Abstract
Introduction:
Although there has been substantial emphasis on the neuromuscular and cardiovascular adaptations following rehabilitation, pulmonary adaptations in individuals with incomplete SCI (iSCI) in response to locomotor training have been less frequently studied. In healthy individuals, effective transition from rest to work is accomplished by a hyperpneic response, which exhibits an exponential curve with three phases. However, the degree to which our current understanding of exercise hyperpnea can be applied to individuals with iSCI is unknown. The purpose of this case series was to characterize exercise hyperpnea during a rest to constant work rate (CWR) transition before and after 12–15 weeks of overground locomotor training (OLT).
Case Presentation:
Six subjects with cervical motor incomplete spinal cord injury participated in 12–15 weeks of OLT. Subjects were trained in 90-min sessions twice a week. All training activities were weight-bearing and under volitional control without the assistance of body-weight support harnesses, robotic devices or electrical stimulation. Six minutes of CWR treadmill walking was performed at self-selected pace with cardiorespiratory analysis throughout the tests before and after OLT. Averaged group data for tidal volume, breathing frequency or VE showed no difference before and after training. VE variability was decreased by 46.7% after OLT.
Discussion:
CWR VE from rest to work was linear throughout the transition. Following OLT, there was a substantial reduction in VE variability. Future research should investigate the lack of a phasic ventilatory response to exercise, as well as potential mechanisms of ventilatory variability and its implications for functional performance.
Similar content being viewed by others
Introduction
Spinal cord injuries (SCI) can substantially impair the pulmonary,1–3 cardiovascular4,5 and skeletal muscle systems.6 Although there has been substantial emphasis on the neuromuscular7–9 and cardiovascular10,11 adaptations following rehabilitation, adaptations of the pulmonary system of individuals with incomplete SCI (iSCI) in response to locomotor training have been less frequently studied.
In healthy individuals, effective transition from rest to work is accomplished by a hyperpneic response. The hyperpneic response is achieved through a concomitant increase in breathing rate and tidal volume to match ventilation with cellular metabolic demands supporting sustained movement.12 Data on exercise hyperpnea in healthy individuals, during a rest to work transition, exhibits an exponential curve with three phases.12 Phase I typically occurs within the first breath and phase II within 15 s creating the sudden rise in minute ventilation (VE),12 while transition into Phase III marks the point of steady-state isocapnia, proportional to metabolic demand.13 Phase I is normally seen only during a rest to constant work rate (CWR) transition.12 Phase II is characterized by an exponential rise toward steady-state ventilation, and is often determined by a time constant of ~60–70 s.12,13 Phase III begins at steady-state ventilation when there is no further rise in theVE during CWR exercise below the anaerobic threshold, and is typically achieved by the 4th minute of CWR exercise.12
In contrast to healthy individuals, impaired pulmonary function contributes to discrepancies in matching internal and external respiration,14 leading to blood gas abnormalities15,16 and potentially resulting in subsequent peripheral muscle fatigue.17,18 In individuals with SCI, altered pulmonary mechanics19,20 and alveolar hypoventilation14 have been observed; however, the degree to which our current understanding of a phasic model of exercise hyperpnea can be applied to individuals with iSCI is unknown. Exercise hyperpnea, and the tri-phasic response, may be an important tool to measure integrated and synchronized function between the cardiovascular, pulmonary and skeletal muscle systems as all three are required to maintain ventilation and arterial blood gas homeostasis.
Various forms of training, including locomotor training,21 aerobic training22 and inspiratory muscle training,23,24 have been shown to increase lung volumes in the SCI population. However, to our knowledge, no studies have reported on the pulmonary response of individuals with chronic motor iSCI (miSCI) during volitional treadmill walking before and after overground locomotor training (OLT). Therefore, the overarching purpose of this study was to document pulmonary performance in individuals with miSCI, which is operationally defined as the variability inVE and the ability to achieve a phasic response to CWR exercise. Specifically following OLT, our study aimed for the following: (1) to compare the conformity of the rest to work transition in individuals with miSCI during self-selected submaximal treadmill walking to the phasic model of exercise hyperpnea typically observed in health individuals during similar transitions; and (2) to measure the variability in the ventilatory response during submaximal CWR treadmill walking. It was hypothesized that the ventilatory response in individuals with miSCI would be a phasic response with a high degree of variability.
Methods
Study design and participants
This study investigated the effects of OLT on breathing response using a pre/post design. Participants were enrolled in an OLT program that was specifically designed to improve overground walking performance. All participants were identified as chronic cervical motor-iSCI on the American Spinal Injury Association Impairment Scale (AIS) as AIS C or D25 (Table 1). The study inclusion criteria required participants to have the following criteria: (1) be 18 years of age or older; (2) be at least 12 months post-injury; (3) be able to stand with no more than minimal assistance from one other person; (4) be able to initiate and complete at least one step using only volitional control, using ambulation aids (walkers, crutches and canes) as necessary; and (5) demonstrate the ability to walk safely on a treadmill. Exclusion criteria included the following: (1) complete spinal cord injury (AIS A) and incomplete spinal cord injury AIS B; (2) any significant orthopedic complications, spasms or contractures preventing safe ambulation on the treadmill; and (3) any history of ischemic heart disease, known cardiovascular, pulmonary or metabolic diseases, HIV infection or use of antiretroviral therapy. We also asked participants to refrain from engaging in any structured locomotor training activities at least 48 h prior to the initial exercise testing session and while they participated in the study.
Overground locomotion training
Detailed explanation of the OLT program is described elsewhere.26 The OLT program required two 90-minute training sessions per week. Each training session involved five consecutive training segments, all with a particular focus as follows: joint mobility; volitional muscle activation; task-isolation; task-integration; and activity rehearsal. Participants were required to perform all exercises under volitional control, but without the assistance of body-weight support harnesses, robotic devices, electrical stimulation or orthoses and other lower-extremity supportive devices. One individual requested the harness because of the fear of falling; no assistance was provided by the harness during training. Participants were allowed to use ambulation aids during training, but were encouraged to attempt as many steps as possible without using any devices at appropriate points during training sessions as their abilities allowed. All training sessions were implemented by a team of physical therapists and exercise physiologists specifically trained in implementing the OLT program developed for this study.
Testing protocol
All testing procedures were strictly observed. We measured each participant’s height and weight using a standard stadiometer (SECA 213) and scale (Health O Meter 400KL). Measurements of cardiorespiratory function were collected with an Ultima CardiO2 gas exchange system (CardiO2 Ultima, MedGraphics Corp., Saint Paul, MN, USA). Flow calibration was completed prior to each test with a 175 milliliter (ml) calibration syringe for rate and volume. Gas calibration was also performed using precision-analyzed gases to ensure accurate oxygen and carbon dioxide concentrations. Twelve lead electrocardiogram were recorded (Mortara: M12A, Milwaukee, WI, USA) with electrodes placed using standardized skin preparation methods and according to the Ultima system guidelines for a Mason-Likar modification. Pulmonary minute ventilation (VE), tidal volume (VT) and breathing frequency (Bf) were measured continuously breath-by-breath during standing quiet rest and exercise at CWR.
All individuals underwent a 6-minute volitional unaided walking bout at a CWR on a motorized treadmill (Trackmaster TMX22). Participants were instructed to stand quietly for 3 min, prior to walking at their preferred walking speed for 6 min. Preferred walking speed was decided by the individuals by starting the treadmill at 0.5 mph and increasing the speed by 0.1 mph until the participant indicated a comfortable walking speed that could be safely sustained. The same testing procedures were repeated after 12 weeks of OLT with the same preferred walking speed used at pre-testing.
Ethical approval
All procedures were approved by the Institutional Review Board of George Mason University (#618911). Adult informed consent, risks and human subject’s rights were verbally reviewed with the participant following an explanation of the experimental protocol. We then provided all time for participants to review the written forms and procedures prior to entering the study. We certify that all applicable institutional and governmental regulation concerning the ethical use of human volunteers were followed during the course of this research.
Data analysis
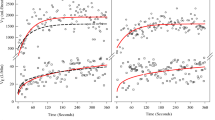
Gas exchange data were analyzed at rest and during the CWR exercise. The averageVE, VT and Bf were averaged taken over the entire CWR. Prior to testing, we assumed that individuals with miSCI would demonstrate a hyperpneic response such as those observed in uninjured healthy individuals (Figure 1). Therefore, the exerciseVE response was fit with the following equation for a phasic response:
where baseline was the 3-minute average of restingVE, amplitude was the difference between baseline restingVE and end-exerciseVE, TD and tau. TD is the time delay, which is the time until the exponential fit begins.27 The tau is the time it takes to reach 63% of the achieved amplitude. However, only one of the six subjects fit the exponential model above (Figure 2). The other five subjects exhibited an exerciseVE response that appeared linear and thus was poorly fit by Equation 1. Therefore, a linear model was also applied to theVE data (Figure 3) for all subjects as follows:
where m is the gradient of the slope, c is the y-intercept and x is the time (seconds) of each data point.
The ventilatory response for a subject with miSCI during a constant work rate test at a self-selected walking speed of 1.7 mph, performed before (a) and following (b) ABR. A mono-exponential fit with residuals and parameters are shown. This was the only subject out of six to display a typical ventilatory response.
The ventilatory response for a representative subject with miSCI during a constant work rate test at a self-selected walking speed of 0.5 mph, performed before (a) and following (b) ABR. A linear fit with residuals are shown. Five of the six subjects had an atypical response, where the mono-exponential model could not be used. A linear model was used instead.
For each subject, the Akaike Information Criterion (AIC) was used to choose between linear and exponential models for the exerciseVE response. The AIC is equal to twice the difference of the number of parameters in the model minus twice its log-likelihood. Smaller AIC values signify a more suitable model. A model was determined to be strictly better than another if its AIC was at least 2% smaller; otherwise the models were considered to be roughly comparable in their ability to predictVE.
Rest to work transition amplitude, which is intensity dependent,28 was calculated from the change in minute ventilation between resting averagedVE and the averagedVE of the first 20 s of exercise.To assessVE variability, the predictedVE utilizing the linear regression fit model was compared to the observedVE (that is,VE variability=VEobs−VEpred) in all participants except for the lone participant whose data were best fit with the exponential model as noted above.
Data are presented as mean (s.d.). Resting and CWR exercise gas exchange data were analyzed using paired sample t-tests (SPSS 22, IBM, Chicago, IL, USA), andVE variability was assessed via an F-statistic for each individual, and the group analyses of variability. Significance was set at P<0.05.
Results
Subjects were five adult males and one female with miSCI between C3 and C6. All participants were between two and five years post injury. Demographics and anthropometric data are presented in Table 1. No changes in anthropometric data were noted following OLT.
The averaged group data for resting VE showed no difference before and after training (Table 2). Group analyses also yielded no significant differences in Bf or VT following OLT at rest (P⩾0.39) or exercise (P⩾0.30; Table 2). Pre-OLT rest to work transition amplitude reported a 4.89 (3.47) l min−1 increase above restingVE. Post-OLT rest to work transition amplitude reported an increase of 4.08 (2.40) (l min−1), which was 0.81 l min−1 less than the rest to work transition before OLT(P=0.45).
The AIC identified the linear model as better than either exponential model for all subjects except one (Participant 6). Participant 6 was therefore excluded from the group analysis of variability. Participant 6’sVE variability significantly increased during rest (6.17–13.62; P=0.01) and exercise (25.09–39.26; P=0.008) following OLT.
Data from a representative subject with linear fit and residuals are shown in Figure 3. ExercisingVE variability was significantly reduced in four of the five participants (Table 3) resulting in a group average reduction of 11.87 arbitrary units (a.u.). The groupVE variability was reduced by 46.7% on average, by 46.7% among the five participants included in this analysis. Participant 6’s data best fit with an exponential curve, which does not allow participant 6’s data to be directly compared to the rest of the variability data where linear models were used in an absence of phasic transitions.
Discussion
Contrary to our hypothesis, the primary finding of this study was that most individuals with cervical miSCI in our study did not show a phasic ventilatory response to treadmill walking at preferred walking speed before or after OLT. In fact, nearly all participants (five out of six) in our study showed a linearVE response to volitional walking that initially presented with a high degree of variability that was subsequently reduced following OLT. Moreover, there were no changes in averaged VE, VT and Bf following OLT training despite the observed changes in variability.
Previous studies of pulmonary dysfunction generally report changes in lung volumes2 and ventilatory muscle mechanics,20,29 which are associated with injury level,1,2,30 physical activity31 and fatigue.23 Pulmonary responses, as measured byVE, during both acute submaximal and maximal exercise in individuals with SCI have demonstrated values similar to32 or lower than30 those in adults without SCI. However, analyses ofVE using data from pre-determined time points that have been averaged may yield skewed descriptions of pulmonary function in SCI as this analytic approach has a limited ability to detect changes inVE resulting from the high degree of VE variability found throughout the first few minutes of exercise. Thus, common reporting of pulmonary data by averaging data during exercise timeframes (for example, entire bout, last 2 min of exercise) may miss potential changes in physiological function in individuals with a SCI (see Table 2 and Figures 1, 3, which may account for the differences between our findings and the results of previous studies). Considering that theVE response is known to have ‘noise’ and follows an exponential time-domain response, averaged ventilatory components may not reach significant changes due to the increasedVE variability (Table 2). ThisVE variability could have two potential sources as follows: (1) variability stemming from the exponential rise inVE in response to exercise; and (2) alterations within mechanical,33,34neural35,36or parenchymal37 constraints potentially stemming from the injury. The methodological approach used in this study aimed to account for the change in the breathing response to physical activity and to quantify the ‘noise’ observed.
Regardless of the therapeutic approach used, the ventilatory musculature is known to respond to several modalities of training. Tiftik et al. investigated locomotor training and pulmonary function in SCI, and reported improvements in vital capacity, forced vital capacity, forced expiratory volume in 1 s and maximum voluntary ventilation in response to body-weight supported treadmill training with varying speeds. However, no measures of ventilation during exercise were reported.21 Additionally, a case report38 described improved locomotor–respiratory coupling in a participant with an incomplete C1/C2 injury walking at 1.0 miles per hour, noting a 10L reduction inVE and a reduction in both Bf and VT. These data demonstrate that locomotor training, under similar walking speeds but not intensity (that is, weight-bearing), is a plausible method for improving pulmonary function in this population. However, other investigators have not reported averaged decreases inVE during end-exercise maximal exertion or submaximal wheelchair interval work.39 These studies illustrate the malleability of the pulmonary system under certain conditions to various forms of exercise training. However, as we note with our reported changes inVE variability, caution should be exercised when evaluating averaged VE during physical exertion in this population. Our data suggest that substantial adaptations to exercise may be obscured because of averaged data. Although our data found no significant difference in averaged VE, Bf or VT during a submaximal bout of volitional treadmill walking, we found significantly reduced variability inVE when the trajectory of VE was accounted for in the methodological approach.
There are a number of potential explanations for the high degree ofVE variability that diminishes following OLT. Decreased variability may be a function of changes in synchronized function between the thoracic and abdominopelvic cavities. Individuals with SCI are known to exhibit paradoxical mechanics of the abdomen and thoracic cavity, which can lead to decrements in lung volumes that are dependent on the level of the lesion,19,20,40 alterations in ventilation perfusion coupling,14 which can disrupt blood gas homeostasis16 and then potentially alter oxygen delivery to the skeletal muscles.41 It is also possible that our participants may have impaired phrenic nerve activity, thus impeding their ability to activate the appropriate musculature1 and maintain transdiaphragmatic pressure.42–44 Difficulties activating musculature and changes in postural control may lead to an increased work of breathing in these participants, which has been associated with the respiratory steal phenomenon17,45 and alterations in peripheral oxygen utilization.18,46,47 OLT may have improved posture that subsequently affected lung volumes, and increased the strength and endurance of the remaining innervated inspiratory and expiratory muscles as non-respiratory movements have been reported to increase diaphragm strength48 and endurance in participants with a SCI.22
These results are clinically relevant as respiratory distress and mechanics are a major impediment to mobility and a leading cause of mortality in incomplete and complete spinal cord injury.49 These data support attempts to promote ventilatory adaptations as a primary outcome of treatment or training to accompany objectives to improve walking performance in individuals with a chronic spinal cord injury.
This study has limitations that should be carefully considered. No measures were taken of the lung volumes, posture or thoracic/abdominal compliance, although these have shown to be involved in ventilation. Thus, we cannot make inferences about the locus of adaptation following our OLT program. The non-phasicVE response may be amenable to several interpretations, none of which can be confirmed by the available data of the current study. Low work rates are associated with faster cardiorespiratory kinetics.28 Therefore, the ventilatory response to exercise may not represent a substantial enough change from resting ventilation to match the metabolic demand of the task that would require an exponential rise inVE. Functionally, the participants may be at or near steady-state ventilation at the onset of exercise, which would result in a linear response along the asymptotic line, and the reduction in ventilatory variability may be a product of more effective ventilatory musculature, posture or pulmonary perfusion, thereby reducing the work of breathing as these individuals also reported a reduction in VO2 following OLT.26 As for the single participant who exhibited increased variability following OLT, this could be a result of improved cardiac function that led to improved ventilation perfusion at the required workload. The improved pulmonary blood flow may have increased the available reserve for ventilation/perfusion; thus large fluctuations in ventilation would not negatively affect arterial blood gases. Additionally, we observed a significant decrease in VT in this participant, which may offer some evidence that more effective ventilatory mechanics resulted in adequate ventilation of the alveoli despite altered airflow measured at the mouth. These results are from a single bout of testing which introduces day-to-day variability, and anxiety/emotional responses to a novel task as potentially limiting factors. Last, we used tools associated with VO2 on-kinetics to allow for a systematic application of our methods, but unlike VO2 on-kinetics, we did not remove any data points outside of the 99% prediction bands, as these data points may be unrelated to gas exchange (for example, coughing/sneezing). Future studies are necessary to determine which of these hypotheses may further explain our findings.
Conclusion
In conclusion, our results demonstrate that ventilatory adaptations to self-selected constant treadmill walking in adults with miSCI are achievable following our novel OLT program. CWRVE from rest to work was linear throughout the transition with no phase III plateau observable within 6 min. A substantial level ofVE variability was observed before OLT, and was reduced by 46.7% after 12–15 weeks of OLT. In these subjects with miSCI, it appears that 12–15 weeks of OLT reduces exercise hyperpnea variability in participants with incomplete cervical spinal cord injuries. Future investigations should examine responses to different modalities of training, potential mechanisms contributing to theVE variability, and an extension of traditional outcome measures typically reported in the literature since the lead cause of death in this population is pulmonary complications.49
References
Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M . Pulmonary function and spinal cord injury. Respir Physiol Neurobiol 2009; 166: 129–141.
Linn WS, Adkins RH, Gong H Jr, Waters RL . Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Arch Phys Med Rehabil 2000; 81: 757–763.
Linn WS, Spungen AM, Gong H Jr, Adkins RH, Bauman WA, Waters RL . Forced vital capacity in two large outpatient populations with chronic spinal cord injury. Spinal Cord 2001; 39: 263–268.
West CR, Bellantoni A, Krassioukov AV . Cardiovascular function in individuals with incomplete spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil 2013; 19: 267–278.
Olive JL, Dudley GA, McCully KK . Vascular remodeling after spinal cord injury. Med Sci Sports Exerc 2003; 35: 901–907.
Hicks AL, Martin Ginis KA, Pelletier CA, Ditor DS, Foulon B, Wolfe DL . The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic review. Spinal Cord 2011; 49: 1103–1127.
Gorgey AS, Poarch H, Harnish C, Miller JM, Dolbow D, Gater DR . Acute effects of locomotor training on neuromuscular and metabolic profile after incomplete spinal cord injury. NeuroRehabilitation 2011; 29: 79–83.
Baldi JC, Jackson RD, Moraille R, Mysiw WJ . Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord 1998; 36: 463–469.
Forrest GF, Sisto SA, Barbeau H, Kirshblum SC, Wilen J, Bond Q et al. Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete AIS-B spinal cord injury. J Spinal Cord Med 2008; 31: 509–521.
Wouda MF, Wejden L, Lundgaard E, Strøm V . Energetic and cardiovascular responses to treadmill walking and stationary cycling in subjects with incomplete spinal cord injury. Spinal Cord 2015; 54: 51–56.
Nash MS, Bilsker S, Marcillo AE, Isaac SM, Botelho LA, Klose KJ et al. Reversal of adaptive left ventricular atrophy following electrically-stimulated exercise training in human tetraplegics. Paraplegia 1991; 29: 590–599.
Wasserman K, Whipp BJ, Casaburi R . Respiratory Control During Exercise. In Comprehensive Physiology. John Wiley & Sons, Inc, 2011, doi:10.1002/cphy.cp030217.
Whipp BJ, Ward SA . Cardiopulmonary coupling during exercise. J Exp Biol 1982; 100: 175–193.
Bergofsky EH . Mechanism for respiratory insufficiency after cervical cord injury; a source of alveolar hypoventilation. Ann Intern Med 1964; 61: 435–447.
Malte CL, Malte H, Wang T . Episodic ventilation lowers the efficiency of pulmonary CO2 excretion. J Appl Physiol 2013; 115: 1506–1518.
Petersson J, Glenny RW . Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J 2014; 44: 1023–1041.
Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB et al. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol 1998; 85: 609–618.
Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB et al. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 1997; 82: 1573–1583.
Estenne M, De Troyer A . Relationship between respiratory muscle electromyogram and rib cage motion in tetraplegia. Am Rev Respir Dis 1985; 132: 53–59.
De Troyer A, Estenne M, Vincken W . Rib cage motion and muscle use in high tetraplegics. Am Rev Respir Dis 1986; 133: 1115–1119.
Tiftik T, Gökkaya NKO, Malas FÜ, Tunç H, Yalçın S, Ekiz T et al. Does locomotor training improve pulmonary function in patients with spinal cord injury? Spinal Cord 2015; 53: 467–470.
Silva AC, Neder JA, Chiurciu MV, Pasqualin DC, da Silva RC, Fernandez AC et al. Effect of aerobic training on ventilatory muscle endurance of spinal cord injured men. Spinal Cord 1998; 36: 240–245.
Rutchik A, Weissman AR, Almenoff PL, Spungen AM, Bauman WA, Grimm DR . Resistive inspiratory muscle training in subjects with chronic cervical spinal cord injury. Arch Phys Med Rehabil 1998; 79: 293–297.
Wang T-G, Wang Y-H, Tang F-T, Lin K-H, Lien I-N . Resistive inspiratory muscle training in sleep-disordered breathing of traumatic tetraplegia. Arch Phys Med Rehabil 2002; 83: 491–496.
Kirshblum S, Waring W 3rd . Updates for the International Standards for neurological classification of spinal cord injury. Phys Med Rehabil Clin N Am 2014; 25: 505.
Gollie JM, Guccione AA, Panza GS, Jo PY, Herrick JE . Effects of overground locomotor training on walking performance in chronic cervical motor-incomplete spinal cord injury: a pilot study. Arch Phys Med Rehabil 2016: 31292–31298.
Whipp BJ, Ward SA, Lamarra N, Davis JA, Wasserman K . Parameters of ventilatory and gas exchange dynamics during exercise. J Appl Physiol 1982; 52: 1506–1513.
Casaburi R, Barstow TJ, Robinson T, Wasserman K . Influence of work rate on ventilatory and gas exchange kinetics. J Appl Physiol 1989; 67: 547–555.
De Troyer A, Heilporn A . Respiratory mechanics in quadriplegia. The respiratory function of the intercostal muscles. Am Rev Respir Dis 1980; 122: 591–600.
Bodin P, Kreuter M, Bake B, Olsén MF . Breathing patterns during breathing exercises in persons with tetraplegia. Spinal Cord 2003; 41: 290–295.
Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E . Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr 1998; 68: 1223–1227.
Loveridge BM, Dubo HI . Breathing pattern in chronic quadriplegia. Arch Phys Med Rehabil 1990; 71: 495–499.
Johnson BD, Saupe KW, Dempsey JA . Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol 1992; 73: 874–886.
Estenne M, De Troyer A . Mechanism of the postural dependence of vital capacity in tetraplegic subjects. Am Rev Respir Dis 1987; 135: 367–371.
Eldridge FL . Relationship between phrenic nerve activity and ventilation. Am J Physiol 1971; 221: 535–543.
Forster HV, Haouzi P, Dempsey JA . Control of breathing during exercise. Compr Physiol 2012; 2: 743–777.
Dempsey JA, Harms CA, Ainsworth DM . Respiratory muscle perfusion and energetics during exercise. Med Sci Sports Exerc 1996; 28: 1123–1128.
Sherman MFB, Lam T, Sheel AW . Locomotor-respiratory synchronization after body weight supported treadmill training in incomplete tetraplegia: a case report. Spinal Cord 2009; 47: 896–898.
Le Foll-de Moro D, Tordi N, Lonsdorfer E, Lonsdorfer J . Ventilation efficiency and pulmonary function after a wheelchair interval-training program in subjects with recent spinal cord injury. Arch Phys Med Rehabil 2005; 86: 1582–1586.
Scanlon PD, Loring SH, Pichurko BM, McCool FD, Slutsky AS, Sarkarati M et al. Respiratory mechanics in acute quadriplegia. Lung and chest wall compliance and dimensional changes during respiratory maneuvers. Am Rev Respir Dis 1989; 139: 615–620.
Richardson RS . Oxygen transport: air to muscle cell. Med Sci Sports Exerc 1998; 30: 53–59.
Hart N, Laffont I, de la Sota AP, Lejaille M, Macadou G, Polkey MI et al. Respiratory effects of combined truncal and abdominal support in patients with spinal cord injury. Arch Phys Med Rehabil 2005; 86: 1447–1451.
Baydur A, Adkins RH, Milic-Emili J . Lung mechanics in individuals with spinal cord injury: effects of injury level and posture. J Appl Physiol 2001; 90: 405–411.
Thomaz S, Beraldo P, Mateus S, Horan T, Leal JC . Effects of partial isothermic immersion on the spirometry parameters of tetraplegic patients. Chest 2005; 128: 184–189.
Harms CA . Effect of skeletal muscle demand on cardiovascular function. Med Sci Sports Exerc 2000; 32: 94–99.
Wetter TJ, Harms CA, Nelson WB, Pegelow DF, Dempsey JA . Influence of respiratory muscle work on VO(2) and leg blood flow during submaximal exercise. J Appl Physiol 1999; 87: 643–651.
Chin LMK, Heigenhauser GJF, Paterson DH, Kowalchuk JM . Pulmonary O2 uptake and leg blood flow kinetics during moderate exercise are slowed by hyperventilation-induced hypocapnic alkalosis. J Appl Physiol 2010; 108: 1641–1650.
DePalo VA, Parker AL, Al-Bilbeisi F, McCool FD . Respiratory muscle strength training with nonrespiratory maneuvers. J Appl Physiol 2004; 96: 731–734.
Frankel HL, Coll JR, Charlifue SW, Whiteneck GG, Gardner BP, Jamous MA et al. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord 1998; 36: 266–274.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Panza, G., Guccione, A., Chin, L. et al. Effects of overground locomotor training on the ventilatory response to volitional treadmill walking in individuals with incomplete spinal cord injury: a pilot study. Spinal Cord Ser Cases 3, 17011 (2017). https://doi.org/10.1038/scsandc.2017.11
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2017.11
This article is cited by
-
Locomotor-respiratory coupling in ambulatory adults with incomplete spinal cord injury
Spinal Cord Series and Cases (2022)
-
Effect of repeated locomotor training on ventilatory measures, perceived exertion and walking endurance in persons with motor incomplete spinal cord injury
Spinal Cord Series and Cases (2020)
-
Effect of overground locomotor training on ventilatory kinetics and rate of perceived exertion in persons with cervical motor-incomplete spinal cord injury
Spinal Cord Series and Cases (2019)