Abstract
Study design:
Case report and literature review.
Objectives:
Gouty arthritis of the spine is rare. Gout presenting as back pain and quadriplegia may be difficult to distinguish from a spinal tumor. Symptoms vary, and the diagnosis is often delayed. We report an unusual case of thoracic spinal cord compression caused by extradural tophaceous deposits whose initial diagnosis had been lymphoid malignancy. To the best of our knowledge, this is only the second report of using single-photon emission computed tomography (SPECT) to diagnose spinal tophus.
Methods:
We retrospectively reviewed the medical records, operative reports and radiographic imaging studies of a single patient.
Results:
A 26-year-old man with severe tophaceous gout presented with a 4-month history of progressive weakness and dyschesia of both lower extremities. Coronal bone slices evaluated by SPECT indicated increased methylene diphosphonate uptake in the T9 and T10 pedicles. Pathology assessment revealed areas of amorphous substance containing urate crystals surrounded by inflammatory cells. The diagnosis was gouty tophus.
Conclusion:
The position of the spinal tophus may be related to the ‘S’ type of spinal anatomical structure. Obesity and inactivity may be the two risk factors for spinal tophus. Every effort should be made to lower the serum uric acid level by maximizing the pharmacological regimen. We believe that laminectomy can effectively relieve spinal cord compression. It also improves the long-term prognosis for spinal gouty tophus. SPECT may be a viable method for differentiating spinal gout and a malignant tumor.
Similar content being viewed by others
Introduction
We report a case of gouty arthritis affecting the thoracic spine in a 26-year-old man with thoracic spinal cord compression caused by extradural tophaceous deposits that had initially been diagnosed as lymphoid malignancy.
Case report
A 26-year-old man weighing 160 kg who had known severe tophaceous gout for more than 4 years presented with a 4-month history of progressive weakness in both lower extremities and dyschesia. The bilateral lower limbs had been paralysed for nearly 10 days. He had lost about 10 kg of weight during the previous 2 months. His medical history included 10 years of significant hypertension. A general physical examination revealed that he had multiple subcutaneous nodules at the back of both feet with swelling and tenderness of the local skin (Figure 1), suggesting gouty tophi. Neurological examination showed somaesthesia deficit below the T10 level. In the lower extremities, he had 1/5 strength and bilateral high muscle tension.
Laboratory evaluations revealed that the serum urate level was elevated at 810 μmmol l−1 (normal range 140−414 μmmol l−1), uric acid 16 mmol per 24 h (normal range 1.49−4.47 mmol per 24 h), blood urea nitrogen 17 mmol l−1 (normal range 10−20 mmol l−1), C-reactive protein (CRP) 15.6 mg l−1 (normal ⩽10 mg l−1) and creatinine 89 μmmol l−1 (normal range 54−106 μmmol l−1). The white blood cell count was 8.9×109 l−1 (normal range 4.0−10.0×109 l−1) with 93% neutrophils, 12% lymphocytes and 5% monocytes.
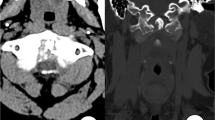
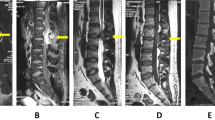
Combining with his history and age, we initially thought that his problems could be caused by a lymphatic hematopoietic systematic malignant tumor. Because the patient’s weight surpassed the examination bed limit, he failed to complete the positron emission tomography–computed tomography (PET–CT) examination. We therefore performed single-photon emission computed tomography (SPECT) bone imaging (the tracer was 99Tcm-MDP at a dose of 30 mCi). The results suggested abnormal tracer distribution at T9–10 with increased uptake at the corresponding sites (Figure 2).
(a) Thoracic CT scan demonstrated lytic cloud-like lesions localized to the facet joints and costovertebral joints of the T10–11 vertebral level and extended to bilateral intervertebral foramens. (b) Axial T1-weighted images showed that the lytic lesions in the left pedicles of T9 and T10 were isointense on T1-weighted imaging. (c) Emission computed tomography (SPECT) bone imaging examination (tracer: 99Tcm-MDP, tracer dose of 30 mCi) indicated an increase in methylene diphosphonate uptake in the right pedicles of T9 and T10.
Although the initial imaging diagnosis was lymphoid malignancy, surgery revealed a large mass with white chalky material in the spinal canal that was eroding into the adjacent pars intra-articularis, granules with smooth edges and blood circulation, separated by their integument from surrounding tissues. Tissue fibrosis was also evident. Pathological results showed areas of an amorphous substance containing urate crystals surrounded by inflammatory cells and a multinucleated giant cell granuloma. The final diagnosis was gouty tophus (Figure 3). No features of a tumorous or an infectious process were seen. Postoperative bacteriological examinations of multiple specimens that included special procedures for detecting mycobacteria were all negative.
(a) During the surgery, a large mass with white chalky material in the vertebrae thoracales was found eroding into the adjacent pars intra-articularis, accompanied by the tissue fibrosis. (b) The granule had a smooth edge and medium blood circulation. (c) Pathological results showed areas of an amorphous substance containing urate crystals surrounded by inflammatory cells and multinucleated giant cell granuloma (hematoxylin and eosin, ×200).
The patient’s lower extremity muscle strength grade and muscle tension returned to normal 6 months after his discharge.
Discussion
Gout is a common metabolic disorder resulting from supersaturation of urate in extracellular fluid and deposition of monosodium urate crystals in tissues. Urate crystals easily deposit to form a tophus in local tissue that has a low pH.1–3 Few tophaceous deposits have been found in the axial skeleton; however, gouty tophus of the thoracic vertebrae is rare. Factors that may increase the precipitation of urate crystals and induce tophus formation include low temperature, decreased pH and trauma.4,5
An analysis of the literature indicated that all segments of the spine could be involved with gout.6–9 The lower lumbar spine is the most common region involved, particularly the L4–5 level.10–13 Only 20 cases of tophus in the dorsal vertebrae have been described in the literature (see Table 16–20). These patients’ ages ranged from 33 to 86 years, 75% were male and most of them had a history of being bedridden.14,15 Our patient had a short history of gout and severe clinical symptoms. The only clue was the prolonged period of poorly controlled hyperuricemia. We think that the position of spinal tophi might be related to ‘S’-type spinal anatomical structure. We also believe that patients who are obese and those with little daily activity could be at risk for axial bone tophus.
High CRP levels have been measured in patients with this diagnosis. Recent studies have suggested that uric acid induces expression of CRP messenger RNA in vascular endothelial and smooth muscle cells.16 In addition, serum urate was associated with serum CRP in patients with gout. It is thus suggested that a high CRP level may be an effective means for predicting a risk of spinal tophus. Additional examinations of more patients with spinal gout are needed, however, to confirm this view.
In this case, the patient sustained three-column spinal disruption from low-energy trauma with only posterior ankylosis; the injury anteriorly goes through the disc space. We posit that biologic remodeling precedes damage to the spinal facet joints structure and that repeated injuries induce persistent inflammation. As intervertebral disc disease progresses, increased stress is applied posteriorly, thus accelerating facet osteoarthritis and urate crystal deposit. We consider that low-energy trauma is the end result of the interplay between subtle alterations in mechanical and biochemical properties of the urate crystal and facet joint complex. As we all know, the facet joint complex has an important role in stabilizing the segmental spinal unit; the resultant spinal gout is likely to change the segmental spinal motion, altering the mechanical forces experienced by the facet joints. These compositional and structural alterations manifest in repeatable patterns of altered motion segment biomechanics. Consequently, in the beginning, spinal gout only affects immediately surrounding tissue with the potential to diminish pressure containment ability, then annulus fibrosus injuries disrupt lamellar fibers sufficiently to compromise the vertebral connectivity and diminish pressure containment, collectively altering spinal column motion.
Computed tomography and especially magnetic resonance imaging should be considered useful instruments in the differential diagnosis of patients who have a prior diagnosis of gout or a history of hyperuricemia who present with symptoms suggesting spinal cord involvement. Histological or cytological analysis is still necessary, however, for a definitive diagnosis, as infectious processes and neoplasms may have similar clinical and imaging aspects.17 There has been no research reported on SPECT imaging of tophi. We found that the imaging in our patient was different from the typical image of bone metastases. Thus, SPECT may prove to be a viable method for preliminarily differentiating between spinal gout and a malignant tumor. Unfortunately, our patient’s weight surpassed the examination bed maximum limit, and he failed to complete the PET–CT examination. At present, only one case report has described the radiological features of spinal gout, and it was based on the results of fluorodeoxyglucose PET.18
Conclusions
The mechanism associated with axial gout is not yet clear, but obesity and inactivity may be two risk factors for spinal tophus. By the time tophaceous deposits in the axial skeleton are identified, the gout is at an advanced stage. Thus, early recognition of spinal gout is extremely important because aggressive medical management of gout could limit the morbidity associated with this condition, avoiding the need for spinal surgery. Conservative treatment could prevent an unnecessary operation, but at present it poses the risk of a diagnostic error in differentiating between gout and tumor. We advocate surgical treatment by segmental spinal decompression, with or without stabilization, followed by long-term treatment with allopurinol to dissolve tophaceous deposits and prevent recurrent attacks of acute gout. However, it would be wise to remember that the differential diagnosis between spinal gout and a malignant tumor is important when patients have a history of gout. An intraoperative frozen section examination should be undertaken to assist with the treatment strategy. The long-term effect of medical treatment for spinal gout has not been reported, and thus prospective studies are needed.
References
Thornton FJ, William CT, Brennan P . Tophaceous gout of the lumbar spine in a renal transplant patient: a case report and literature review. Eur J Radiol 2000; 36: 123–125.
Dhote R, Roux FX, Bachmeyer C, Tudoret L, Daumas-Duport C, Christoforov B . Extradural spinal tophaceous gout: evolution with medical treatment. Clin Exp Rheum 1997; 15: 421–423.
Mekelburg K, Rahimi AR . Gouty arthritis of the spine: clinical presen-tation and effective treatments. Geriatrics 2000; 55: 71–73.
Alarcon GS, Reveille JD . Gouty arthritis of the axial skeleton including the sacroiliac joints. Arch Intern Med. 1987; 147: 2018–2019.
Pankhania AC, Patankar T, Du Plessis D . Neck pain: an unusual presentation of a common disease. Br J Radiol 2006; 79: 537–539.
Suk KS, Kim KT, Lee SH, Park SW, Park YK . Tophaceous gout of the lumbar spine mimicking pyogenic discitis. Spine J 2007; 7: 94–99.
Yasuhara K, Tomita Y, Takayama A, Fujikawa H, Otake Y, Takahashi K . Thoracic myelopathy due to compression by the epidural tophus: a case report. J Spinal Disord Tech. 1994; 7: 82–85.
Cabot J, Mosel L, Kong A, Hayward M . Tophaceous gout in the cervical spine. Skeletal Radiol. 2005; 34: 803–806.
Hasegawa EM, de Mello FM, Goldenstein-Schainberg C, Fuller R . Gout in the spine. Rev Bras Reumatol 2013; 53: 296–302.
Wang LC, Hung YC, Lee EJ, Chen HH . Acute paraplegia in a patient with spinal tophi: a case report. J Formos Med Assoc. 2001; 100: 205–208.
Lam HY, Cheung KY, Law SW, Fung KY . Crystal arthropathy of the lumbar spine: a report of 4 cases. J Orthop Surg (Hong Kong) 2007; 15: 94–101.
Magid SK, Gray GE, Anand A . Spinal cord compression by tophi in a patient with chronic polyarthritis: case report and literature and literature review. Arthritis Rheum. 1981; 24: 1431–1434.
Vervaeck M, De Keyser J, Pauwels P, Frecourt N, D'Haens J, Ebinger G . Sudden hypotonic paraparesis caused by tophaceous gout of the lumbar spine. Clin Neurol Neurosurg. 1991; 93: 233–236.
Leaney BJ, Calvert JM . Tophaceous gout producing spinal cord compression. J Neurosurg. 1983; 58: 580–582.
Souza AW, Fontenele S, Carrete H, Fernandes AR, Ferrari AJ . Involvement of the thoracic spine in tophaceous gout. A case report. Clin Exp Rheum 2002; 20: 228–230.
Kang DH, Park SK, Lee IK, Johnson RJ . Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am SocNephrol 2005; 16: 3553–3562.
Duprez TP, Malghem J, Vande Berg BC, Noel HM, Munting EA, Maldague BE . Gout in the cervical spine: MR pattern mimicking diskovertebral infection. AJNR Am J Neuroradiol 1996; 17: 151–153.
Popovich T, Carpenter JS, Rai AT, Carson LV, Williams HJ, Marano GD . Spinal cord compression by tophaceous gout with fluorodeoxyglucose-positron-emission tomographic/ MR fusion imaging. AJNR Am J Neuroradiol 2006; 27: 1201–1203.
Yoon JW, Park KB, Park H, Kang DH, Lee CH, Hwang SH et al. Tophaceous gout of the spine causing neural compression. Korean J Spine 2013; 10: 185–188.
Kwan BY, Osman S, Barra L . Spinal gout in a young patient with involvement of thoracic, lumbar and sacroiliac regions. Joint Bone Spine 2013; 80: 667–668.
Gongidi P, Gough-Fibkins S . Spondyloarthritis: a gouty display. J Radiol Case Rep 2010; 4: 13–18.
Ntsiba H, Makosso E, Moyikoua A . Thoracic spinal cord compression by a tophus. Joint Bone Spine 2010; 77: 187–188.
Kao MC, Huang SC, Chiu CT, Yao YT . Thoracic cord compression due to gout: a case report and literature review. J Formos Med Assoc 2000; 99: 572–575.
Chan AT, Leung JL, Sy AN, Wong WW, Lau KY, Ngai WT et al. Thoracic spinal gout mimicking metastasis. Hong Kong Med J 2009; 15: 143–145.
Tsai CH, Chen YJ, Hsu HC, Chen HT . Bacteremia coexisting with tophaceous gout of the spine mimicking spondylodiscitis: a case report. Spine (Phila Pa 1976) 2009; 34: E106–E109.
Hsu CY, Shih TT, Huang KM, Chen PQ, Sheu JJ, Li YW . Tophaceous gout of the spine: MR imaging features. Clin Radiol 2002; 57: 919–925.
St George E, Hillier CE, Hatfield R . Spinal cord compression: an unusual neurological complication of gout. Rheumatology (Oxford) 2001; 40: 711–712.
Hausch R, Wilkerson M, Singh E, Reyes C, Harrington T . Tophaceous gout of the thoracic apine presenting as back pain and fever. J Clin Rheumatol 1999; 5: 335–341.
Bret P, Ricci AC, Saint-Pierre G, Mottolese C, Guyotat J . Thoracic spinal cord compression by a gouty tophus. Case report. Review of the literature. Neurochirurgie 1999; 45: 402–406.
Pfister AK, Schlarb CA, O'Neal JF . Vertebral erosion, paraplegia and spinal gout. AJR Am J Roentgenol 1998; 171: 1430–1431.
Dhôte R, Roux FX, Bachmeyer C, Tudoret L, Daumas-Duport C, Christoforov B . Extradural spinal tophaceous gout: evolution with medical treatment. Clin Exp Rheumatol 1997; 15: 421–423.
Downey PR, Brophy BP, Sage MR . Four unusual cases of spinal cord compression. Australas Radiol 1987; 31: 136–141.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr Zhu had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study conception and design was by TZ, TL and HL. Acquisition of data was by TL and HL. Analysis and interpretation of data was by TZ, TL and HL.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, T., Liu, H. & Zhu, T. Thoracic spinal cord compression by extradural tophus: a case report and review of the literature. Spinal Cord Ser Cases 1, 15015 (2015). https://doi.org/10.1038/scsandc.2015.15
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2015.15
This article is cited by
-
Beyond Medical Treatment: Surgical Treatment of Gout
Current Rheumatology Reports (2021)
-
A rare cause of back pain and radiculopathy – spinal tophi: a case report
Journal of Medical Case Reports (2019)