed after each attempt to maintain motivation. Standardized rest breaks were administered to avoid fatigue. The goal was to achieve ~1000 repetitions in a given session. During the study period, participants did not participate in any other occupational therapy program involving arm and hand training.
MRI-DTI data acquisition
Data were acquired on a Philips (Houston, TX, USA) 3.0T Achieva scanner using a SENSE receive neurovascular coil. The MRI protocol included conventional MRI and three-dimensional T1-weighted magnetization prepared rapid acquisition of gradient echo. The T1-weighted sequence spatial resolution was 1×1×1 mm and field of view was 256×256 mm. Diffusion-weighted image data were acquired axially with the balanced and alternating polarity Icosa21 tensor encoding scheme.29,30 The b-factor=1000 s mm−2, repetition time (TR)/echo time (TE)=11 000/65 μs, field of view (FOV)=320×320 mm and slice thickness/gap/#slices=4 mm/0 mm/80. The echo planar imaging (EPI) phase encoding used a SENSE k-space undersampling factor of two, with an effective k-space matrix of 160×160 and an image matrix after zero filling of 320×320. The constructed image spatial resolution for the diffusion-weighted image data was ~1×1×4 mm. The values for FA range from 0 to 1, where higher values suggest highly oriented water diffusion (that is, anisotropic diffusion), and therefore highly organized white matter structure.31
MRI data processing
The MRI data processing pipeline used in this work are described in more detail elsewhere.32
Brain diffusion tensor fiber tractography analysis
We have used a brute force and multiple regions-of-interest (ROI) tracking method, and fiber assignment with continuous tractography algorithm33 b (DTI Studio, Johns Hopkins University, Baltimore, MD, USA; http://cmrm.med.jhmi.edu) to reconstruct CST. Reproducibility of fiber construction in both hemispheres was tested on all participants by two experienced raters (ZK, KH). Once a fiber tract was reconstructed, its entire trajectory was verified on a slice-by-slice basis to compare with established anatomical landmarks described in the human brain neuroanatomy atlases.34 Regions of interests used for CST were subcortical white matter of motor cortex, posterior limb of internal capsule, cerebral peduncle, pons and medulla.35 FA values of the tracts were obtained by averaging the FA values in these regions of interests where CST goes through. The researcher who performed the analysis was blinded to participants’ group assignment.
Arm and hand function measurements
Testing for arm and hand function
The Jebsen–Taylor Hand Function Test (JTHFT) was used as the primary outcome measure of arm and hand functions. The test36 has shown to have good to excellent inter-rater and intrarater reliability,37 and capacity for detecting performance change in activities that resemble daily life activities. Time to perform seven everyday activities (for example, writing, feeding) was tested. We excluded the writing task because of heavy dependence on the side with less impairment.38 Administration of the JTHFT subtests was discontinued after 120 s if a subject could not complete the task in that time. Scores were recorded in number of items completed/total time (in s).39 This modified method of recording had superiority compared with traditional recordings of total time only. Thus, changes in number of items completed within 120 s could be reflected as an increase or decrease of performance.
Self-report of arm function with Motor Activity Log
Participants were asked to report ‘how much’ and ‘how well’ they have used their arm during 30 daily activities such as brushing hair, drinking from a glass and picking up the phone. Two scores were generated from this self-report; however, for the purpose of this study, only the amount of use was used as we were interested in increased activity of the arm in daily life.40 A score of 0.50 was used to report as minimal clinically important difference in stroke population.41
Assessments were performed by the same senior therapist who was blinded to study assignment.
Results
Participant demographics and clinical scores at baseline
Four participants with impairment in upper-limb motor functions caused by incomplete, cervical SCI were randomized into active versus sham stimulation groups. The characteristics of the participants are presented in Table 1. All four participants successfully completed the 2-week treatment intervention.
Motor function changes after active tDCS and repetitive training
After 10 sessions of active treatment, both participants demonstrated improvement in trained arms and, to a certain extent, in the non-trained arm as measured with JTHFT. Individual improvements were observed in subtests of JTHFT. For example, subject 1 completed the ‘simulated feeding’ subtest in 20 s, after scoring ‘0’ at baseline. The task consists of scooping five beans with a spoon from the top of a table and dropping them one by one into a can. This task requires a firm grip of the spoon and proximal arm movements. Subtests of subject 2 showed significant improvement in page turning with the non-trained arm, in which he turned all five pages in 27 s, compared with being able to turn just one page in 120 s before treatment. He also showed improvements in picking up small objects with his non-trained side by picking up four items in 120 s versus ‘0’ items in 120 s before treatment.
Amount of use (Motor Activity Log-Amount of Use) of the trained arm increased significantly by exceeding minimal clinical significant difference of 0.5.
Motor function changes after sham tDCS and repetitive training
Ten sessions of sham stimulation with repetitive robotic training resulted in minimal improvement of arm and hand functions. For example, subject 3 was able to initiate the page-turning task and turned two pages in 120 s with her trained arm, compared with zero pages before treatment. In addition, she was able to pick up and move six small items with the non-trained arm within 97 s, compared with ‘0’ items before treatment, and was able to initiate simulated feeding by scooping and moving one bean with the non-trained arm within 120 s, compared with ‘0’ before treatment. Subject 4 showed slightly higher improvements in the non-trained arm compared with his trained side.
Amount of use (Motor Activity Log-Amount of Use) of the trained arm did not show significant change (Table 2).
Brain diffusion tensor fiber tractography analysis
We found an overall positive trend in FA values in participants regardless of group assignments and arm being trained. FA was not found to be sensitive for the effects of tDCS in our small cohort. The mean FA change in active versus sham stimulation groups are shown in Table 3 and demonstrated in Figure 1.
Illustration of the corticospinal tracts of each subject from two time points: before (a, c, e, g) and after (b, d, f, h). Regions of interest used to construct the pathways were subcortical white matter of the motor cortex, posterior limb of the internal capsule, cerebral peduncle, pons and medulla (ROIs are illustrated in the descending order in a, respectively). As background, b0 map from diffusion tensor imaging maps is used.
Discussion
In this pilot case series, individuals with chronic, incomplete cervical SCI underwent an intervention consisting of 10 sessions of combined anodal tDCS with RAT or RAT only. Neural correlates related to treatment-induced changes before and after treatment were measured with DTI. After treatment, our findings demonstrate (a) modest improvement in motor functions in patients treated with anodal tDCS and repetitive training and (b) a positive trend in DTI measures demonstrated as an overall increase in FA change.
In contrast to a large number of studies in stroke recovery, very few studies have examined the effects of primary motor cortex stimulation in improving arm and leg motor functions after SCI. Several researchers have reported improvement in International Neurological Classification motor and sensory scores, hand functions,24,26,42 or gait functions43,44 when stimulating M1 with high-frequency repetitive transcranial magnetic stimulation. On the basis of findings from our study, one can speculate that anodal tDCS, when applied at 2 mA for 20 min before a high-intensity repetitive arm training protocol, has the potential to facilitate movement recovery, but this must be interpreted with caution because of the very small sample size, differences in elapsed time since injury (longer in the control group), age (higher in control group) and differences in baseline motor function (lower in the control group).
When comparing groups for amount of arm use in real-life environments, three out of four patients reported minimal use at the beginning of the study. As expected, high-functioning patients had the highest score on Motor Activity Log-Amount of Use. However, after treatment, patients in the active group started to use their arms during daily activities more than patients in the control group. The so-called ‘learned non-use’ was first proposed by Taub et al.45 Regardless of the etiology (that is, stroke or SCI), learned non-use usually develops in response to excess motor disability after central nervous system injury.45 Unfortunately, most patients with long-term paralysis tend to use their paretic hand less in daily life, despite residual motor function. This acquired non-use can further lead to reversible loss of neural and behavioral function. Therefore, overcoming learned non-use and decreasing compensatory movements should be carefully integrated into successful therapeutic strategies that aim to improve motor functions after SCI. In the present study, we were able to demonstrate positive changes in the amount of use in participants who received M1 stimulation in addition to intense exercise. However, further studies are needed to explore short- and long-term effects of learned non-use in patients with SCI and its negative effect on poor recovery.
To the best of our knowledge, no previous studies in adults have investigated changes in DTI values in response to relatively short intensive repetitive treatments of upper limbs. In previous studies, recovery of motor functions after SCI has been highly correlated to preservation of longitudinally oriented axonal tracts in the white matter46 and myelination of intact axons. Tract quality has been roughly defined to correlate positively with FA. In our study, we observed a positive FA change in both right and left corticospinal tracts and across all participants. Interestingly, JTHFT performance not only improved in the trained arm, but also in the non-trained side. Despite unilateral training, this finding is particularly interesting and needs to be further explored. One can speculate that increased FA values might be a reflection of training-induced modification in white matter microstructure and may indicate increased coherence of CST fibers, increased myelination or increased axon density with resulting improvement in fine motor control, but results should be interpreted with caution.
The results from this study demonstrated that in vivo quantification of the CST, the main motor pathway, is feasible in adults with chronic cervical incomplete SCI using 2 mm slices at 3.0 T. Furthermore, this study has shown that this pathway is sensitive to the extent of functional recovery induced by a combination therapy protocol.
The main limitation of this study is the small sample size (n=4). A larger sample size with groups homogeneous in, that is, baseline motor function and time since injury is warranted to draw a stronger conclusion. In addition, interpretation of the changes in diffusion in white matter is not straightforward. In the future, we expect to see the use of DTI in mainstream clinical practice to both prognosticate and to monitor recovery in patients with spinal cord disease. In the long term, we believe our work will shed light onto controlled clinical trials with larger sample sizes.
References
Kirshblum S, Millis S, McKinley W, Tulsky D . Late neurologic recovery after traumatic spinal cord injury. Arch Phys Med Rehabil 2004; 85: 1811–1817.
Anderson KD . Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 2004; 21: 1371–1383.
Snoek GJ, Ijzerman MJ, Hermens HJ, Maxwell D, Biering-Sorensen F . Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord 2004; 42: 526–532.
Ditunno JF Jr, Cohen ME, Hauck WW, Jackson AB, Sipski ML . Recovery of upper-extremity strength in complete and incomplete tetraplegia: a multicenter study. Arch Phys Med Rehabil 2000; 81: 389–393.
Choe AS, Belegu V, Yoshida S, Joel S, Sadowsky CL, Smith SA et al. Extensive neurological recovery from a complete spinal cord injury: a case report and hypothesis on the role of cortical plasticity. Front Hum Neurosci 2013; 7: 290.
Potter K, Saifuddin A . Pictorial review: MRI of chronic spinal cord injury. Br J Radiol 2003; 76: 347–352.
Blight AR, Decrescito V . Morphometric analysis of experimental spinal cord injury in the cat: the relation of injury intensity to survival of myelinated axons. Neuroscience 1986; 19: 321–341.
Totoiu MO, Keirstead HS . Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol 2005; 486: 373–383.
Harrison BM, McDonald WI . Remyelination after transient experimental compression of the spinal cord. Ann Neurol 1977; 1: 542–551.
Topka H, Cohen LG, Cole RA, Hallett M . Reorganization of corticospinal pathways following spinal cord injury. Neurology 1991; 41: 1276–1283.
Lotze M, Laubis-Herrmann U, Topka H, Erb M, Grodd W . Reorganization in the primary motor cortex after spinal cord injury-a functional magnetic resonance (fMRI) study. Restor Neurol Neurosci 1999; 14: 183–187.
Laubis-Herrmann U, Dichgans J, Bilow H, Topka H . Motor reorganization after spinal cord injury: evidence of adaptive changes in remote muscles. Restor Neurol Neurosci 2000; 17: 175–181.
Kriz J, Kozak J, Zedka M . Primary motor cortex inhibition in spinal cord injuries. Neuro Endocrinol Lett 2012; 33: 431–441.
Petersen JA, Wilm BJ, von Meyenburg J, Schubert M, Seifert B, Najafi Y et al. Chronic cervical spinal cord injury: DTI correlates with clinical and electrophysiological measures. J Neurotrauma 2012; 29: 1556–1566.
Freund P, Schneider T, Nagy Z, Hutton C, Weiskopf N, Friston K et al. Degeneration of the injured cervical cord is associated with remote changes in corticospinal tract integrity and upper limb impairment. PLoS ONE 2012; 7: e51729.
Chang Y, Jung TD, Yoo DS, Hyun JK . Diffusion tensor imaging and fiber tractography of patients with cervical spinal cord injury. J Neurotrauma 2010; 27: 2033–2040.
Koskinen E, Brander A, Hakulinen U, Luoto T, Helminen M, Ylinen A et al. Assessing the state of chronic spinal cord injury using diffusion tensor imaging. J Neurotrauma 2013; 30: 1587–1595.
Beekhuizen KS, Field-Fote EC . Massed practice versus massed practice with stimulation: effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabil Neural Repair 2005; 19: 33–45.
Kohlmeyer KM, Hill JP, Yarkony GM, Jaeger RJ . Electrical stimulation and biofeedback effect on recovery of tenodesis grasp: a controlled study. Arch Phys Med Rehabil 1996; 77: 702–706.
Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI . Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord 2006; 44: 143–151.
Yozbatiran N, Berliner J, O'Malley MK, Pehlivan AU, Kadivar Z, Boake C et al. Robotic training and clinical assessment of upper extremity movements after spinal cord injury: a single case report. J Rehabil Med 2012; 44: 186–188.
Zariffa J, Kapadia N, Kramer JL, Taylor P, Alizadeh-Meghrazi M, Zivanovic V et al. Effect of a robotic rehabilitation device on upper limb function in a sub-acute cervical spinal cord injury population. IEEE Int Conf Rehabil Robot 2011; 2011: 5975400.
Cortes M, Elder J, Rykman A, Murray L, Avedissian M, Stampa A et al. Improved motor performance in chronic spinal cord injury following upper-limb robotic training. NeuroRehabilitation 2013; 33: 57–65.
Belci M, Catley M, Husain M, Frankel HL, Davey NJ . Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 2004; 42: 417–419.
Kumru H, Murillo N, Samso JV, Valls-Sole J, Edwards D, Pelayo R et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair 2010; 24: 435–441.
Gomes-Osman J, Field-Fote EC . Improvements in hand function in adults with chronic tetraplegia following a multiday 10-Hz repetitive transcranial magnetic stimulation intervention combined with repetitive task practice. J Neurol Phys Ther 2015; 39: 23–30.
Yozbatiran N, Keser Z, Davis M, Stampas A, O'Malley MK, Cooper-Hay C et al. Transcranial direct current stimulation (tDCS) of the primary motor cortex and robot-assisted arm training in chronic incomplete cervical spinal cord injury: a proof of concept sham-randomized clinical study. NeuroRehabilitation 2016; 39: 401–411.
Fitle KD, Pehlivan AU, O'Malley MK. A robotic exoskeleton for rehabilitation and assessment of the upper limb following incomplete spinal cord injury. Proceedings - IEEE International Conference On Robotics and Automation; 4960–4966 May; Seattle, WA. Robotics and Automation: USA, 2015.
Hasan KM, Narayana PA . Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: theoretical analysis and validation. Magn Reson Med 2003; 50: 589–598.
Hasan KM . A framework for quality control and parameter optimization in diffusion tensor imaging: theoretical analysis and validation. Magn Reson Imaging 2007; 25: 1196–1202.
Chabert S, Scifo P . Diffusion signal in magnetic resonance imaging: origin and interpretation in neurosciences. Biol Res 2007; 40: 385–400.
Hasan KM, Walimuni IS, Abid H, Hahn KR . A review of diffusion tensor magnetic resonance imaging computational methods and software tools. Comput Biol Med 2011; 41: 1062–1072.
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007; 36: 630–644.
Blumenfeld H. Neuroanatomy Through Clinical Cases. Sinauer Ass., Inc.,: Sunderland, MA, USA, 2010.
Keser Z, Yozbatiran N, Francisco GE, Hasan KM . A note on the mapping and quantification of the human brain corticospinal tract. Eur J Radiol 2014; 83: 1703–1705.
Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA . An objective and standardized test of hand function. Arch Phys Med Rehabil 1969; 50: 311–319.
Beebe JA, Lang CE . Relationships and responsiveness of six upper extremity function tests during the first six months of recovery after stroke. J Neurol Phys Ther 2009; 33: 96–103.
Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005; 128 (Pt 3): 490–499.
Resnik L, Borgia M, Latlief G, Sasson N, Smurr-Walters L . Self-reported and performance-based outcomes using DEKA arm. J Rehabil Res Dev 2014; 51: 351–362.
Taub E, Miller NE, Novack TA, Cook EW 3rd, Fleming WC, Nepomuceno CS et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil 1993; 74: 347–354.
van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM . Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke 1999; 30: 2369–2375.
Kuppuswamy A, Balasubramaniam AV, Maksimovic R, Mathias CJ, Gall A, Craggs MD et al. Action of 5 Hz repetitive transcranial magnetic stimulation on sensory, motor and autonomic function in human spinal cord injury. Clin Neurophysiol 2011; 122: 2452–2461.
Kumru H, Benito J, Murillo N, Valls-Sole J, Valles M, Lopez-Blazquez R et al. Effects of high-frequency repetitive transcranial magnetic stimulation on motor and gait improvement in incomplete spinal cord injury patients. Neurorehabil Neural Repair 2013; 27: 421–429.
Calabro RS, Naro A, Leo A, Bramanti P . Usefulness of robotic gait training plus neuromodulation in chronic spinal cord injury: a case report. J Spinal Cord Med 2017; 40: 118–121.
Taub E, Crago JE, Burgio LD, Groomes TE, Cook EW 3rd, DeLuca SC et al. An operant approach to rehabilitation medicine: overcoming learned nonuse by shaping. J Exp Anal Behav 1994; 61: 281–293.
Nathan PW . Effects on movement of surgical incisions into the human spinal cord. Brain 1994; 117 (Pt 2): 337–346.
Acknowledgements
This study was supported by a pilot research grant from Mission Connect, a program of TIRR Foundation, and by The Institute for Rehabilitation and Research (TIRR) at Memorial Hermann.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yozbatiran, N., Keser, Z., Hasan, K. et al. White matter changes in corticospinal tract associated with improvement in arm and hand functions in incomplete cervical spinal cord injury: pilot case series. Spinal Cord Ser Cases 3, 17028 (2017). https://doi.org/10.1038/scsandc.2017.28
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.28